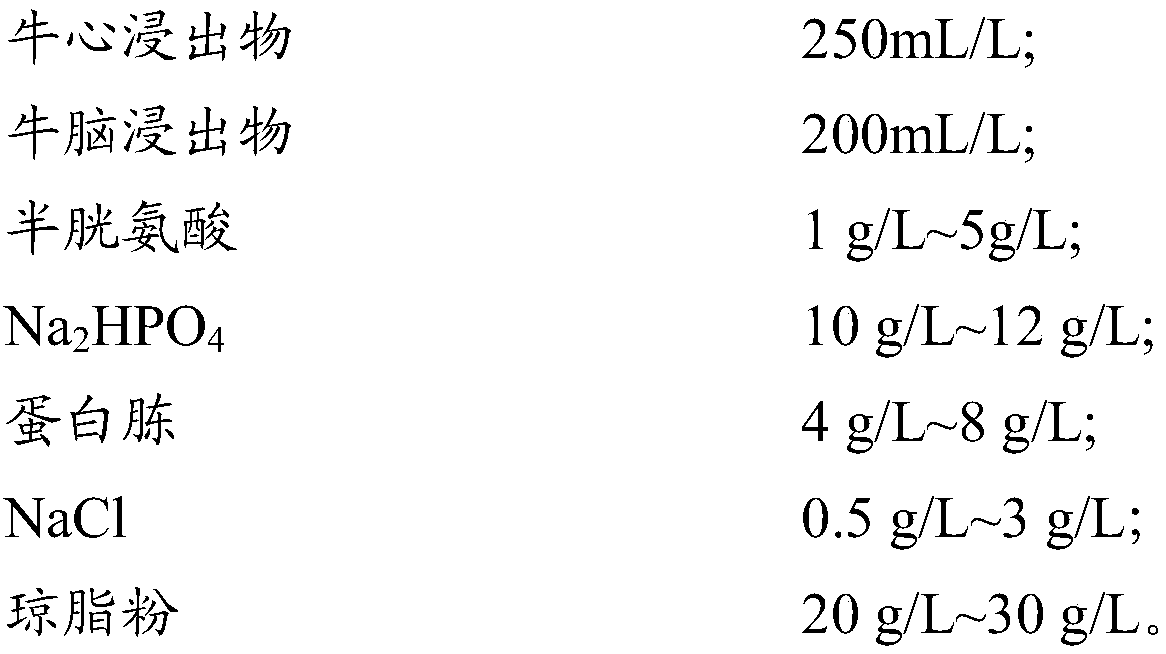Patents
Literature
75 results about "Drug allergy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An immune mediated adverse reaction to a drug.
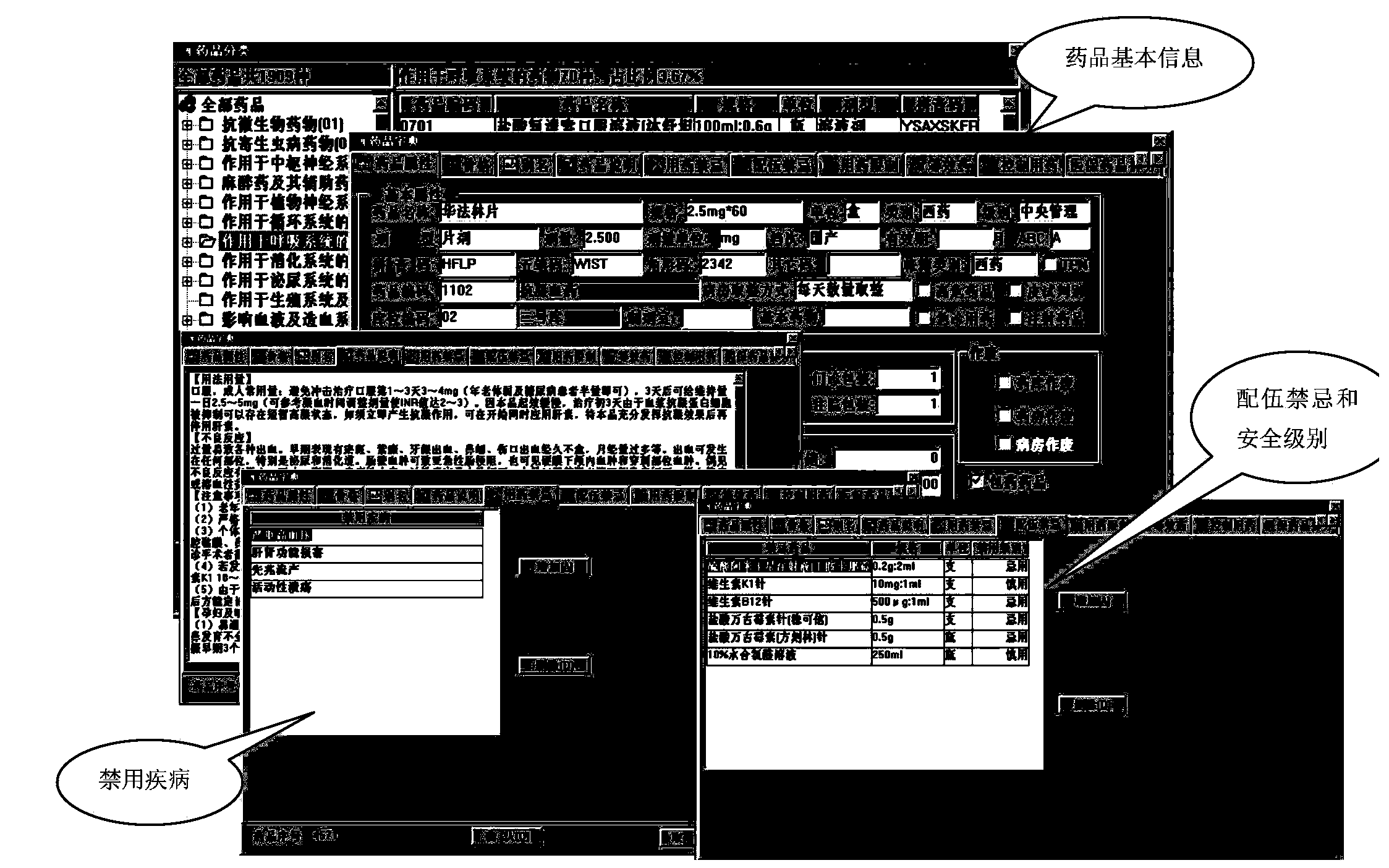
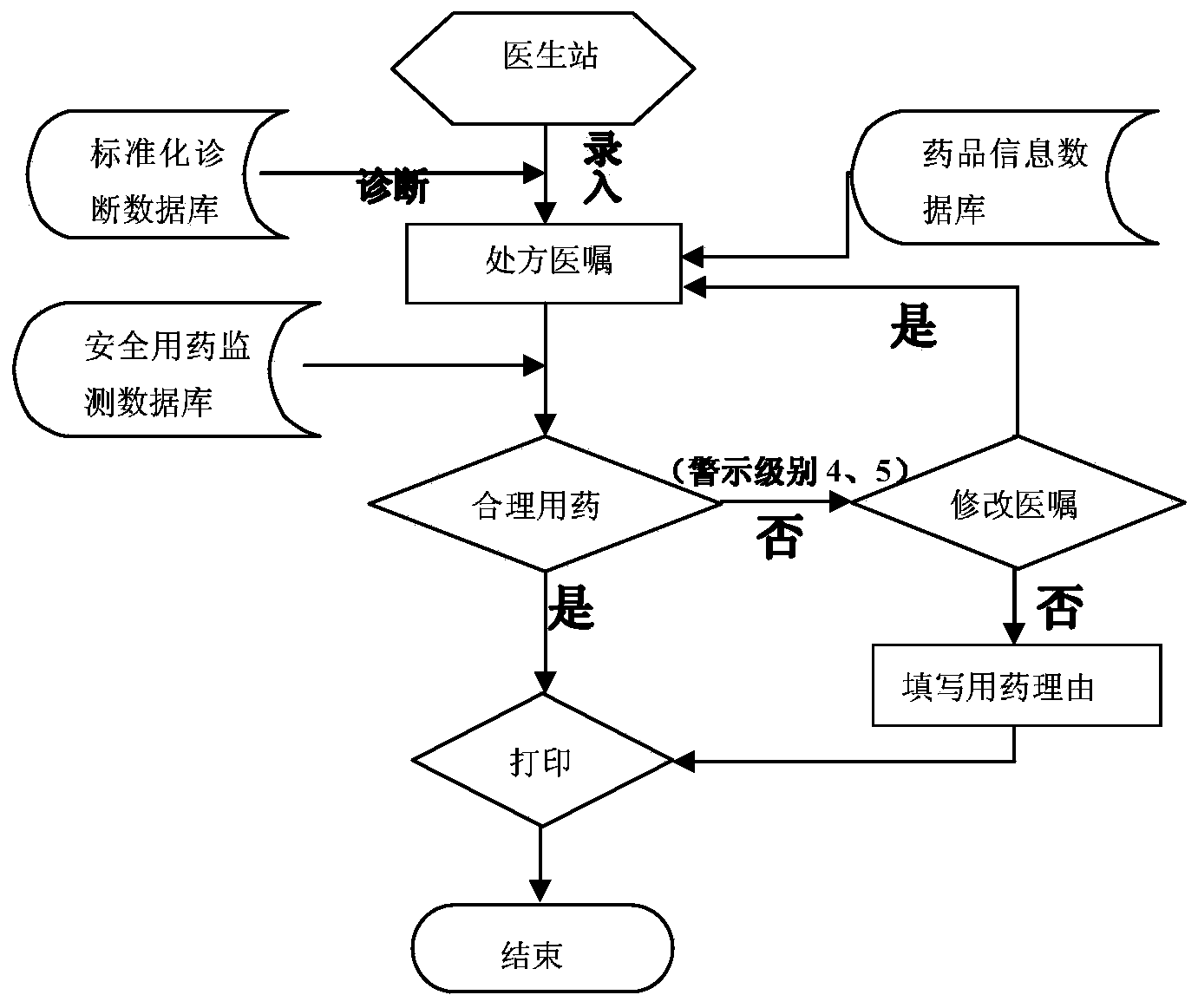
Hospital medication management system with medication safety monitoring and intervening functions
The invention relates to a hospital medication management system with the medication safety monitoring and intervening functions. The hospital medication management system with the medication safety monitoring and intervening functions is composed of a medication safety monitoring system and an early informatization intervening system. The computer database technology and other technologies are adopted for the medication safety monitoring system. According to the professional examination principle in the medicine science and the pharmaceutical science, with the specialized knowledge in the medicine science and the pharmaceutical science as standards, relevant medicine data information can be provided when doctor's advice is entered and examination of drug allergy histories, drug interactions, contraindications, side effects, external injection medicine compatibility and the like is performed on the doctor's advice to assist a doctor in correctly screening medicines and determining the doctor's advice, a prompt and an alarm can be given timely when problems are found, and thus the possibility of errors is reduced.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Interleukin-2 stimulated T lymphocyte cell death for the treatment of autoimmune diseases, allergic responses, and graft rejection
A method for the treatment or prevention of autoimmune diseases, allergic or atopic disorders, and graft rejection is provided, comprising inducing the death by apoptosis of a subpopulation of T lymphocytes that is capable of causing such diseases, while leaving substantially unaffected the majority of other T lymphocytes. Cell death is achieved by cycle(s) comprising challenging via immunization these T cells with antigenic substance at short time intervals, or by immunization followed by administering interleukin-2 (IL-2) when these T cells are expressing high levels of IL-2 receptor so as to cause these T cells to undergo apoptosis upon re-immunization with the antigenic peptide or protein. These methods are applicable to the treatment of autoimmune diseases such as, for example, multiple sclerosis, uveitis, arthritis, Type I insulin-dependent diabetes, Hashimoto's thyroiditis, Grave's thyroiditis, autoimmune myocarditis, etc., allergic disorders such as hay fever, extrinsic asthma, or insect bite and sting allergies, food and drug allergies, as well as for the treatment or prevention of graft rejection.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Food for improving constitutional trait and preparation technology of food
ActiveCN104256616AEasy to manufactureLow costImmunological disordersFood ingredient functionsDiseaseDrug allergy
The invention relates to food, in particular to Food for improving the constitutional trait and a preparation technology of the food. According to the food for improving the constitutional trait, synergy is achieved by efficacy of medicines through reasonable compatibility, so that the food has the functions of improving symptoms such as poor season and climate adaptability, easiness of scratch on skin, proneness to forming of wheal and urticaria, proneness to hay fever, asthma and the like, proneness to trigger of inveterate disease, drug allergy, food allergy and the like for allergic constitution.
