Quantitative determination and drug allergy determination kits for helicobacter pylori viable bacteria and determination method
A technology for the quantitative determination of Helicobacter pylori, which is applied in the field of rapid screening methods and kits for anti-Helicobacter pylori sensitive drugs, can solve the problems of high injury and pain for patients, can only be qualitatively determined, and is expensive, and achieve cheap reagents and methods Simple, cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
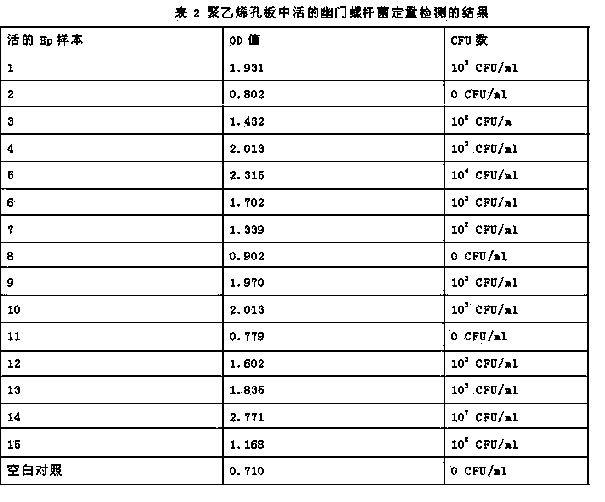
[0027] Quantitative determination method of viable bacteria of Helicobacter pylori
[0028] 8-10 parts of wt2% urea, 0.1-0.5 parts of wt 0.04% phenol red, wt 0.04% NaH 2 PO 4 ﹒ h 2 O 3-5 parts, wt 0.1%Na 2 HPO 4 2-3 parts are configured as sterile urease reagent.
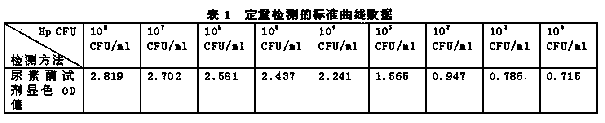
[0029] Draw the standard curve of the CFU / ml value corresponding to the OD value, that is, the number of individual bacteria in each ml of bacterial solution. The standard curve of the CFU corresponding to the OD value is determined by the gold standard of bacteriological detection (colony plate count), and the specific operation of the colony plate count is briefly described as follows:
[0030] Dilute a certain concentration of bacterial solution by a 10-fold gradient, take 100 microliters of the diluted bacterial solution and spread it on the medium, parallel 3 plates, count the number of colonies after 72 hours of cultivation, and dilute the gradient until there are only 1-5 colonies on the plate
[00...
Embodiment 2
[0044] Helicobacter pylori drug susceptibility testing method
[0045] 8-10 parts of wt2% urea, 0.1-0.5 parts of wt 0.04% phenol red, wt 0.04% NaH 2 PO 4 ﹒ h 2 O 3-5 parts, wt 0.1%Na 2 HPO 4 2-3 parts are configured as sterile urease reagent.
[0046] Draw the standard curve of the CFU / ml value corresponding to the OD value, that is, the number of individual bacteria in each ml of bacterial solution.
[0047] The standard curve of the CFU corresponding to the OD value is determined by the gold standard of bacteriological detection (colony plate count), and the specific operation of the colony plate count is briefly described as follows:
[0048] Dilute a certain concentration of bacterial solution by a 10-fold gradient, and take 100 microliters of the diluted bacterial solution and smear it on the culture plate.
[0049] On the base, parallel 3 plates, count the number of colonies after 72 hours of culture, and dilute the gradient until there are only 1-5 colonies on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com