Ceftazidime injectable powder for injection and preparation method of ceftazidime injectable powder for injection
A technology for ceftazidime and injection, which is applied in the field of pharmaceutical preparations, can solve the problems of reducing the content of active pharmaceutical ingredients, increasing the content of polymer impurities, allergic reactions in the human body, etc., and achieves the effects of reducing the dosage, improving the purity, and enhancing the solubilizing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
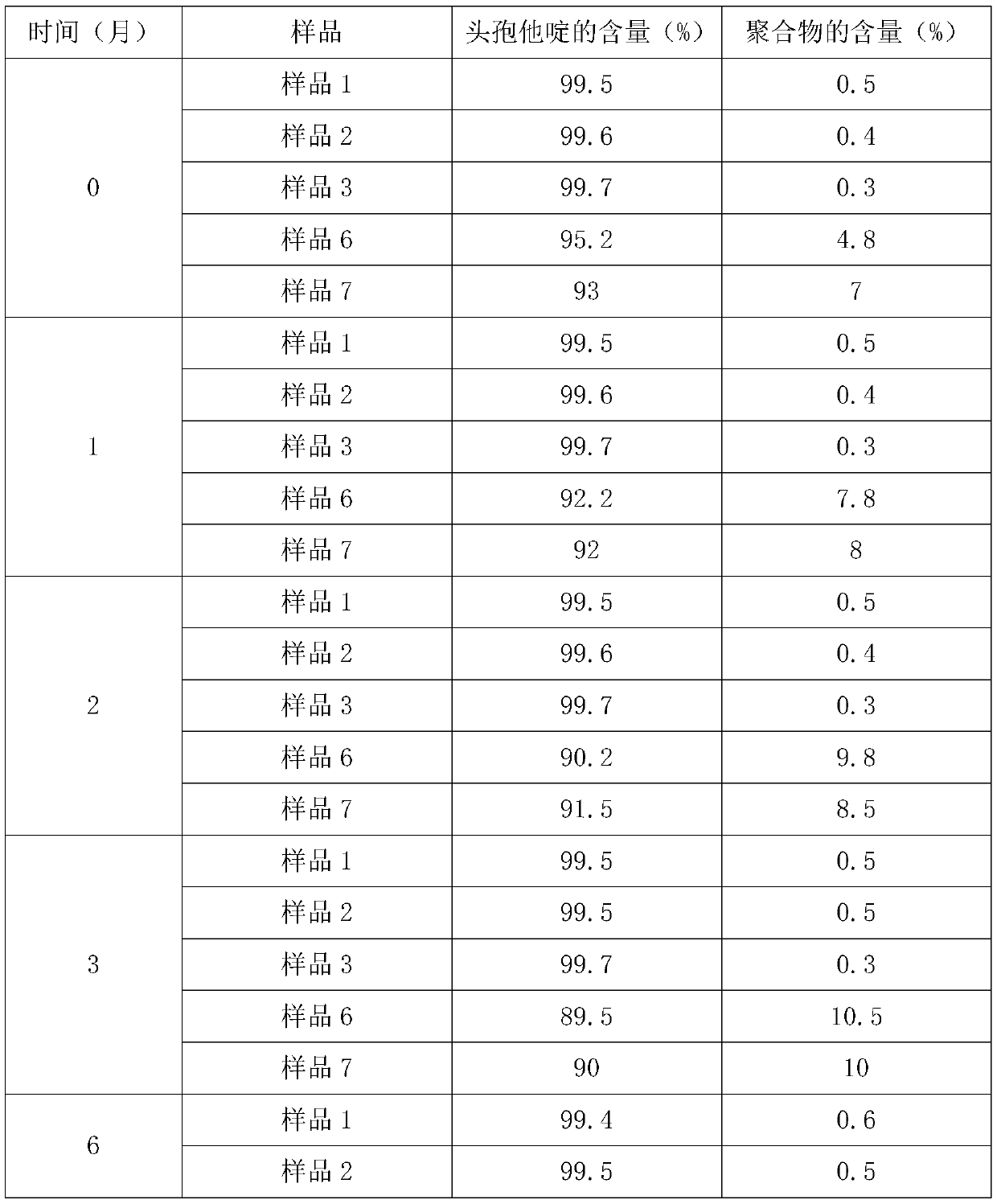
Examples
Embodiment 1
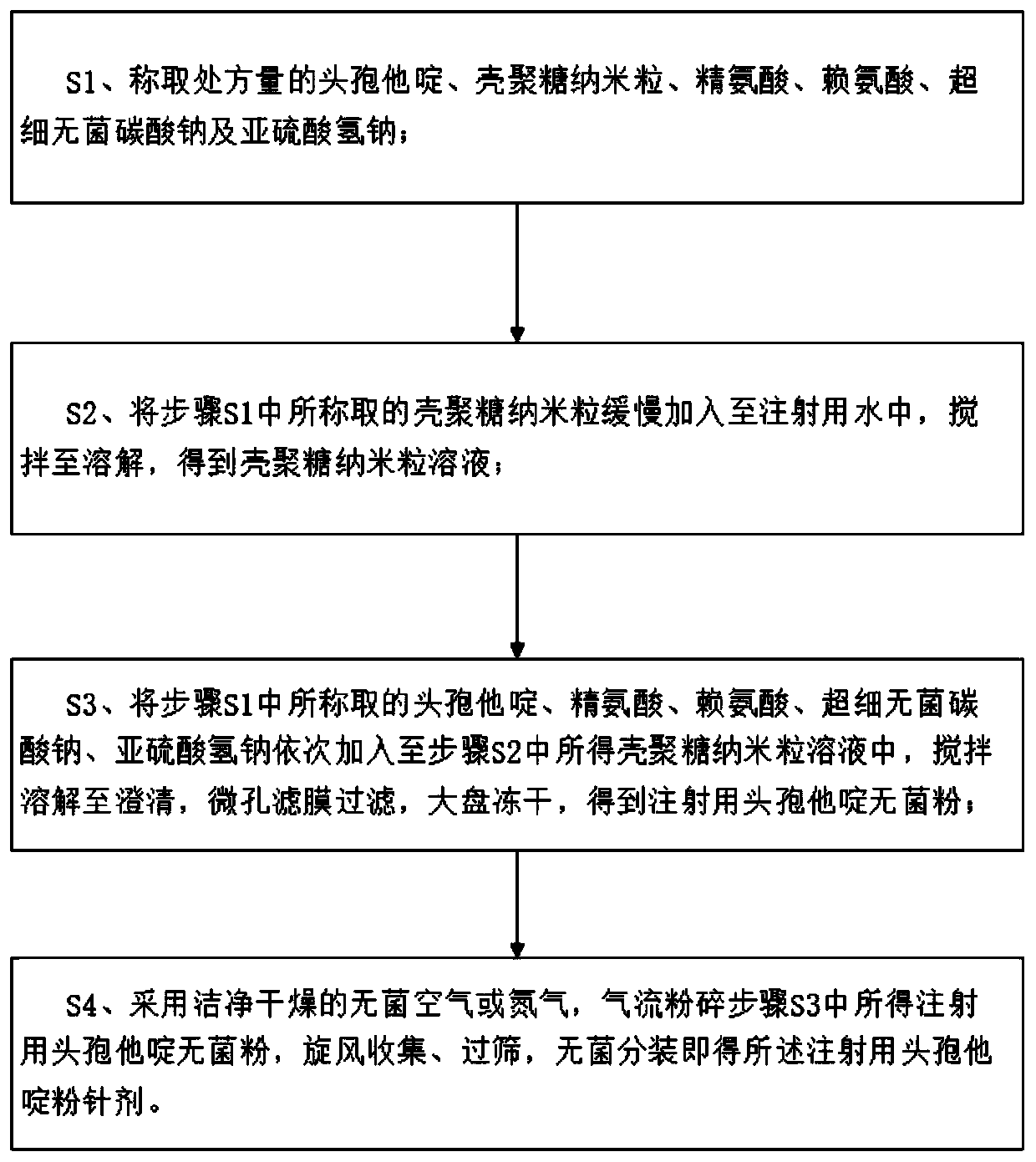
[0036] A kind of ceftazidime powder injection for injection, comprises the raw material of following weight: ceftazidime 8.3g, chitosan nano particle 6.5g, arginine 0.06g, lysine 0.07g, superfine sterile sodium carbonate 0.85g, hydrogen sulfite Sodium 0.00005g.
[0037] Wherein, the particle diameter of the ultrafine sterile sodium carbonate in the present embodiment is ≤20 μm.
[0038] Wherein, the particle size of the chitosan nanoparticles in this embodiment is ≤100nm.
