Clinical drug allergy management method, auxiliary device and system
A technology for drugs and allergies, applied in the medical field, can solve the problems of low efficiency of information recording, unstructured, and the system cannot be used directly.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
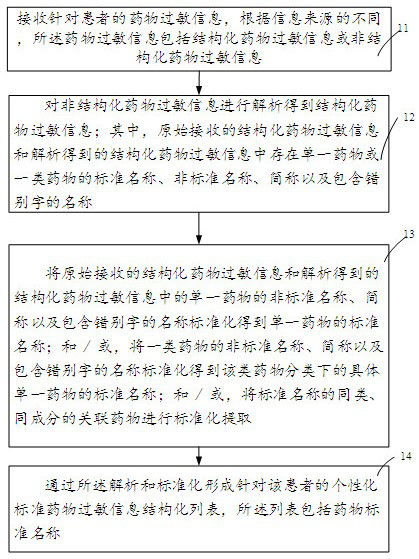
[0034] A schematic flow chart of a clinical drug allergy management method proposed in the embodiment of the present invention is as follows figure 1 As shown, it is applied to the clinical drug allergy management auxiliary device, including the following steps:
[0035] Step 11. Receive drug allergy information for the patient. According to different information sources, the drug allergy information includes structured drug allergy information or unstructured drug allergy information;
[0036] When a patient sees a doctor or is admitted to the hospital, the clinician will ask the patient's history of allergies such as drugs, food, and items, and record it in the business device through structured list selection or unstructured free text input. For structured selection record results, That is, structured drug allergy information needs to be standardized. For the recorded results of unstructured text, that is, unstructured drug allergy information, it needs to be parsed and st...
Embodiment 2
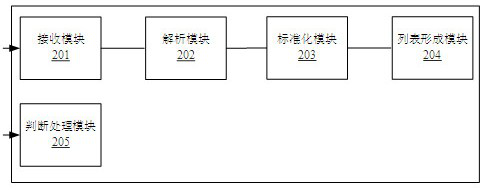
[0072] Based on the same inventive concept, an embodiment of the present invention provides an auxiliary device for clinical drug allergy management, the structural diagram is as follows figure 2 shown, including:
[0073] The receiving module 201 receives drug allergy information for the patient, and according to different information sources, the drug allergy information includes structured drug allergy information or unstructured drug allergy information;
[0074] The parsing module 202, which parses the unstructured drug allergy information to obtain structured drug allergy information; wherein, there are standard names of a single drug or a class of drugs in the originally received structured drug allergy information and the parsed structured drug allergy information , non-standard names, abbreviations, and names containing typos;
[0075] The standardization module 203, standardizes the non-standard name, abbreviation and names containing typos of a single drug in the ...
Embodiment 3
[0095] In terms of clinical application of allergy record results, the following situations exist:
[0096] Situation 1: The use of drug allergy information record results is a closed-loop problem. The allergy records are only recorded in the system, and have not been referenced and used at the system and business levels. The information about drug allergies must be passed on through oral question and answer;
[0097] Situation 2: Records are directly quoted and used by the system through structured entry, but only limited to penicillin and other antibiotics and other skin test drugs, and the allergy records of other drugs are not actually clinically applied;
[0098] Case 3: The accuracy review and verification of allergy information records is closed-loop, the quality review of the record filling link is not performed, and the verification of skin test results for allergy records automatically updates the results of allergy information records;
[0099] Situation 4: Closed-l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com