Owner:北京王琦医学研究院
Extracorporal building method of tumor microenvironment and application of method to drug allergy screening
InactiveCN104342405ANormal physiological functionGood biocompatibilityMicrobiological testing/measurementTumor/cancer cellsDrug allergyTumor microenvironment
The invention relates to an extracorporal building method of a tumor microenvironment and an application of the method. By using the method, a three-dimensional stent similar with a cell internal microenvironment is built and total cells of tumor tissues are inoculated to promote recovery of physiological activity of all cells, so that a tumor microenvironment is built for drug screening for individual-based tumor treatment.
Owner:SUZHOU CANCERCELL BIOTECH
Quantitative determination and drug allergy determination kits for helicobacter pylori viable bacteria and determination method
InactiveCN103757088ASolve the deficiency of only qualitative detection of Helicobacter pyloriSimple methodMicrobiological testing/measurementUrocaninaseDrug allergy
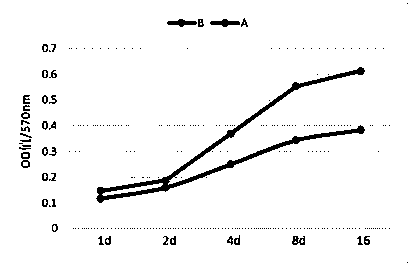
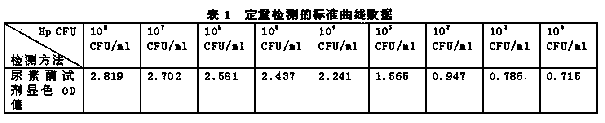
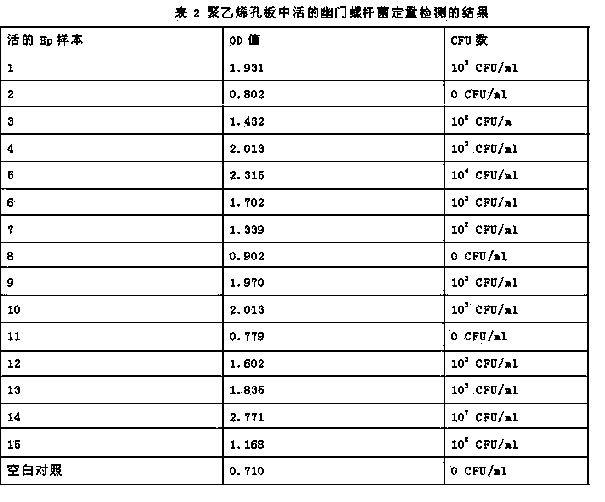
The invention discloses quantitative determination and drug allergy determination kits for helicobacter pylori viable bacteria and a determination method. The method adopts viable helicobacter pylori as a sample to be detected; the property that the viable helicobacter pylori can generate urease is used for rapidly and accurately reading the quantity of the helicobacter pylori by a helicobacter pylori colony counting standard method (CFU / ml, namely the bacterium individual quantity in each ml of bacterium liquid and a colony quantity standard curve); the detection result is accurate and sensitive and has high reliability; the counting difficulty of existing helicobacter pylori clinic microbiological identification, caused by difficult culture and complicated operation, is solved; the drug allergy determination kit and a preparation method, which are expanded by the invention, can be used for carrying out various drug allergy tests at the same time; clinicians can rapidly and conveniently screen suitable anti-helicobacter pylori medicines so that the time and the labor are saved and the cost is saved, so as to provide a beneficial technical solution for clinical scientific treatment.
Owner:SICHUAN VACCINE TECH
System for visually identifying and analyzing as well as identifying drug allergy and drug allergy detecting method
ActiveCN104749180ACompact structureRealize intelligent drug susceptibility identificationMaterial analysis by optical meansFiltrationBarcode
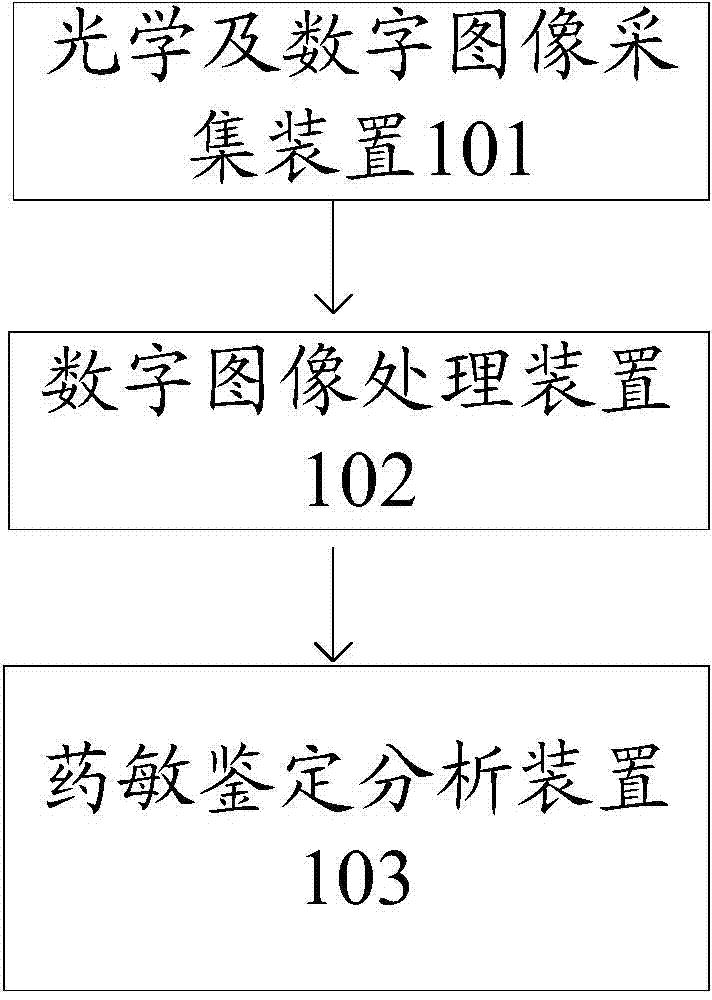
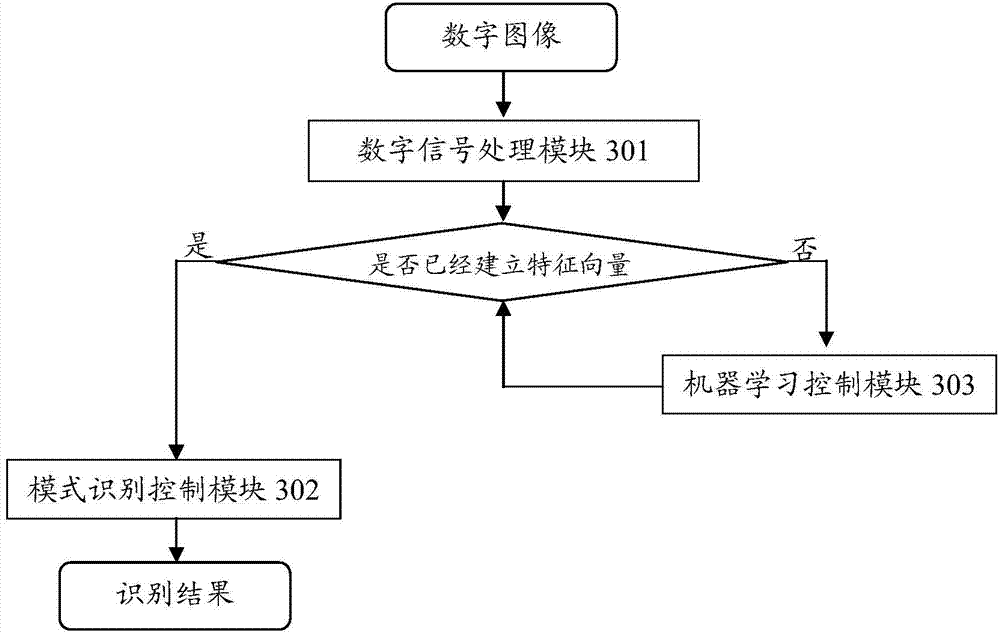
The invention discloses a system for visually identifying and analyzing as well as identifying drug allergy. The system comprises an optical and digital image acquiring device, a digital image processing device and a drug allergy identifying and analyzing device, wherein the optical and digital image acquiring device is used for providing a light source and acquiring the digital image, and sending the digital image to the digital image processing device; a polarizer and an analyzer in the optical and digital image acquiring device are used for strengthening the digital image of the detected object, and the cubic aberration of the digital image is corrected through a lens; the digital image processing device is used for receiving the digital image, capturing motion, performing filtration and feature enhancement on the image after sensing a target code, determining a target specimen type according to target specimen characteristics and bar code information after obtaining the target specimen image, selecting a detecting method to obtain detected results, processing and sending the detected results to the drug allergy identifying and analyzing device; the drug allergy identifying and analyzing device is used for identifying drug allergy by combining a mode identifying algorithm with preset CLIS (cost-of-living index) identifying standards in the drug allergy identifying and analyzing device, and obtaining the identifying conclusions.
Owner:北京浩辰星月科技有限公司 +1
Omeprazole sodium combined medicament and preparation method thereof
InactiveCN101766614AGood treatment effectAvoid allergic reactionsOrganic active ingredientsDigestive systemOmeprazole SodiumMedicine
The invention discloses an omeprazole sodium combined medicament and a preparation method thereof. The omeprazole sodium combined medicament comprises the following medicinal effect compositions in part by weight: 17.3 to 34.8 parts of the omeprazole sodium, 8.7 to 13.0 parts of reduced glutathione and 43.5 to 52.2 parts of sodium glutamate. The omeprazole sodium combined medicament provided by the invention has the effect of resisting drug allergy and can lighten the damage effect and the adverse reaction of the omeprazole sodium on liver; the medicament has high quality; and the preparation method is energy-saving and environment-friendly.
Owner:蔡海德
Biological anti-allergy compound
InactiveCN107469064AAllergy hasAnti-inflammatoryHydrolysed protein ingredientsAntipyreticDrug allergyInflammation
The invention provides a biological anti-allergy compound. The compound comprises the following raw materials: water, glycerin, a collagen crosslinked body, a Paeonia lactiflora extract product, a Tilia europaea extract product, an Arnica montana extract product, an Althaea officinalis root extract product, oligopeptide-1, and oligopeptide-6. The product contains effective components of Paeonia lactiflora, Tilia europaea, and the like, and has efficacies for diminishing inflammation, resisting inflammation, relieving itching, relieving pain, and preventing allergy; the product aims at drug allergy, diet allergy, seasonal allergy, eczema allergy, eczema in infant, and infantile eczema, and even aims at skin allergy due to improper usage of cosmetics with good control and repair effects; the product can effectively alleviate allergy, and long-term usage of the product has effects for preventing and improving sequelae of allergy.
Owner:广州艾玫格生物科技有限公司
Method for constructing ovarian cancer transplantation tumor model based on organoid method and application of method
InactiveCN109392843AConvenient researchNormal food intakeMicrobiological testing/measurementMicrocarriersDrug allergyImmunodeficiency
The invention discloses a method for constructing an ovarian cancer transplantation tumor model based on an organoid method, and belongs to the field of medicines. According to the invention, an "organoid" culture technology is adopted, and a micro-carrier is used as a support material for culturing a patient-derived ovarian cancer cell-3D material complex; then the cell-3D material complex is directly inoculated under the skin of a normal immune mouse, and a patient-derived ovarian cancer transplantation tumor model is constructed, so that a problem that immune-deficiency mice such as severecombined immunodeficiency (SCID) mice, nude mice and the like are adopted in existing human ovarian cancer transplantation tumor models, the price is high, breeding is difficult, the tumor forming rate is low and the like can be solved, especially a problem that the existing immune-deficiency mouse human ovarian cancer transplantation tumor model cannot reflect impotent effects of an organism immune system in occurrence and development processes of tumors is solved, and a problem that an immune-deficiency mouse human transplantation tumor model is long in tumor forming period and cannot meet drug allergy requirements of patients urgently needing clinic drug therapy is solved. The method is applied to the field of mouse model construction.
