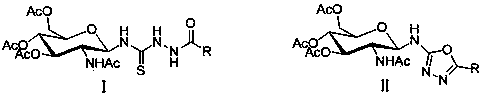
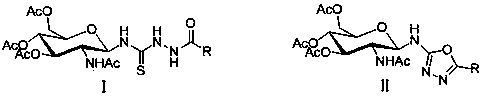
1,3,4-oxadiazole derivative containing glucosamine fragment as well as synthetic method and use of derivative
A synthesis method and technology of glucose, applied in the directions of sugar derivatives, sugar derivatives, sugar derivatives preparation, etc., can solve the problems of harsh reaction conditions, many by-products, unfriendly environment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1, a glucosamine-based 1,3,4-oxadiazole, its molecular formula is as follows: Formula III:
[0044]
[0045] Wherein, the R is selected from CH 3 -, C 4 h 9 -, C 6 h 5 -, 4-CH 3 C 6 h 4 -, 3-CH 3 C 6 h 4 -,2-CH 3 C 6 h 4 -, 4-CH 3 OC 6 h 4 -, 3,4-di-CH 3 OC 6 h 4 -, 3-CH 3 OC 6 h 4 -, 4-FC 6 h 4 -, 2,3-di-FC 6 h 4 -, 4-ClC 6 h 4 -, 2-ClC 6 h 4 -, 4-BrC 6 h 4 -, 4-IC 6 h 4 -, 3-IC 6 h 4 -, 4-OHC 6 h 4 -, 4-NO 2 C 6 h 4 -,
[0046] 3-NO 2 C 6 h 4 -, 3-C 6 h 4 N-, 2-C 5 h 3 S-,5-Cl-2-C 5 h 2 S-, 4-( N , N -di-CH 3 )-C 6 h 4 -.
[0047]The 1,3,4-oxadiazole derivatives containing glucosamine fragments of the present invention, as a class of novel compounds, have higher inhibitory activity on urease. The following is the urease activity experiment process and its results. Urease activity test method: urease activity was detected using the method of Tanaka et al. (Tanaka T, Kawase M, Tani S. (2003). Urease inh...
Embodiment 2
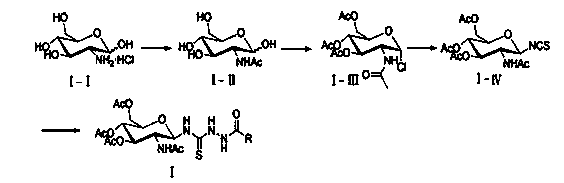
[0049] Embodiment 2, a kind of synthetic method of the 1,3,4-oxadiazole derivative containing glucosamine fragment as described in embodiment 1, its steps are as follows:
[0050] (1) the compound shown in formula I N -(2-Acetamido-3,4,6 - Tris-O-acetyl-2-deoxy- β -D-pyranose)- N' -amide thiosemicarbazide, p-toluenesulfonyl chloride and organic base were dissolved in tetrahydrofuran and reacted at 20°C for 3 hours, the reaction solution was distilled under reduced pressure, and purified by silica gel column chromatography to obtain the compound shown in formula II N -(2-Acetylamino-3,4,6-tri-O-acetyl-2-deoxy- β -D-pyranose)-5-substituted-1,3,4-oxadiazol-2-amine; the molar ratio of the compound shown in formula I, p-toluenesulfonyl chloride and organic base is 1:1:1; The organic base is pyridine or triethylamine;
[0051]
[0052] R in formula I and formula II is the same as R in formula III;
[0053] (2) React the compound represented by formula II in a mixed solution...
Embodiment 3
[0054] Embodiment 3, a kind of synthetic method of the 1,3,4-oxadiazole derivative containing glucosamine fragment as described in embodiment 1, its steps are as follows:
[0055] (1) the compound shown in formula I N -(2-Acetamido-3,4,6 - Tris-O-acetyl-2-deoxy- β -D-pyranose)- N' -amide thiosemicarbazide, p-toluenesulfonyl chloride and organic base were dissolved in tetrahydrofuran and reacted for 10 hours at 65°C. The reaction solution was distilled under reduced pressure and purified by silica gel column chromatography to obtain the compound shown in formula II. N -(2-Acetylamino-3,4,6-tri-O-acetyl-2-deoxy- β -D-pyranose)-5-substituted-1,3,4-oxadiazol-2-amine; the molar ratio of the compound shown in formula I, p-toluenesulfonyl chloride and organic base is 1: 1.5: 2.1; The organic base is pyridine or triethylamine;
[0056]
[0057] R in formula I and formula II is the same as R in formula III;
[0058] (2) React the compound represented by formula II in a mixed s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com