Escherichia coli heat-labile enterotoxin gene fragment and application thereof
A heat-resistant enterotoxin and gene fragment technology, which can be used in applications, genetic engineering, plant genetic improvement, etc., can solve the problems of inability to eradicate bacterial infection, toxicity of large ADP-ribosyltransferase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
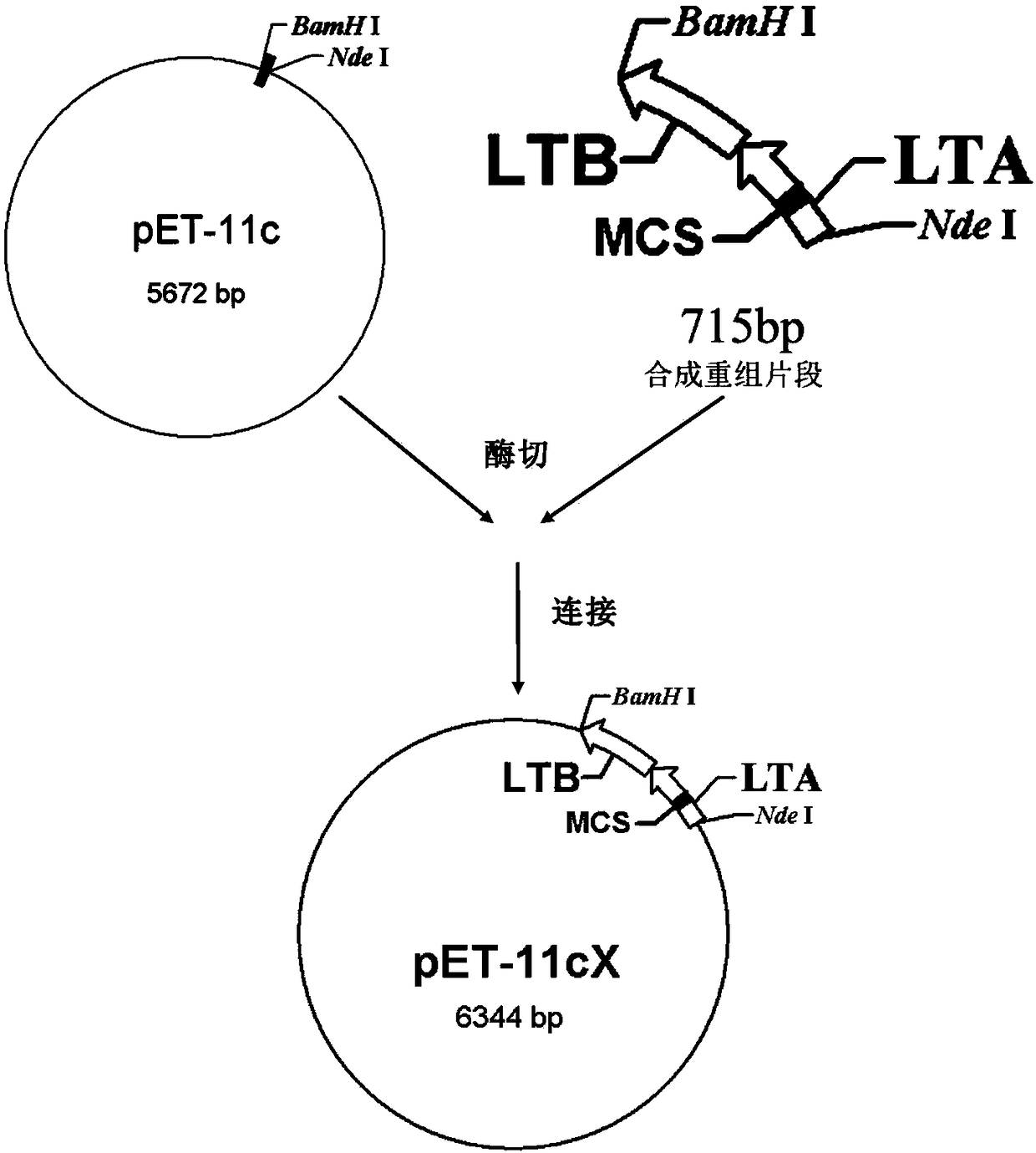
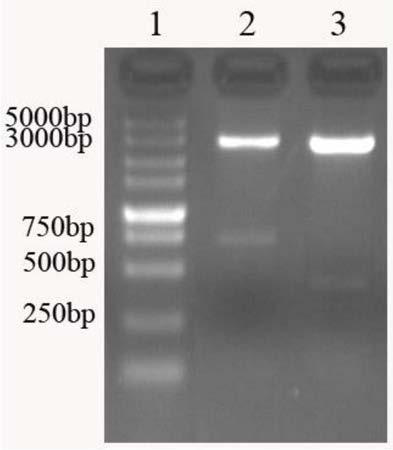
[0048] Construction of recombinant plasmids:
[0049] Using the nucleotide sequence of the Escherichia coli heat-labile enterotoxin gene (GenBank: AB011677.1) published by GenBank as a template, the complete nucleotide sequence is shown in Seq ID No.1, the modified sequence was designed, and a multiple cloning site was introduced. The specific design is as follows:
[0050] The gene encoding LT is 1148bp in length, including: LTA signal peptide (18 amino acids) gene, LTA (240 amino acids) gene, LTB signal peptide (21 amino acids) gene and LTB (103 amino acids) gene . There is a frameshift reading frame between the end of the gene encoding LTA and the beginning of the gene encoding LTB signal peptide, so the gene encoding the entire LT is a continuous DNA sequence.
[0051] The nucleotide sequence of the gene encoding LTA is shown in Seq ID No.2. After bioinformatics analysis and structural analysis, the 100-585bp coding nucleotide sequence of the LTA subunit gene was removed...
Embodiment 2
[0059] Helicobacter pylori adhesin HpaA gene fragment inserted into pET-11cX to express recombinant protein to prepare vaccine:
[0060] (1) Obtaining of HpaA gene fragment hpaA1 of Helicobacter pylori adhesin:
[0061] The nucleotide sequence of the Helicobacter pylori adhesin hpaA1 gene fragment is shown in Seq ID No.4, the gene sequence was synthesized by Beijing Liuhe Huada Gene Technology Co., Ltd., the NcoI restriction site was introduced upstream, and the XhoI enzyme was introduced downstream Insert the cutting site into the commonly used cloning vector pUC19 to obtain the pUC19 / hpaA1 synthetic plasmid.
[0062] (2) Digestion:
[0063] The synthesized pET-11cX and pUC19 / hpaA1 synthetic plasmids were double-digested with restriction endonucleases NcoI+XhoI, and operated according to the instructions. The enzyme digestion reaction system was prepared as follows:
[0064] Table 2 Recombinant plasmid construction enzyme digestion reaction system
[0065]
[0066] The...
Embodiment 3
[0092] Adenovirus capsomer protein Hexon gene fragment inserted into pET-11cX to express recombinant protein to prepare vaccine:
[0093] (1) Acquisition of adenovirus capsomer protein Hexon gene fragment hexon-1:
[0094] The nucleotide sequence of the adenovirus capsomer protein Hexon gene fragment hexon-1 is shown in Seq ID No.5. The gene sequence was synthesized by Beijing Liuhe Huada Gene Technology Co., Ltd., and the NcoI restriction site was introduced upstream, and the XhoI enzyme was introduced downstream Insert the cutting site into the commonly used cloning vector pUC19 to obtain the pUC19 / hexon-1 synthetic plasmid.
[0095] (2) Digestion:
[0096] The synthesized pET-11cX and pUC19 / hexon-1 plasmids were double-digested with restriction endonucleases NcoI+XhoI, and operated according to the instructions. The enzyme digestion reaction system was prepared as follows:
[0097] Table 3 Recombinant plasmid construction enzyme digestion reaction system
[0098]
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com