Owner:上海美峰生物技术有限公司
Lactobacillus plantarum capable of adjusting ampicillin induced intestinal flora disturbance
Owner:JIANGNAN UNIV
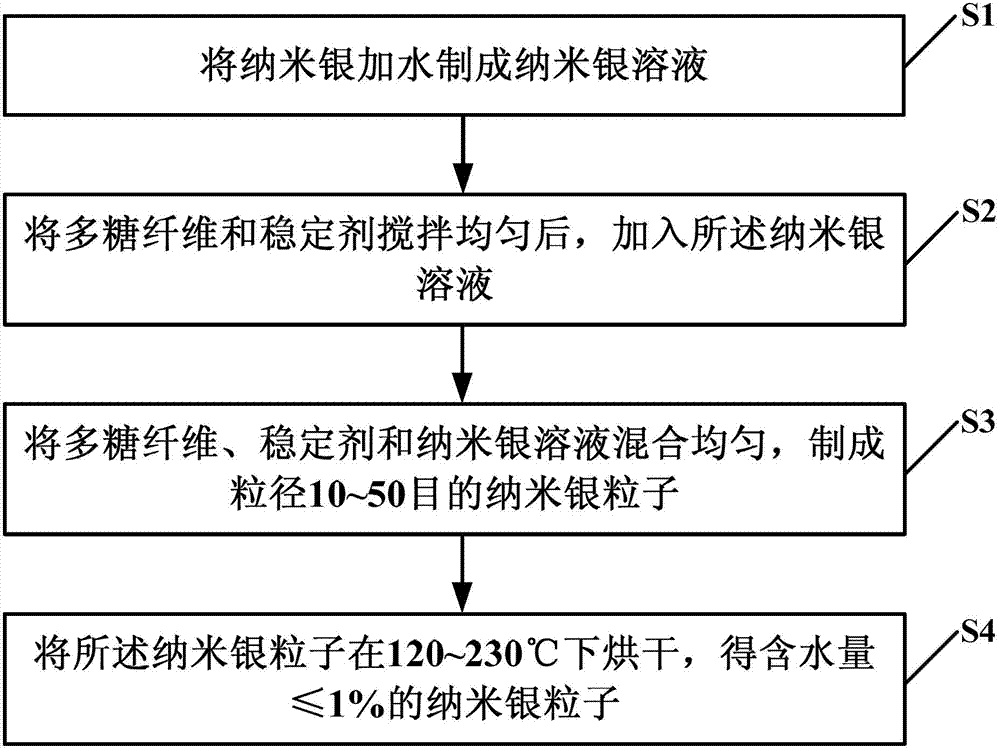
Silver nanoparticles, preparation method thereof and nano silver dressing
The invention provides silver nanoparticles used for solving the problems that the existing hydrogel nano silver external patches or medical dressings have low adsorption capacities and the nano silver external patches or medical dressings have single function or complex components, thus easily causing user skin harms, heavy metal poisoning and drug allergy. The silver nanoparticles comprise the following components by weight percent: 90-98% of polysaccharide fibers, 0.5-6.5% of stabilizing agent and 0.03-3.5% of nano silver. The silver nanoparticles have the following beneficial effects: the silver nanoparticles are larger, have low moisture content, have higher absorbed amount of blood and fluid oozing from the wounds and can form a 2-3mm thick gel after absorbing the blood and fluid oozing from the wounds, thus not only preventing oozing fluid pollution and adhesion, isolating the external air and sources of infection and protecting the skins from friction or infection but also ensuring the silver nanoparticles to release slowly and ensuring the antibacterial and sterilization capabilities to be stronger and more lasting; and besides, the silver nanoparticles do not contain drug components, are nontoxic and harmless and can avoid user skin harms, heavy metal poisoning and drug allergy.
Owner:张利波
Wound healing composition having function of promoting growth of granulation tissues
InactiveCN104415081APromote generationImprove protectionAntibacterial agentsAntimycoticsWestern medicineSide effect
The invention relates to a wound healing composition having a function of promoting growth of granulation tissues; the wound healing composition contains a base substance extracted from plants and a base body, wherein the base substance comprises a honeysuckle extract, a folium artemisiae argyi extract and ginkgo, and the weight percentage ratio of the honeysuckle extract to ginkgo is ranged between 0.28 and 3.5. The composition has the efficacies that the wound healing composition can effectively promote the formation of the granulation tissues, and in addition, honeysuckle, folium artemisiae argyi, gingko and the like are all natural plant ingredients and are extracted, so compared with a western medicine composition adopted by the prior art, the wound healing composition has low drug resistance, does not easily produce drug allergy or doubts likely to cause skin cancer and other side effects.
Owner:LI TZU PAO MEI REGIMAN BEAUTY
Method used for extracting antibacterial composition from limulus reagent production waste materials
The invention relates to a method used for extracting an antibacterial composition from limulus reagent production waste materials. The method comprises following steps: 1, raw material treatment is carried out, wherein a lower layer precipitate waste material obtained in centrifugation of an emulsified product in limulus reagent production is collected, and is taken as a raw material I, and discarded blood plasma in limulus reagent production is collected, and is taken as a raw material II; 2, extraction of a tachyplesin crude extracted solution is carried out, wherein acidolysis is carried out, pH is adjusted so as to remove a precipitate product, and thermal denaturation is carried out; 3, preparation of a hemocyanin crude extracted solution from blood plasma is carried out; 4, preparation of a SOD crude extracted solution is carried out; 5, 60 to 80 parts of the tachyplesin crude extracted solution,10 to 20 parts of the hemocyanin crude extracted solution, and 10 to 20 parts of the SOD crude extracted solution are mixed, pH value is adjusted to 4 to 5, and filtering is carried out so as to obtain the antibacterial composition. According to the method, the crude extracted solutions are prepared rapidly and conveniently at low cost; the antibacterial composition is capable of inhibiting growth of a plurality of kinds of bacteria and fungus; compared with conventional chemical antibacterial products, the antibacterial composition possesses following advantages: the antibacterial composition is safe, mild, and effective, no toxic or side effect is caused, and generation of drug resistance and drug allergy caused by conventional antibacterial products can be avoided.