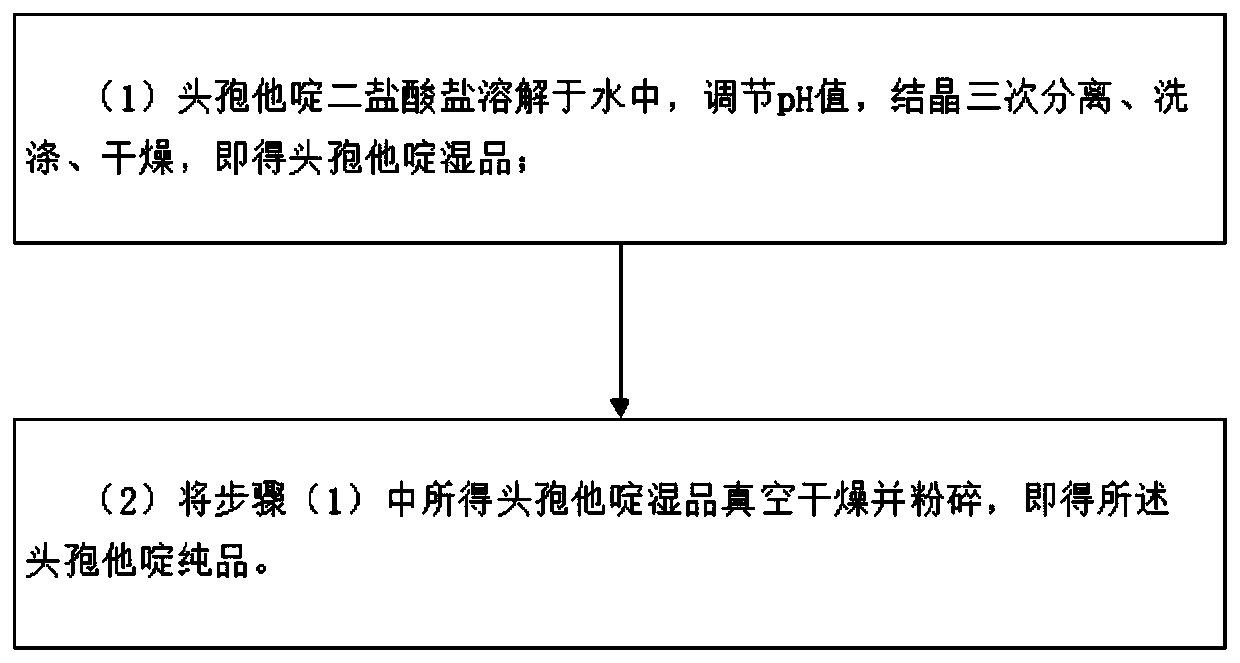
[0039] refer to figure 1 Shown, the preparation method of the ceftazidime in the present embodiment comprises the following steps:
[0040] (1) Ceftazidime dihydrochloride is dissolved in water, the pH value is adjusted, the crystallization is separated three times, washed, and dried to obtain the wet product of ceftazidime;
[0041] (2) The wet product of ceftazidime obtained in the step (1) is vacuum-dried and pulverized to obtain the pure product of ceftazidime.
[0042]Wherein, in step (1), the pH i...
Embodiment 2
[0052] A kind of ceftazidime powder injection for injection, comprises the raw material of following weight: ceftazidime 12.3g, chitosan nano particle 9.0g, arginine 1.5g, lysine 1.5g, superfine sterile sodium carbonate 5.2g, hydrogen sulfite Sodium 0.0019g.
[0053] Wherein, the particle diameter of the ultrafine sterile sodium carbonate in the present embodiment is ≤20 μm.
[0054] Wherein, the particle size of the chitosan nanoparticles in this embodiment is ≤100nm.
[0055] refer to figure 1 Shown, the preparation method of the ceftazidime in the present embodiment comprises the following steps:
[0056] (1) Ceftazidime dihydrochloride is dissolved in water, the pH value is adjusted, the crystallization is separated three times, washed, and dried to obtain the wet product of ceftazidime;
[0057] (2) The wet product of ceftazidime obtained in the step (1) is vacuum-dried and pulverized to obtain the pure product of ceftazidime.
[0058] Wherein, in step (1), the pH is ...
Embodiment 3
[0068] A kind of ceftazidime powder injection for injection, comprises the raw material of following weight: ceftazidime 9.24g, chitosan nano particle 7.2g, arginine 1.8g, lysine 1.8g, superfine sterile sodium carbonate 1.39g, hydrogen sulfite Sodium 0.0011g.
[0069] Wherein, the particle diameter of the ultrafine sterile sodium carbonate in the present embodiment is ≤20 μm.
[0070] Wherein, the particle size of the chitosan nanoparticles in this embodiment is ≤100nm.
[0071] refer to figure 1 Shown, the preparation method of the ceftazidime in the present embodiment comprises the following steps:
[0072] (1) Ceftazidime dihydrochloride is dissolved in water, the pH value is adjusted, the crystallization is separated three times, washed, and dried to obtain the wet product of ceftazidime;
[0073] (2) The wet product of ceftazidime obtained in the step (1) is vacuum-dried and pulverized to obtain the pure product of ceftazidime.
[0074] Wherein, in step (1), the pH is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com