Recombinant helicobacter pylori protein vaccine and preparation method thereof
A technology of Helicobacter pylori and protein is applied in the field of recombinant Helicobacter pylori protein vaccine and its preparation, and achieves the effects of important social value, economic value and high immune effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthetic gene:
[0033] Using the nucleic acid sequence of Escherichia coli heat-labile enterotoxin gene (GenBank: AB011677.1) published by GenBank as a template for transformation, the specific design is as follows:
[0034] The gene encoding LT is 1148bp in length, including: LTA signal peptide (18 amino acids) gene, LTA (240 amino acids) gene, LTB signal peptide (21 amino acids) gene and LTB (103 amino acids) gene . There is a frameshift reading frame between the end of the gene encoding LTA and the beginning of the gene encoding LTB signal peptide, so the gene encoding the entire LT is a continuous DNA sequence. Its complete gene nucleotide sequence is shown in Seq ID No.4.
[0035] After bioinformatics analysis and structural analysis, the 100-585bp coding nucleotide sequence of the LTA subunit gene was removed, and the Helicobacter pylori urease A subunit gene fragment ureA 1 Insert it to replace the removed LT A subunit gene coding nucleotide sequence 100-585...
Embodiment 2
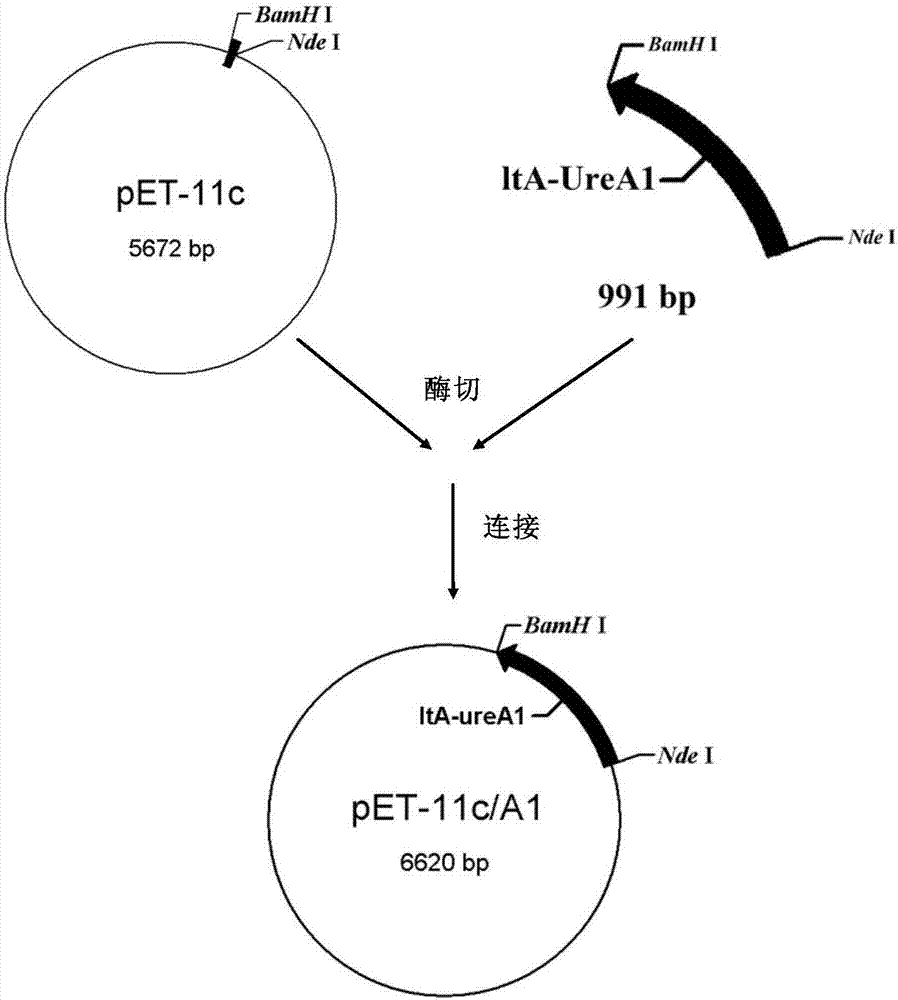
[0038] Recombinant plasmid pET-11c / lt-ureA 1 construction, such as figure 1 Shown:
[0039] The expression vector was pET-11c, which was purchased from Novagen. The expression vector carries the ampicillin resistance gene, is a mode of chemical substance IPTG induction control, and has a high expression level. Before induction, lacIq encodes a repressor protein, which can be combined with the operator gene in the operon to control the transcription of adjacent structural genes; when the inducer exists, the repressor protein is affected by the inducer, and its configuration occurs Changes, so that the loss of affinity with the operator is released, and the polymerase can transcribe the structural gene and then express it. Insert the recombinant target gene containing LTA signal peptide and the ltB gene containing LTB signal peptide behind the NdeI site of the plasmid, and pET-11c can co-express the recombinant target protein LTA in the host bacteria 1 -UreA 1 and LTB prote...