Owner:GENOBIO PHARM CO LTD
Helicobacter pylori drug allergy detection culture medium and kit
PendingCN108486211AReasonable formulaEasy to controlMicrobiological testing/measurementBiological material analysisInhibition zoneHelicobacter pylori
The invention provides a culture medium for testing helicobacter pylori drug allergy and a determination method. The culture medium is unique in formula, can efficiently and stably promote the growthof helicobacter pylori, ensures that an inhibition zone in a drug allergy test detection result and a size of the inhibition zone are only related to the type of antibiotics and concentration of the antibiotics, so that the sensitivity of different helicobacter pylori isolate strain for different antibiotics can be scientifically determined by virtue of a set determination method and standard according to the presence and the size of the inhibition zone, and the helicobacter pylori drug allergy detection culture medium has the characteristics and advantages of rapidness, accuracy, intuitiveness and convenience. The helicobacter pylori drug allergy detection culture medium and kit are a unique and reliable novel drug allergy detection method for various pathogenic bacteria. Compared with the standard drug allergy test, the detection accuracy can reach 100 percent.
Owner:SHENZHEN BLOT BIOTECH
Method of screening for drug hypersensitivity reaction
ActiveUS20070148641A9High incidenceIncreased riskSugar derivativesMicrobiological testing/measurementHypersensitive responseDrug allergy
Methods of assessing the risk of clinical signs of hypersensitivity reaction to nucleoside antiviral compounds, including abacavir, are described. The methods include genotyping subjects for polymorphisms in the TNFα gene, the class 1 HLA genes, or a combination of both the TNFα and HLA genes.
Owner:VIIV HEALTHCARE UK LTD
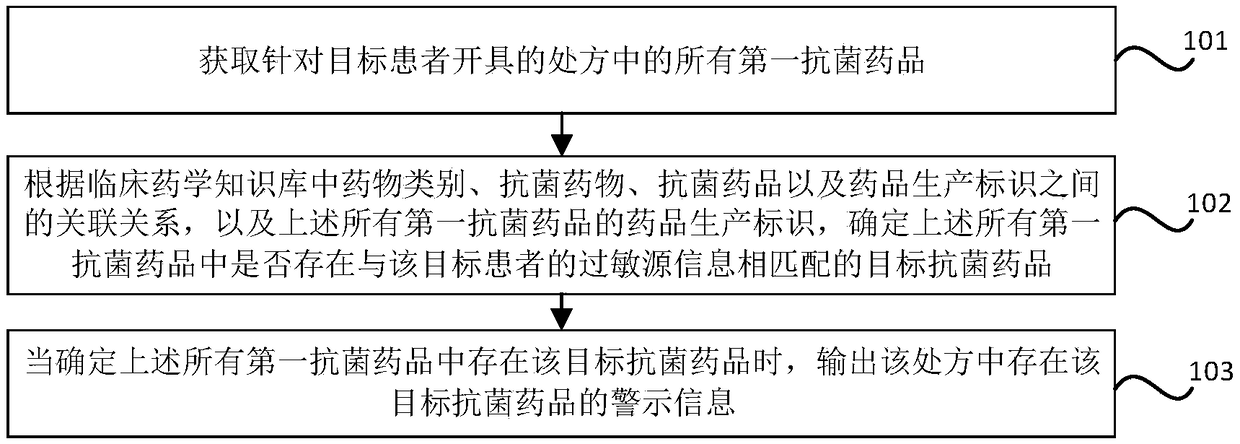
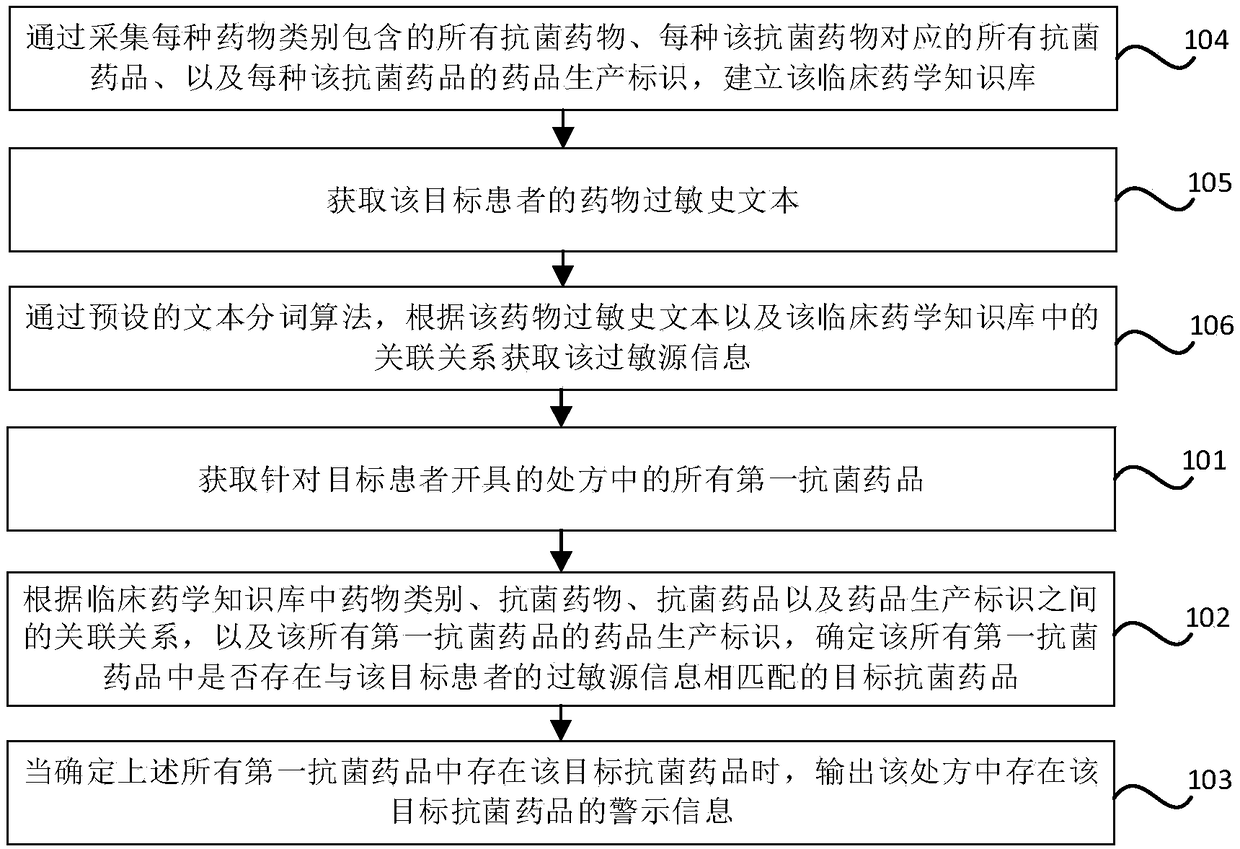
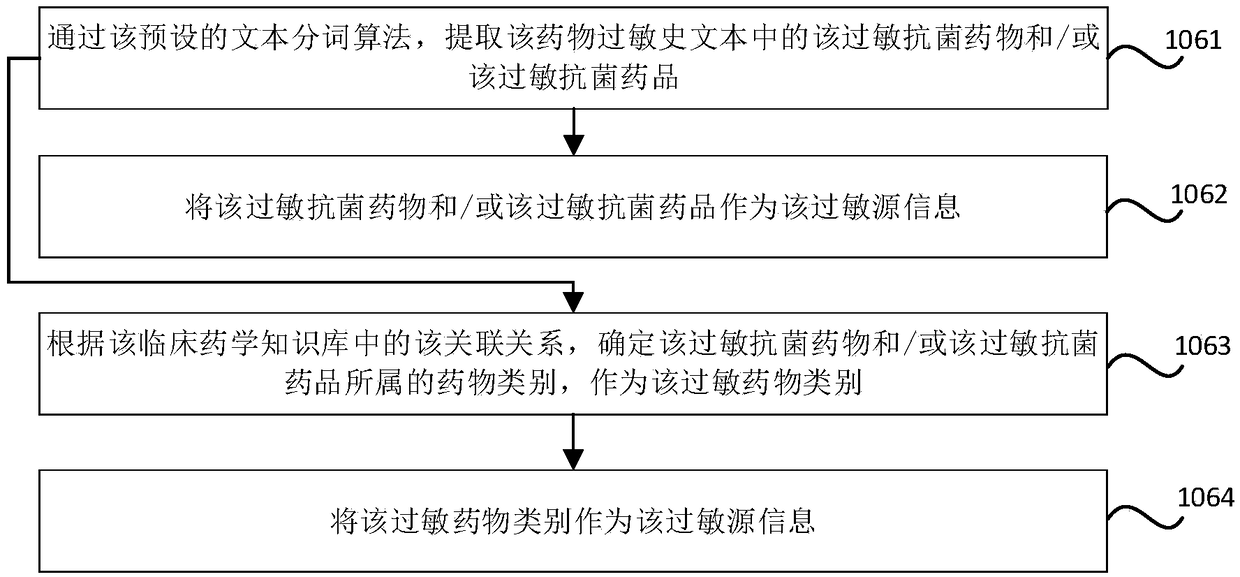
Antibacterial drug prescription checking method and device, storage medium and electronic equipment
ActiveCN109243617AGuarantee drug safetyMedical data miningDrug referencesAntimicrobial drugDrug allergy
The invention relates to an antibacterial drug prescription checking method and device, a storage medium and electronic equipment. The method comprises the steps: obtaining all the first antibacterialdrugs in the prescription made for the target patient; determining whether there is a target antibacterial drug matching the allergen information of the target patient in all the first antibacterialdrugs according to the association between the drug class, the antibacterial drug, the antibacterial agent and the drug production identifier in the clinical pharmaceutical knowledge base and the drugproduction identifier of all the first antibacterial drugs; and outputting the warning information of presence of the target antimicrobial drug in the prescription when it is determined that the target antimicrobial agent exists in all the first antimicrobial agents,. The antimicrobial allergy in prescription made by the doctor can be checked and intervened according to the clinical pharmaceutical knowledge base and the drug allergy history of the patient so as to reduce the possibility of misuse of the antimicrobial drugs and ensure the medication safety of the patient.
Owner:NEUSOFT CORP
A bacterial reverse-inoculation identification method in blood testing
InactiveCN104561225AThe experiment process is simpleLow costBacteriaMicrobiological testing/measurementDiseaseMicroorganism
