Helicobacter pylori IgG antibody enzyme linked immunosorbent assay (ELISA) diagnostic kit
A technology for Helicobacter pylori and immunodiagnosis, which is applied in the field of medical biology, can solve the problems of poor stability and short storage period, and achieve the effects of high accuracy, improved storage period and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
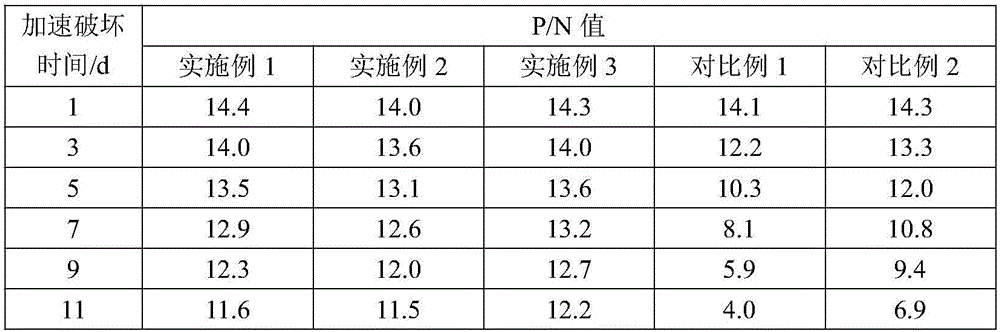
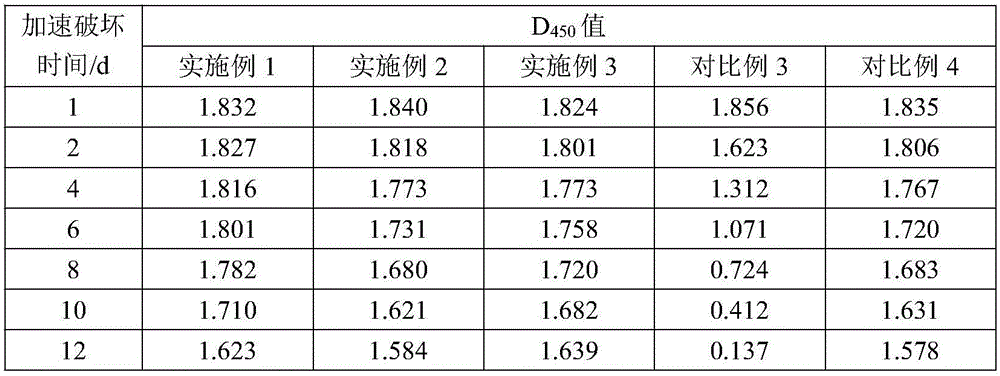
Embodiment 1
[0025] Embodiment 1, Helicobacter pylori IgG antibody ELISA diagnostic kit of the present invention
[0026] The kit of this embodiment consists of Helicobacter pylori antigen-coated plate (96 wells), 1 bottle of sample diluent, 1 bottle of enzyme labeling solution, 1 bottle of washing solution, 1 bottle of chromogenic solution, 1 bottle of negative control substance, and 1 bottle of positive control substance 1 bottle and 1 bottle of stop solution.
[0027] Wherein, the preparation process of the Helicobacter pylori antigen-coated plate is as follows: after the Helicobacter pylori antigen for coating is diluted with 0.05mol / L carbonate buffer solution of pH 9.5, the plate is coated with 0.1mL / well. After absorbing at 4°C for 24 hours, wash the plate with 0.05mol / L pH9.5 phosphate buffer, then block with blocking solution at 4°C for 18 hours, discard the excess blocking solution, and vacuum After drying, the Helicobacter pylori antigen-coated plate was prepared.
[0028] The...
Embodiment 2
[0036] Embodiment 2, Helicobacter pylori IgG antibody ELISA diagnostic kit of the present invention
[0037] The kit of this embodiment consists of Helicobacter pylori antigen-coated plate (96 wells), 1 bottle of sample diluent, 1 bottle of enzyme labeling solution, 1 bottle of washing solution, 1 bottle of chromogenic solution, 1 bottle of negative control substance, and 1 bottle of positive control substance 1 bottle and 1 bottle of stop solution.
[0038]Wherein, the preparation process of the Helicobacter pylori antigen-coated plate is as follows: after the Helicobacter pylori antigen for coating is diluted with 0.05mol / L carbonate buffer solution of pH 9.5, the plate is coated with 0.1mL / well. After absorbing in the microwell plate at 8°C for 26 hours, wash the plate with 0.05mol / L pH9.5 phosphate buffer, then block with blocking solution at 8°C for 20 hours, discard the excess blocking solution, and vacuum After drying, the Helicobacter pylori antigen-coated plate was p...
Embodiment 3
[0047] Embodiment 3, Helicobacter pylori IgG antibody ELISA diagnostic kit of the present invention
[0048] The kit of this embodiment consists of Helicobacter pylori antigen-coated plate (96 wells), 1 bottle of sample diluent, 1 bottle of enzyme labeling solution, 1 bottle of washing solution, 1 bottle of chromogenic solution, 1 bottle of negative control substance, and 1 bottle of positive control substance 1 bottle and 1 bottle of stop solution.
[0049] Wherein, the preparation process of the Helicobacter pylori antigen-coated plate is as follows: after the Helicobacter pylori antigen for coating is diluted with 0.05mol / L carbonate buffer solution of pH 9.5, the plate is coated with 0.1mL / well. After absorbing in the microwell plate at 6°C for 24 hours, wash the plate with 0.05mol / L pH9.5 phosphate buffer, then block with blocking solution at 6°C for 18 hours, discard the excess blocking solution, and vacuum After drying, the Helicobacter pylori antigen-coated plate was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com