Embodiment 3
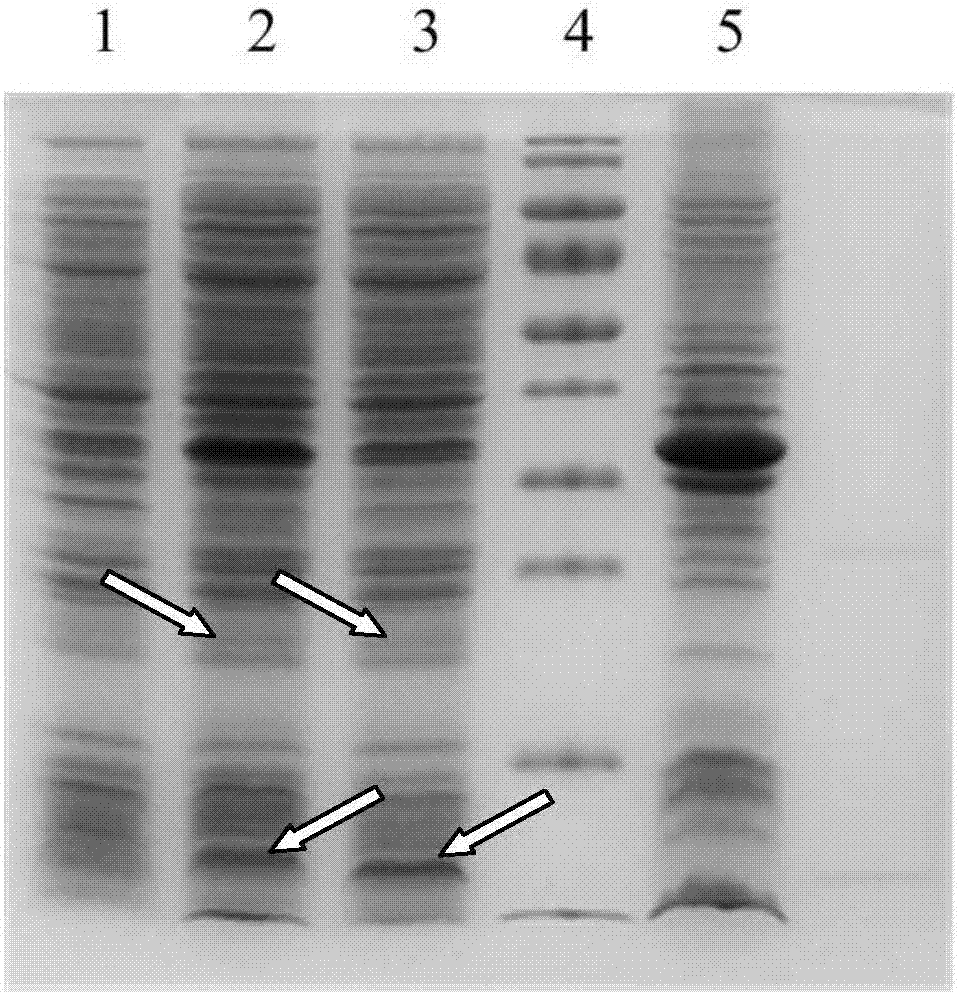
[0046] Expression, purification and identification of target protein:
[0047] The host bacteria used was E.coli / BL21(DE3), which was purchased from Tiangen Biochemical Technology (Beijing) Co., Ltd. Genotype: F-ompT gal dcm lon hsdSB(rB-mB-)λ(DE3[lacI lacUV5-T7 gene 1 ind1 sam7 nin5]). The bacterial strain is used as a host for protein expression of high-efficiency exogenous genes using T7 RNA polymerase as an expression system. The expression of the T7 phage RNA polymerase gene is controlled by the lacUV5 promoter in the DE3 region of the lambda phage, which is integrated on the chromosome of BL21. The strain is suitable for the expression of non-toxic protein.
[0048] (1) Conversion
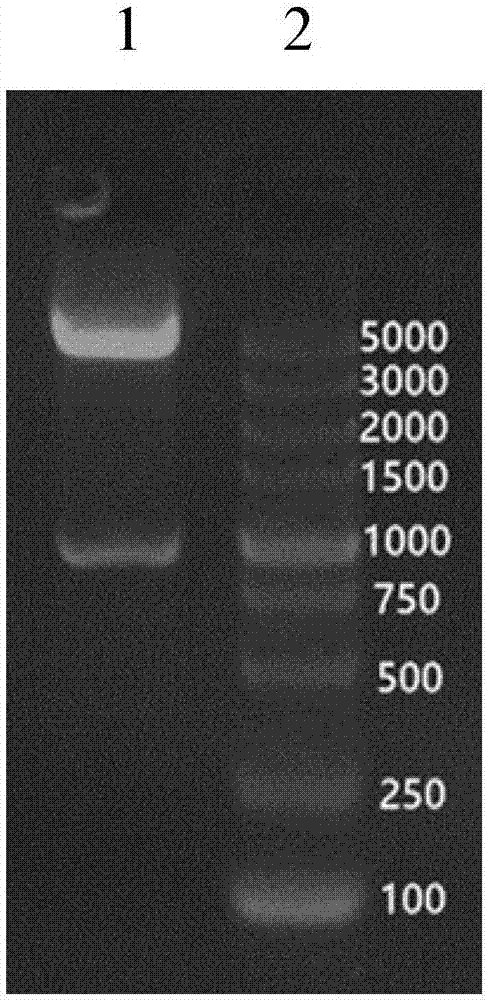
[0049] Take the recombinant plasmid pET-11c / lt-ureA 1 E.coli / BL21(DE3) competent cells (TIANGEN, Code: CB105) were transformed according to the operating instructions, and positive recombinants were screened on ampicillin-resistant LB plates.
[0050] (2) Identification of recombinant en...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com