Through reverse-inoculation after suspected disease microorganisms are subjected to inoculated culture, different types of the microorganisms are distinguished by utilization of different growth characteristics and colony characteristics for the different types of the microorganisms after bacterial culture, and a suspected disease bacterium is selected and subjected to drug allergy testing so as to provide basis for a certain type of infection for clinicians. The method provided by the invention has advantages of simple experiments, low costs, easy operation and implementation, good repeatability, and the like, and overcomes limitation of traditional clinical examination to some extent.
Owner:晏健强
Anti-wrinkle, freckle-removing and whitening itching relieving dew
InactiveCN104398427AImprove actual functionsPromote blood circulationCosmetic preparationsToilet preparationsBletilla striataCuticle
The invention relates to anti-wrinkle, freckle-removing and whitening itching relieving dew. The itching relieving dew is prepared by eggplant juice, kunzea, bletilla striata, lucid ganoderma, aloe juice, feverfew, liquorice and traditional Chinese medicinal materials, has the efficacies of promoting blood circulation to remove blood stasis, beautifying skin and removing freckles, removing wrinkles, treating rhagadia manus and pedalis and diminishing inflammation and itching, has a strong medicine permeation effect and is free from rebound, and liquid medicine can be absorbed by skin within 3 minutes; the itching relieving dew can promote cell functions and blood circulation, enhance the activity of epidermic cells, promote metabolism and regulate endocrine and nervous functions and is fed three times a day, the color of spots is changed from deep to shallow after seven days, and the facial spots disappear within about 30 days, so that the skin is polished, is smooth and glows with good health; the problems that the conventional beautifying product only treats symptoms, not root causes, easily rebounds and reappears and the like are solved. The anti-wrinkle, freckle-removing and whitening itching relieving dew is mainly suitable for freckles, dark spots, pigmented spots, chloasma, butterfly rashes, acne, yellowish skin, darker skin, wrinkle removal, eye bag removal, drug allergy, pollen allergy, mosquito bite and the like.
Owner:王献美
Chinese medicinal embrocation for treating dermatosis of dogs and cats
InactiveCN102106933ANon-irritatingTo promote metabolismAnthropod material medical ingredientsHydroxy compound active ingredientsDiseasePeriostracum
The invention relates to Chinese medicinal embrocation for treating dermatosis of dogs and cats, which comprises common cnidium fruit, great burdock achene, rheum officinale, whitebackleaf mallotus leaf, sophorae flavescens, borneol, thinleaf adina fruit, rumex japonicus root, cattail pollen, Japanese premna herb and periostracum cicadae. The Chinese medicinal embrocation is prepared by a conventional method and is a specific medicament which is scientifically extracted and refined and used for treating various stubborn skin diseases; with a specific skin penetrant, the Chinese medicinal embrocation can penetrate through the outer layer of the skin and is directly acted at the pathological changes parts to achieve the aim of externally curing internal disease; and through clinical verification, the Chinese medicinal embrocation has specific effects for treating various dermatitises, miliaria, acne, drug allergy, and the like. In addition, the Chinese medicinal embrocation is a pure Chinese medicinal preparation, has no simulation on the skin, high cure speed and favorable curative effect and can promote the metabolism of the skin.
Owner:QINGDAO VLAND BIOTECH INC +1
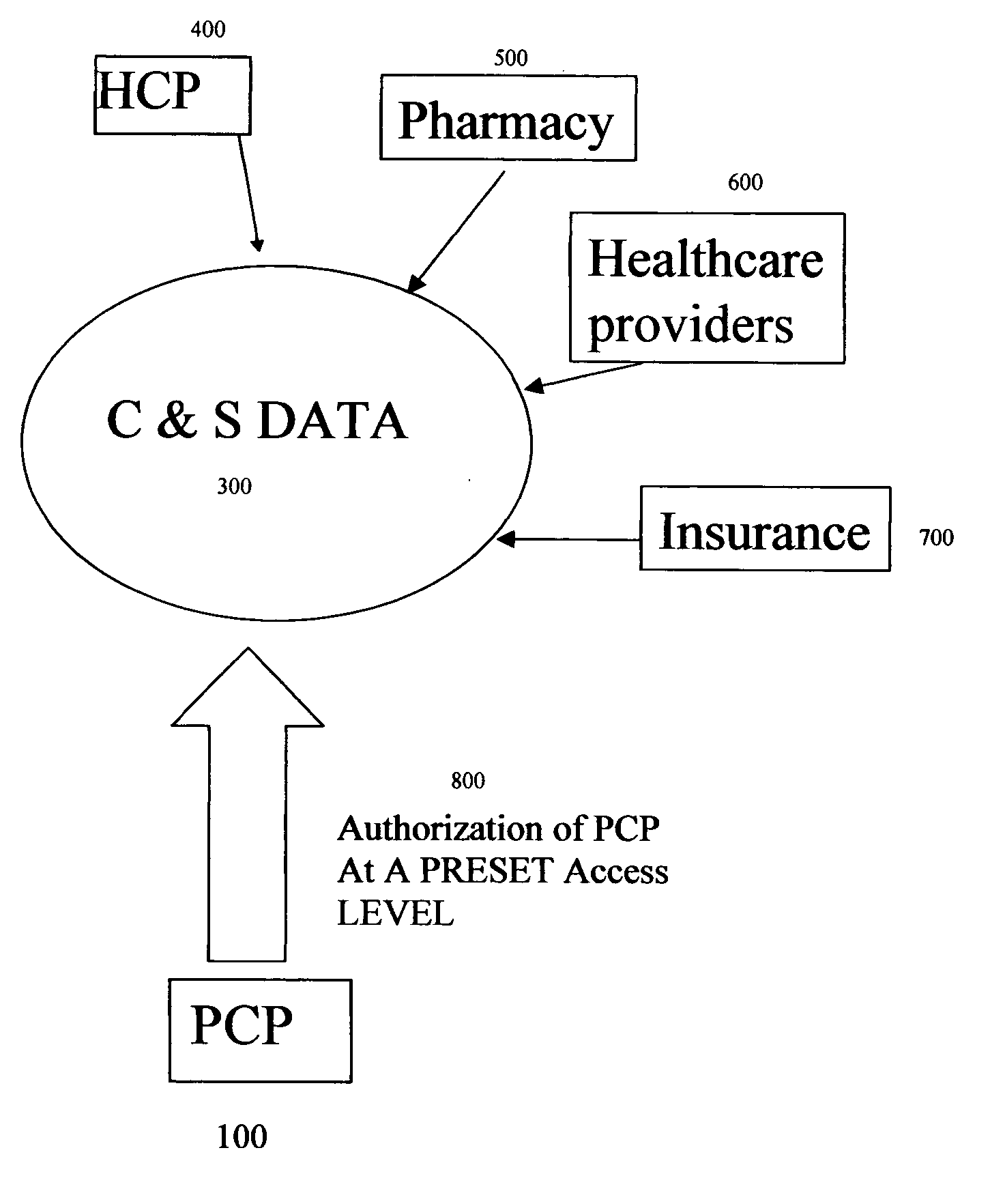
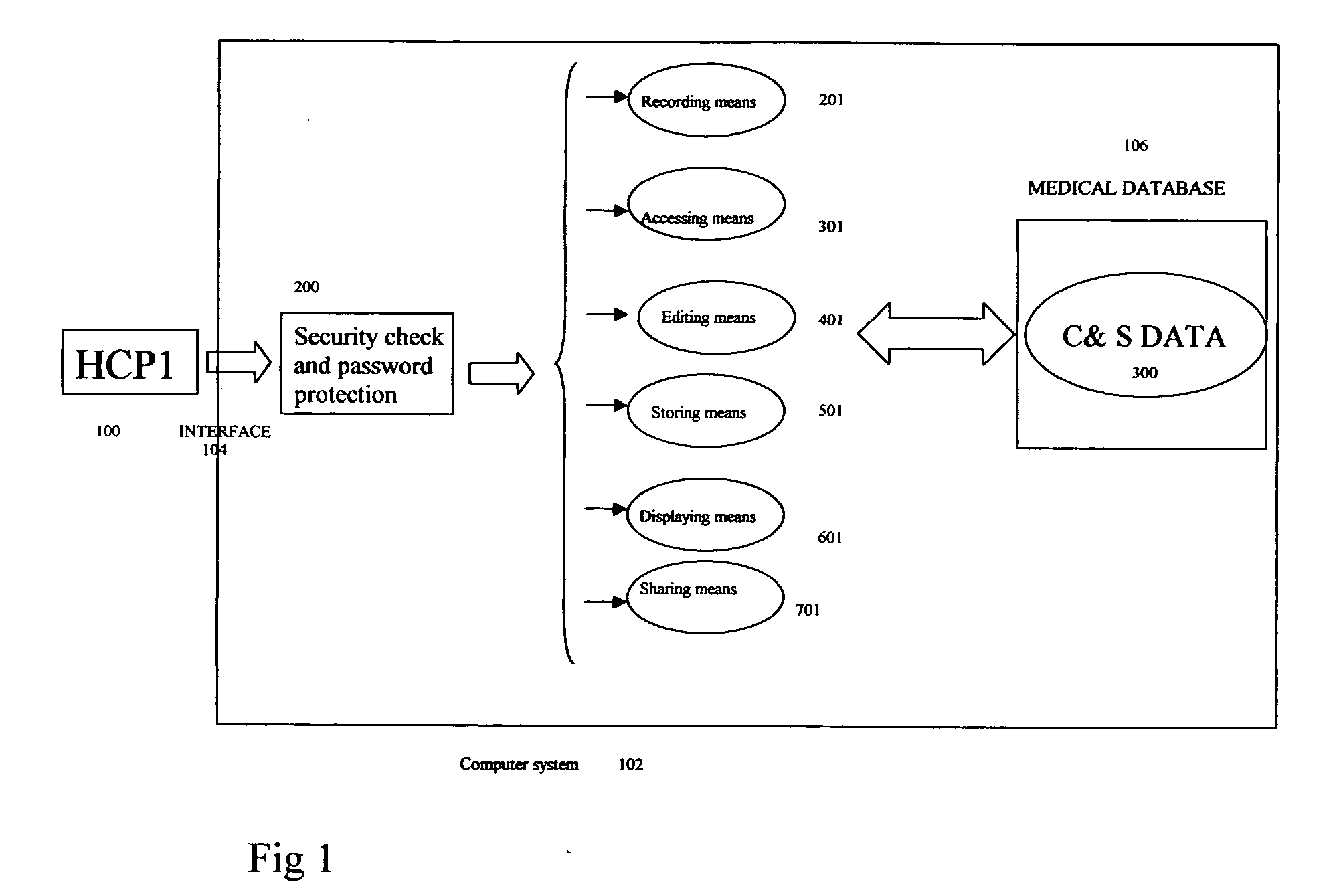
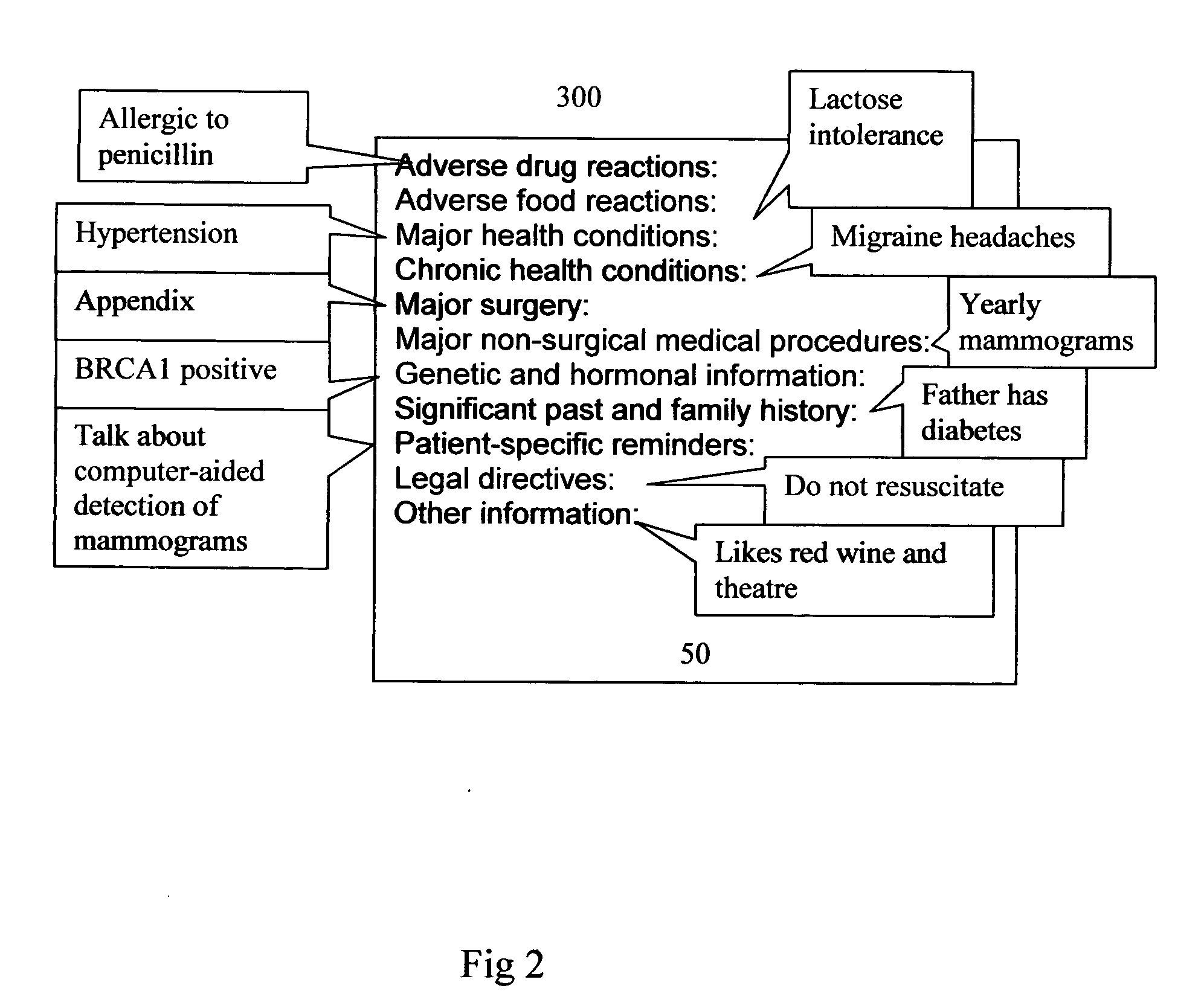
Crucial and significant (C&S) patient information management and display
InactiveUS20050246203A1Data processing applicationsPatient personal data managementMedical recordDispensary
Method, product and system for Health Care Providers (HCP) to extract, store, organize, password protect, and share crucial and significant (C&S) health, medical, dental, and pharmacy information without regard to healthcare provider systems are provided. All the C&S information contained in a patient's medical record(s) is entered in a customizable screen that can be accessed as soon as a doctor types in the name of the patient or presses a key. Crucial and Significant (C&S) patient information includes but not limited to important history, major diagnoses; major medical procedures and investigations, allergies, drug allergies and blood type. This may also include alerts for important patient directives including but not limited to “do not resuscitate,”“organ donation” etc.
Owner:NARAYAN M S LAXMI +1
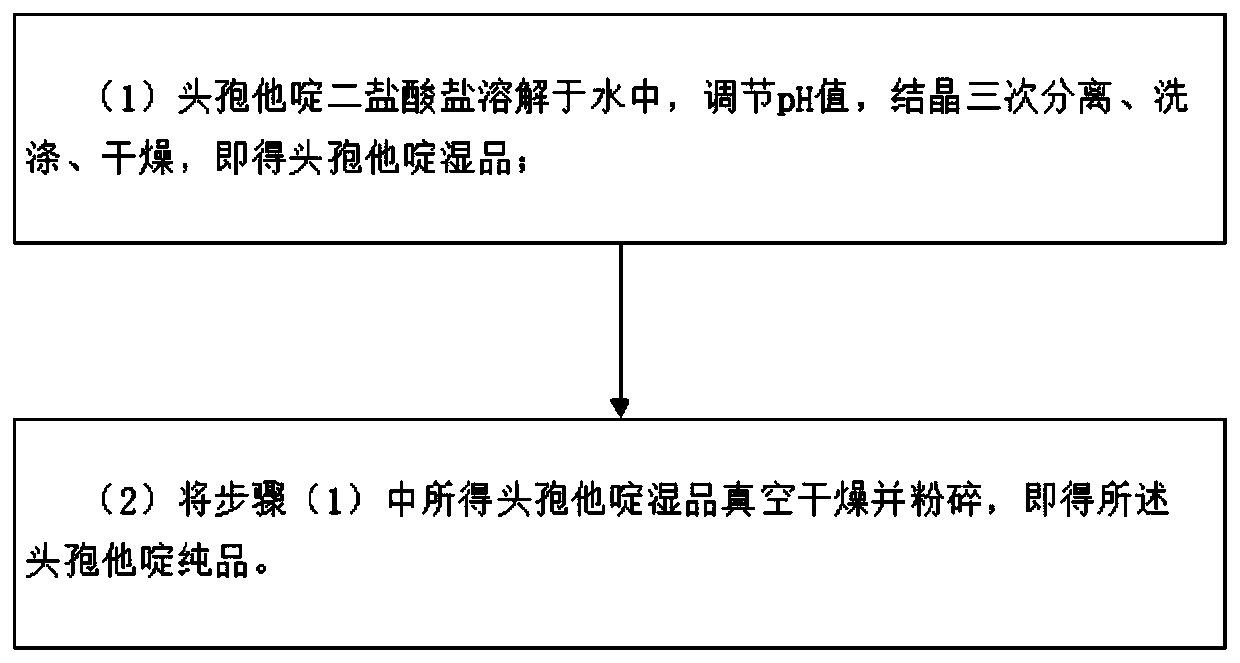
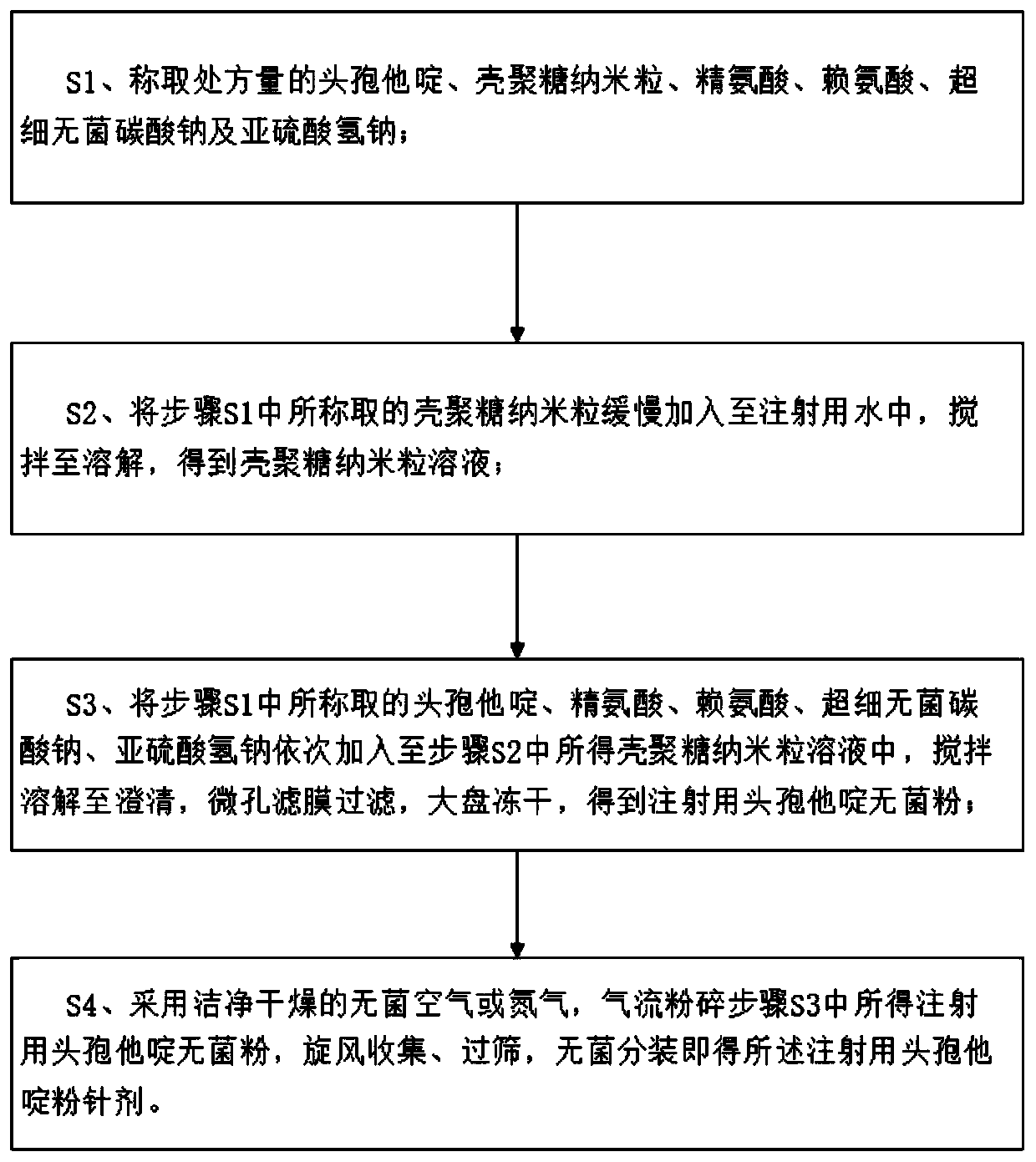
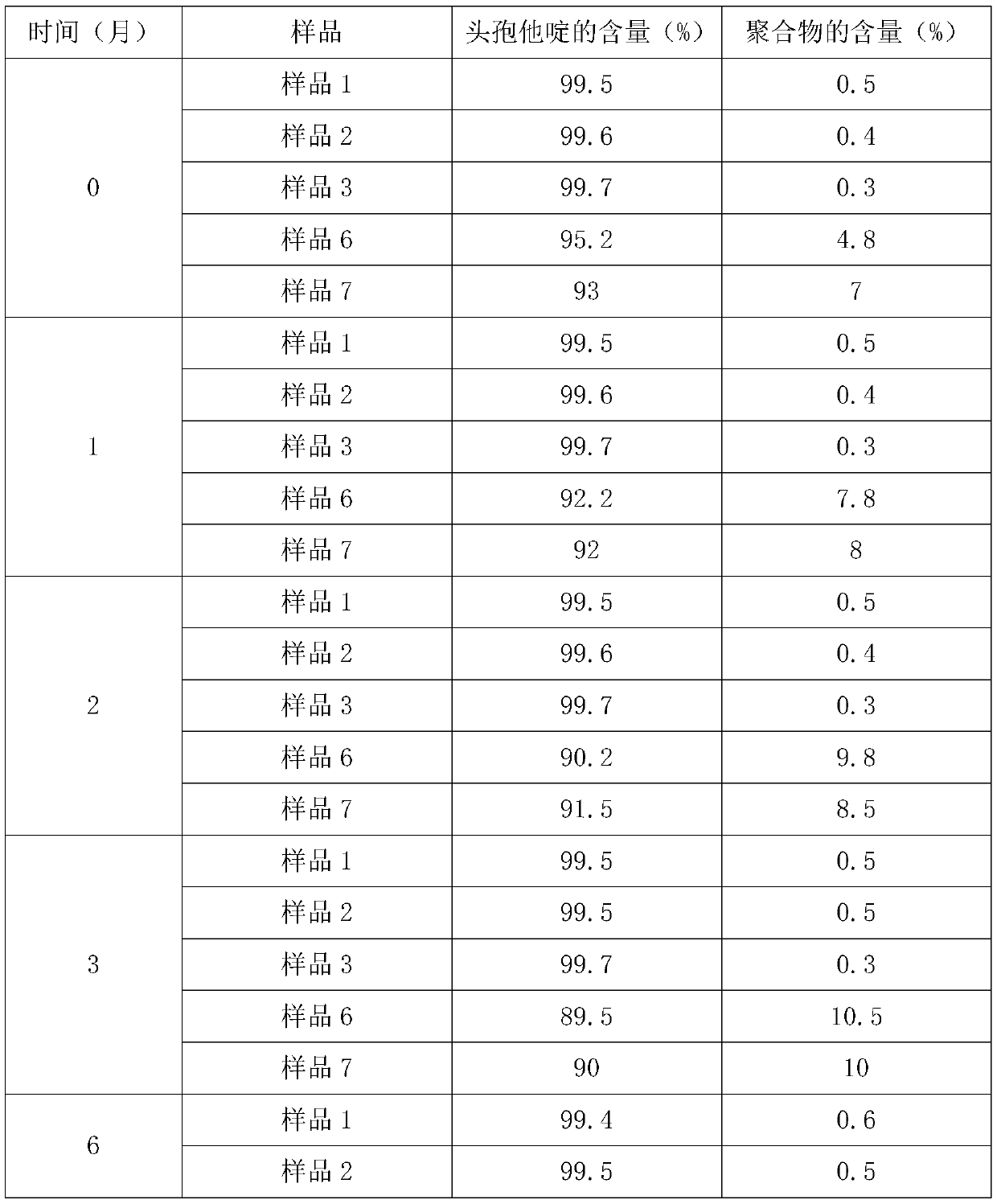
Ceftazidime injectable powder for injection and preparation method of ceftazidime injectable powder for injection
InactiveCN110893173ABroad spectrum antibacterialImprove antibacterial propertiesAntibacterial agentsPowder deliveryBiotechnologyChitosan nanoparticles
The invention discloses ceftazidime injectable powder for injection and a preparation method of the ceftazidime injectable powder for injection. The ceftazidime injectable powder for injection comprises the following raw materials in parts by weight of 8.26-12.31 parts of ceftazidime, 6.45-9.22 parts of chitosan nanoparticles, 0.06-3.8 parts of arginine, 0.06-3.8 parts of lysine, 0.82-5.3 parts ofsuperfine bacteria-free sodium carbonate and 0.00005-0.0019 part of sodium bisulfite. The ceftazidime injectable powder for injection disclosed by the invention is high in purity, particularly low inimpurity content, good and stable in quality, better in clarity and wide in in vitro antibacterial activity spectrum, the antibacterial activity of the ceftazidime on Gram-negative bacteria can be significantly strengthened, the content of polymers is low, drug allergy reactions are reduced, the dissolving speed is high, the ceftazidime injectable powder for injection can quickly dissolve even atlow temperature of 10 DEG C, and the ceftazidime injectable powder for injection is convenient in clinical use. The preparation method of the ceftazidime injectable powder for injection disclosed bythe invention is simple and convenient to operate, low in cost, good in safety and suitable for industrial production.
Owner:上海欣峰制药有限公司
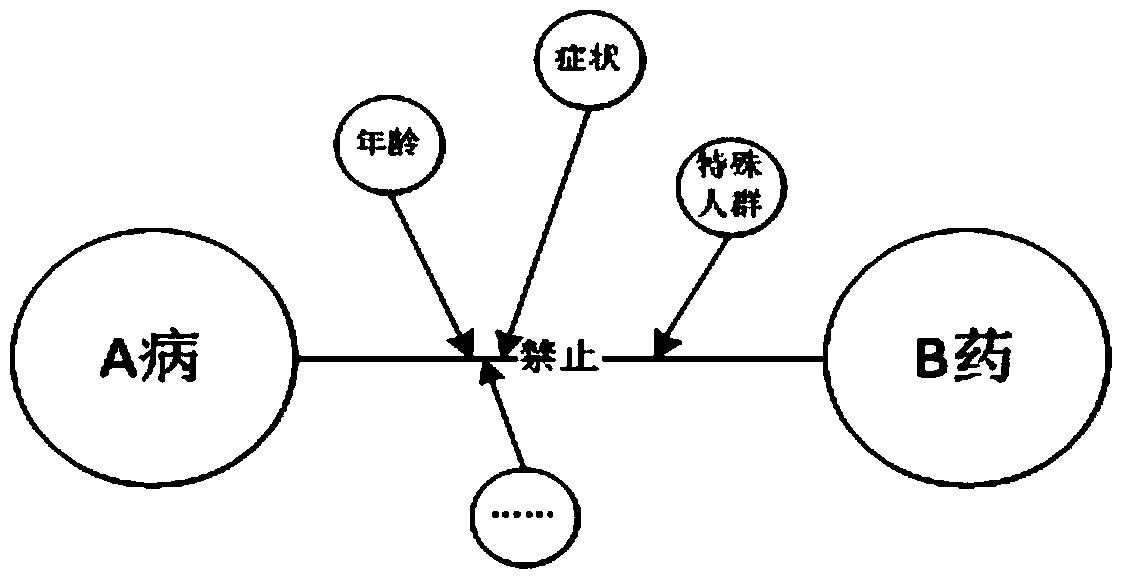
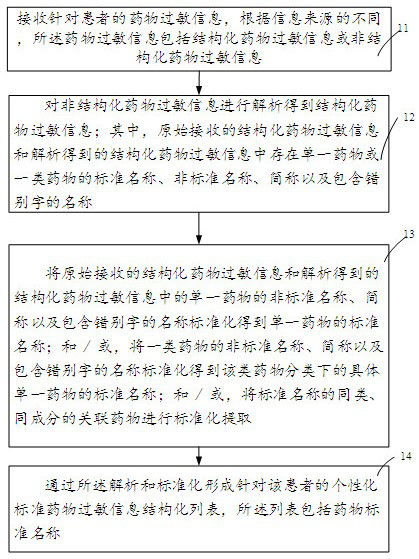
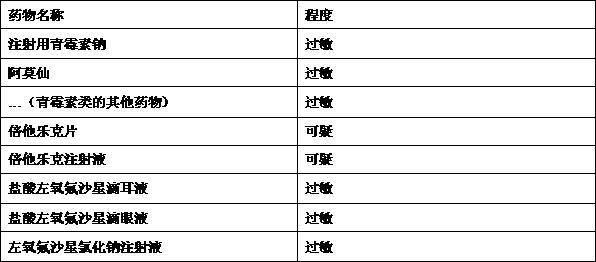
Clinical drug allergy management method, auxiliary device and system
InactiveCN113393945AStandardize the allergy management processGuarantee drug safetyDrug referencesDrug utilisationDrug allergy
The invention discloses a clinical drug allergy management method, auxiliary device and system. The method comprises the steps: receiving drug allergy information of a patient, and enabling the drug allergy information to comprise structured drug allergy information or unstructured drug allergy information according to different information sources; analyzing the unstructured drug allergy information to obtain structured drug allergy information; standardizing the non-standard name of the single medicine to obtain a standard name of the single medicine; and / or standardizing the non-standard names of the medicines to obtain the standard names of the specific single medicines under the classification of the medicines; and / or carrying out standardized extraction on similar and same-component associated drugs with standard names; forming a personalized standard drug allergy information structured list for the patient through analysis and standardization, so that the effective management is achieved in the diagnosis and treatment link of the patient, and the medication safety of the patient is effectively guaranteed. According to the invention, the completeness and availability of the drug allergy information record can be effectively improved.
Owner:FUWAI HOSPITAL CHINESE ACAD OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
Method for differentiating ureaplasma urealyticum and ureaplasma parvum in ureaplasma with culture method
ActiveCN107746875AEasy to operateEasy for clinical promotion and useMicrobiological testing/measurementBiological material analysisUreaplasma parvumManganese
The invention discloses a method for differentiating ureaplasma urealyticum and ureaplasma parvum in ureaplasma with a culture method. The method comprises the following steps: determining two detection micropores which are respectively marked as a first hole and a second hole, wherein the first hole does not contain additive, a manganese and magnesium solution is added into the second hole, and magnesium and manganese ions are attached to the inner wall of the second hole after being dried; adding a sample to be tested into a ureaplasma culture medium, after even mixing, respectively adding into the first hole and the second hole, and culturing mixture for 24-48 hours at 37 DEG C; according to the color change situation of the first hole and the second hole, determining whether the ureaplasma urealyticum and the ureaplasma parvum are contained in a sample or not. The preparation of a kit through the method disclosed by the invention is more convenient and practical. The authenticationmethod is simple in operation, whether a sample contains the ureaplasma urealyticum and the ureaplasma parvum or not can be accurately differentiated through one step, meanwhile, the method can be combined with drug sensitivity detection, and meanwhile, an authentication and drug allergy result can be carried out. The detection kit prepared according to the method principle disclosed by the invention has the advantages of visual and accurate authentication result and is convenient for clinical popularization and use.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Medical composition for treating mucosa infection and wound infection and auxiliarily treating breast cancer
InactiveCN103006913ANo scarsGood curative effectAntibacterial agentsDermatological disorderSide effectTreatment effect
The invention discloses a medical composition for treating mucosa infection and wound infection and auxiliarily treating a breast cancer, which is prepared from following raw materials based on the mass percentage: 35-45% of lantana camara, 15-25% of bidens biternata, 15-25% of clerodendrum fortunatum and 15-25% of radix astragali. The medical composition for treating the mucosa infection and the wound infection and auxiliarily treating the breast cancer disclosed by the invention can rapidly and effectively cure various mucosa infection, drug allergy and wound infection of human bodies, poultries and domestic animals, and is effective in treatment; and scars are not formed on wounds of patients and the medical composition has no side effects. The medical composition for treating the mucosa infection and the wound infection and auxiliarily treating the breast cancer disclosed by the invention has good treating effect on auxiliarily treating the breast cancer and is effective in treatment.
Owner:梁灿东
Chinese herb medicine or natural material pesticide effect detecting method
InactiveCN101067610ASave development costsComprehensiveDiagnostic recording/measuringSensorsDiseaseSide effect
The invention relates to an examination method of Chinese traditional medicine or the natural material drug efficacy, which belongs to the Chinese medicine, the western medicine examination domain. Uses the quantum resonance detector and puts the human body or the animal sample on the test plate, detects the disease information code, clicks on the handle to calculate the disease code degree counting value, then puts the Chinese medicinal plant or the natural matter sample on the test plate, detects the medicine effect value. The difference between medicine effect value and the disease code degree value is the Chinese native medicine or natural matter drug efficacy value. The method can real, accurate reflect the function trend of medicine to disease, is advantageous to select and screen the Chinese medicine, the western medicine drug. The method is simple and quickly, the data accurate is reliable, the method may avoid the phenomena of drug hyper susceptibility, serious side effect or unwell because of unknown the individual drug allergy.
Owner:闫群
Method of screening for drug hypersensitivity reaction
ActiveUS7550261B2High incidenceIncreased riskSugar derivativesMicrobiological testing/measurementAbacavirDrug allergy
Methods of assessing the risk of clinical signs of hypersensitivity reaction to nucleoside antiviral compounds, including abacavir, are described. The methods include genotyping subjects for polymorphisms in the TNFα gene, the class 1 HLA genes, or a combination of both the TNFα and HLA genes.
Owner:VIIV HEALTHCARE UK LTD
Health electronic information card
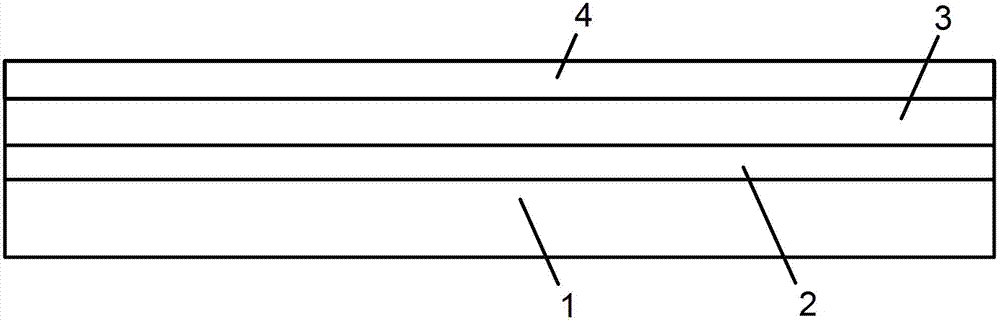
PendingCN108960396ARealize contactless reading and writingNo data interfaceRecord carriers used with machinesDrug allergyElectronic information
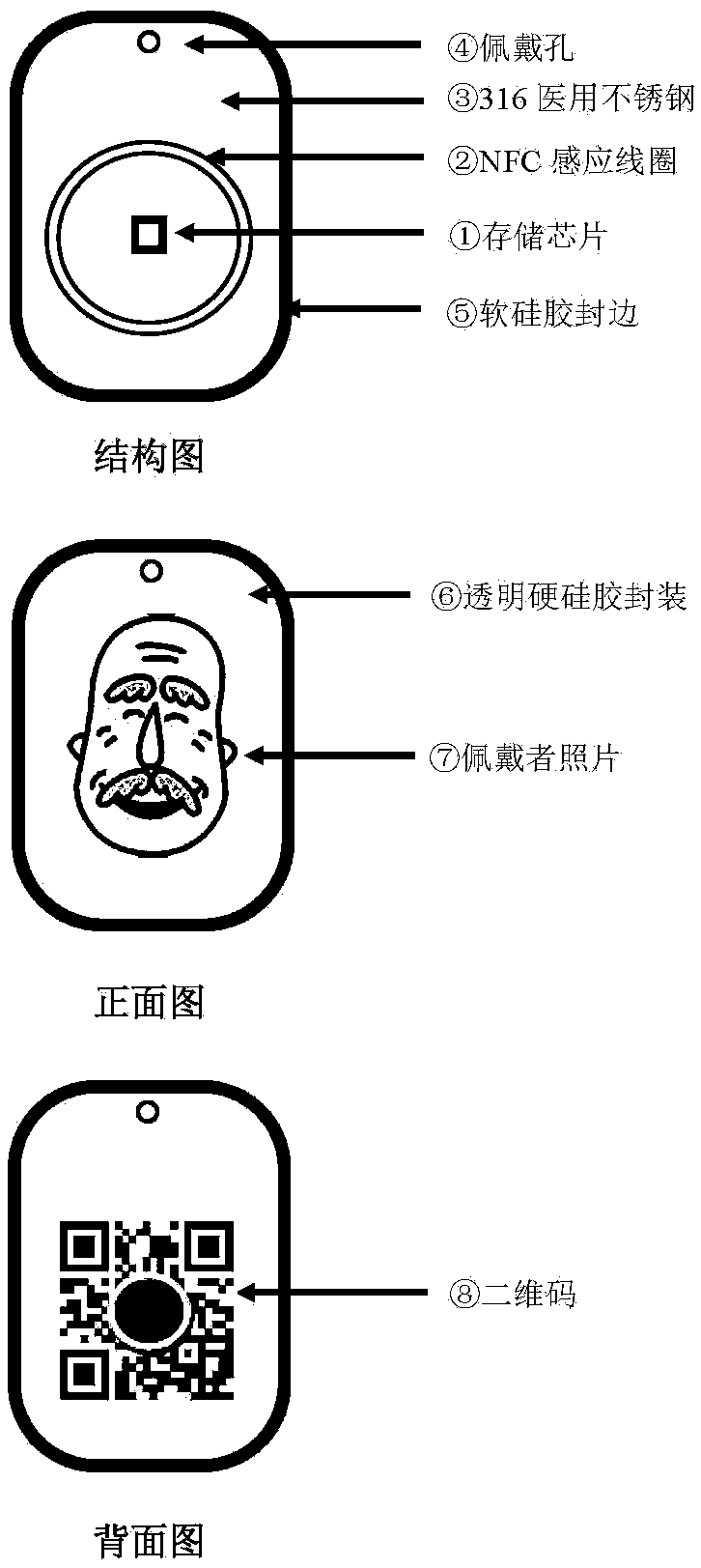
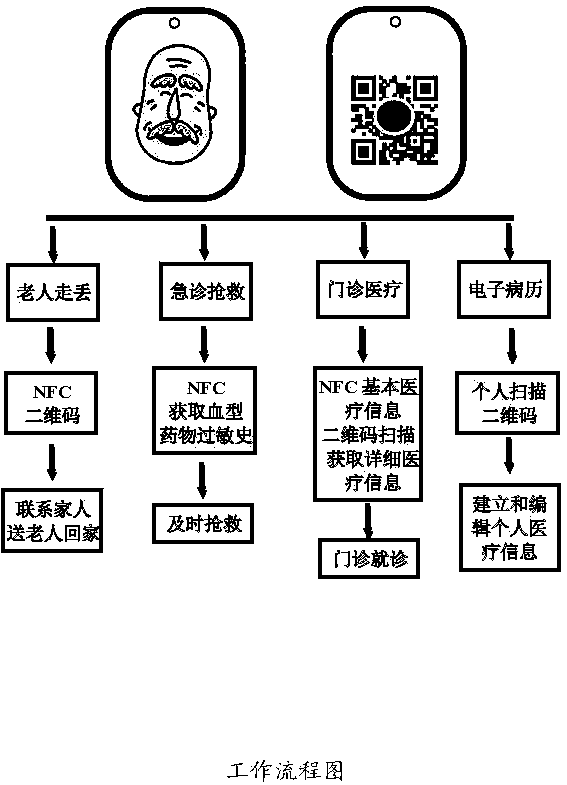
The invention discloses a health electronic information card, which organically integrates an electronic storage chip, a near field communication (NFC) technology, a personal intelligent mobile terminal and a personal health information webpage. The system comprises the electronic storage chip 1, an NFC induction coil 2, a 316 medical stainless steel card body 3, a wearing hole 4, a soft silica gel sealed edge 5, a transparent hard silica gel package 6, a wearer photo 7, a rear-positioned two-dimensional code 8, and a personal health homepage 9. The health electronic information card can realize functions of preventing loss of the elderly with dementia, acquiring key medical information such as blood type, drug allergy history and chronic disease history of a user by medical personnel timely during emergency treatment, being capable of reading detailed medical health information of the user by the medical personnel after being authorized by the wearer in the clinic, and being capable of performing maintenance such as filling in, updating and modification on the personal medical health information by the user through using a computer or an intelligent mobile terminal.
Owner:张航向
Method for screening the sensitizing properties of chemical compounds
InactiveUS20110245104A1Prevent sensitizationAvoid developmentElectrolysis componentsMicrobiological testing/measurementDiseaseChemical compound
A method is provided for screening the sensitizing properties of chemical compounds. The method is based on keratinocytes or cells that share important hallmarks of these cells, but other components e.g. proteins could also be used. This method is of importance for several conditions, including but not limited to allergic contact dermatitis (ACD), drug hypersensitivity reactions (DHRs) and autoimmune diseases.
Owner:BROO KERSTIN S +4
Method for distinguishing ureaplasma urealyticum and ureaplasma parvum in ureaplasma by using culture method
ActiveCN107619849AEasy to operateEasy for clinical promotion and useMicrobiological testing/measurementBiotechnologyUrease enzyme
The invention discloses a method for distinguishing ureaplasma urealyticum and ureaplasma parvum in ureaplasma by using a culture method. The method comprises the following steps: firstly, confirmingtwo detection micro-pores, namely a pore A and a pore B; putting a proper amount of glucose or / and a urease inhibitor into the pore A, putting a manganese-magnesium solution into the pore B, drying, adhering the glucose or / and the urease inhibitor to the inner wall of the pore A, and adhering manganese ions and magnesium ions to the inner wall of the pore A; putting a sample to be tested into a ureaplasma culture medium, uniformly mixing, respectively putting into the pore A and the pore B, and culturing for 24-48 hours at 37 DEG C; and according to the color change situations of the pore A and the pore B, confirming whether the sample has ureaplasma urealyticum and ureaplasma parvum or not. A kit which is relatively convenient to use is prepared according to the method. The method disclosed by the invention is simple to operate, whether a specimen contains the ureaplasma urealyticum and the ureaplasma parvum or not can be precisely distinguished at one step, meanwhile, the method canbe implemented together with drug allergy detection, and identification and drug allergy results can be achieved; and identification results are visible and accurate, and clinical popularization and application are facilitated.
Owner:AUTOBIO DIAGNOSTICS CO LTD
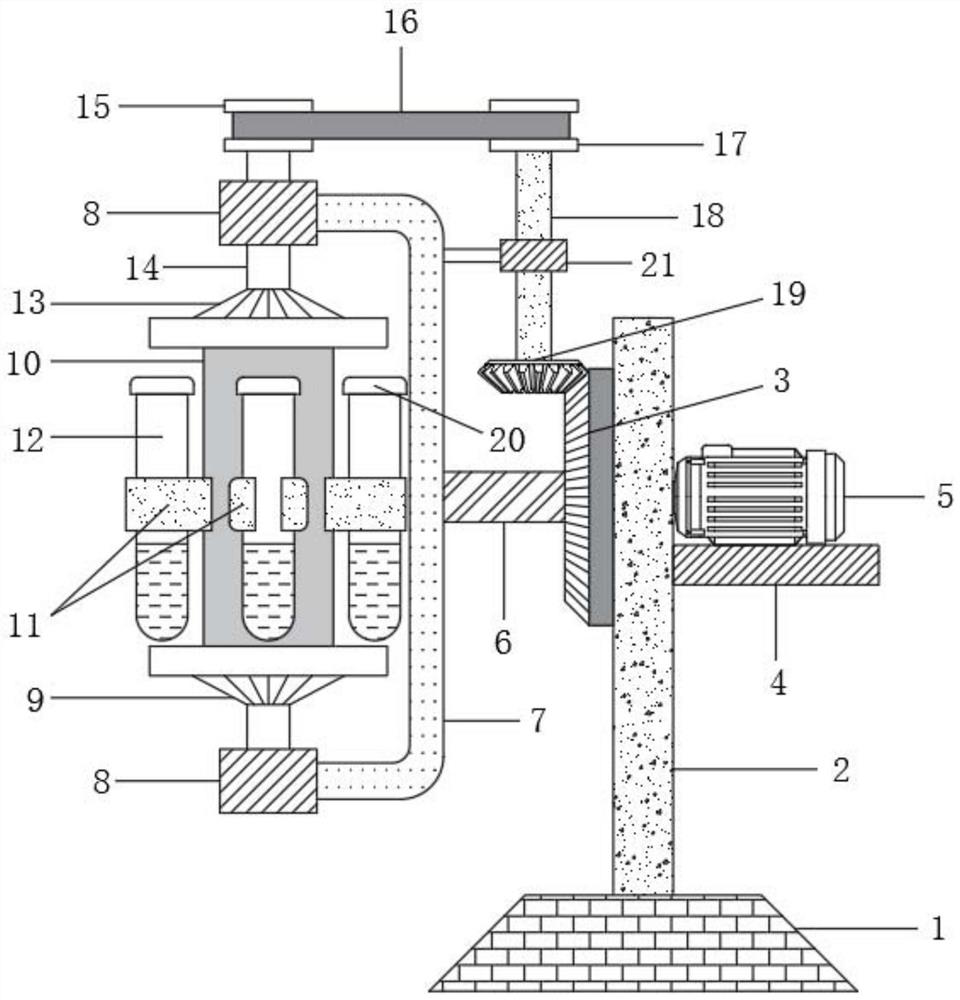
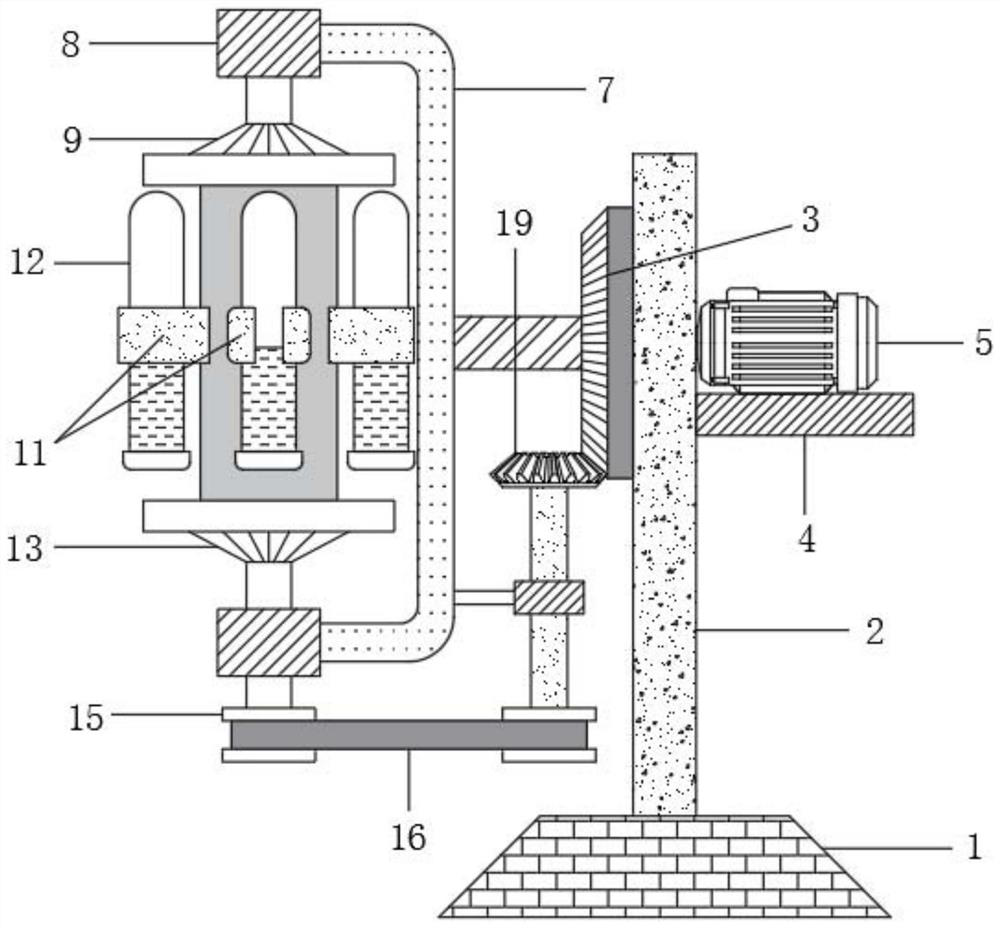
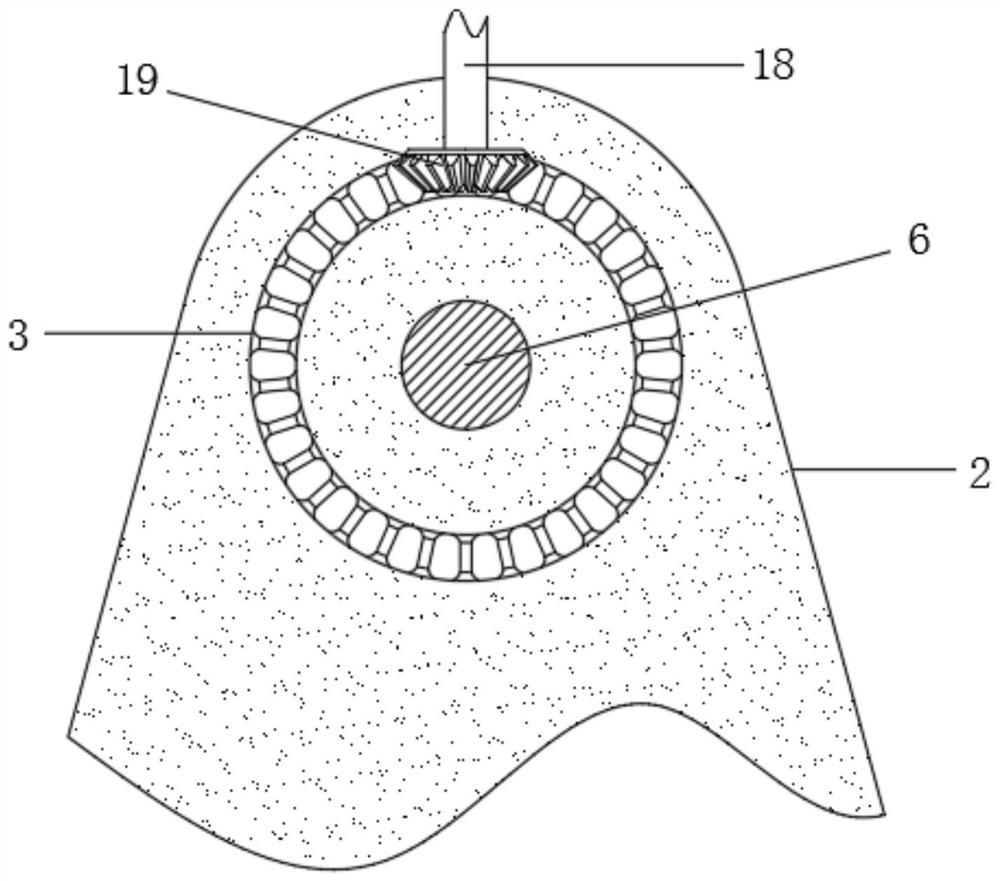
Drug allergy detection device and method for cancer patients
InactiveCN111812310AAchieve the effect of allergy detectionWell mixedBiological testingDrug allergyPharmaceutical drug
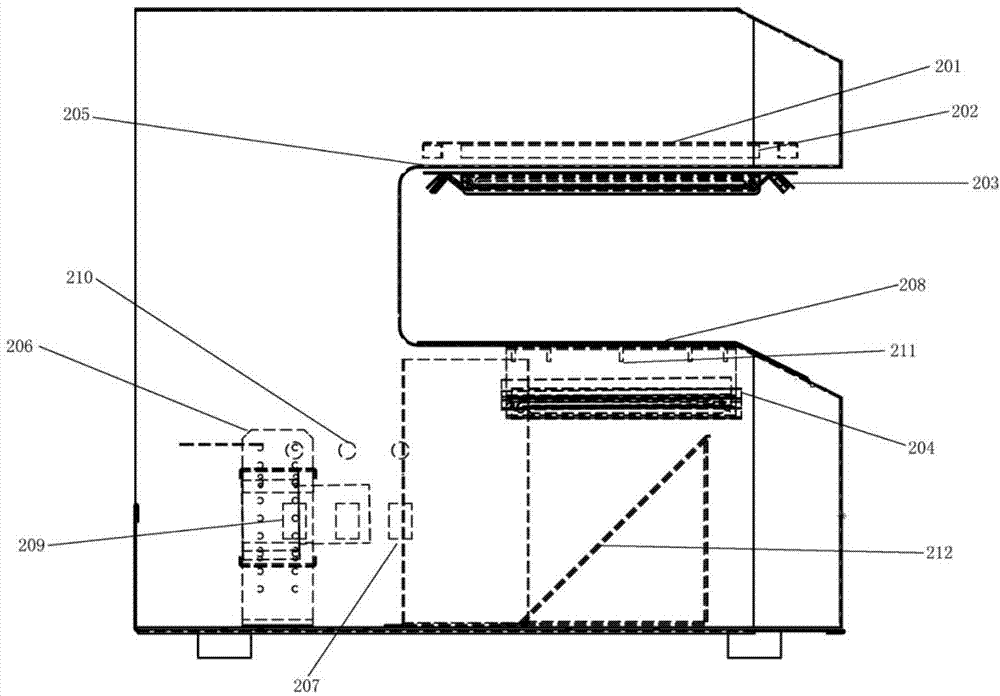
The invention relates to the technical field of drug allergy detection and further discloses a drug allergy detection device for cancer patients. A base is included. A mounting plate is fixedly connected to the upper end of the base, a conical gear ring is fixedly connected to the left side of the mounting plate, a supporting plate is fixedly connected to the right side of the mounting plate, a motor is fixedly connected to the upper end of the supporting plate, a rotating shaft is fixedly connected to an output end of the motor, and a U-shaped rod is fixedly connected to the end, away from the motor, of the rotating shaft. According to the drug allergy detection device for cancer patients, the U-shaped rod drives a connecting column to synchronously rotate so as to drive a detection testtube to synchronously rotate; a bevel gear is matched to revolve around an axis of the bevel gear ring and autoroatation is generated at the same time; and a first transmission wheel is indirectly driven to synchronously rotate so that the detection test tube is driven to synchronously rotate through the connecting column, and the detection test tube rotates around the axis of the rotating shaft and the axis of the connecting column at the same time so that effects of more uniform medicament mixing and high result reliability are achieved.
Owner:刘燕
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com