Staphylococcus aureus FnBPA-A protein mimic epitope peptides having immunizing protection, mimic epitope peptide composition, and applications of mimic epitope peptides and mimic epitope peptide composition
A Staphylococcus, protein simulation technology, applied in the direction of antibody mimics/scaffolds, medical preparations containing active ingredients, combined chemistry, etc., can solve the difficulty of mastitis in dairy cows, the hazards of the dairy industry, and affect the quality and safety of milk and dairy products human health issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Preparation and purification of rabbit anti-FnBPA-A protein antibody
[0043] 1.1 Antibody Preparation
[0044] Mix 1 mL of rFnBPA-A purified protein (prepared in our laboratory) with a concentration of 1 mg / mL and 42 μL of Tween-80 as the water phase, and then add 3126 μL of white oil and shake it repeatedly on a vortex shaker to emulsify. The emulsified immunogen was subcutaneously injected into the neck and back of experimental rabbits at multiple points, and the immunization dose was 1 mg / rabbit. The second immunization was performed on the 14th day after the first immunization, and the immunization dose and immunization route were the same. Three immunizations were performed on the 10th day after the second immunization, and no immune adjuvant was added during the third immunization. The immunization dose and immunization route were the same as those of the first immunization. Rabbit blood was collected before immunization and on the 32nd day after imm...
example 2
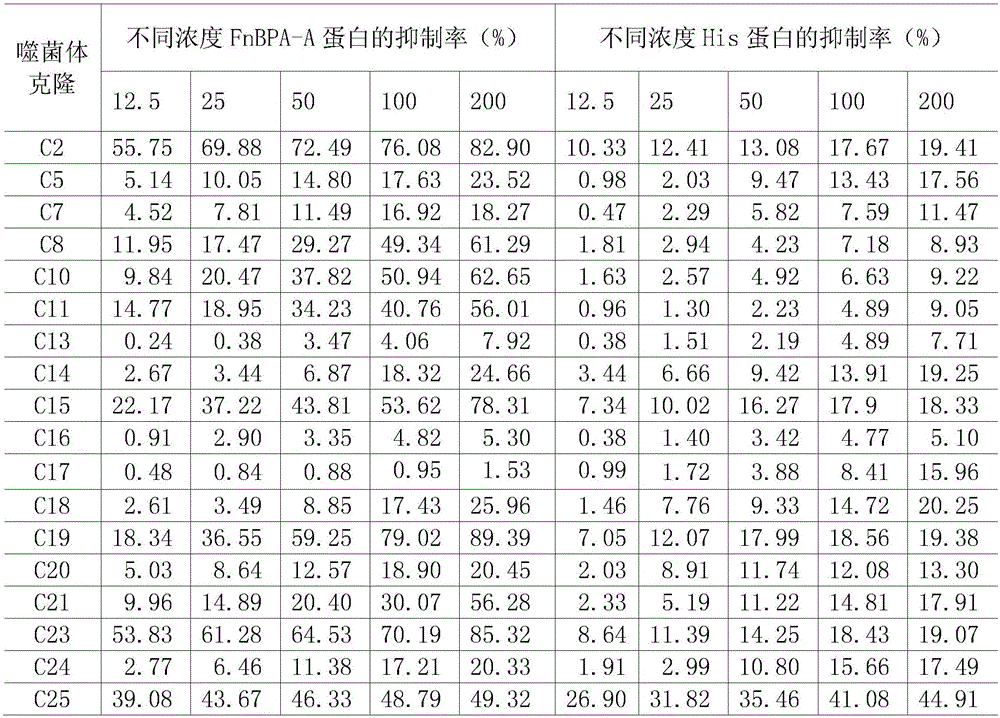
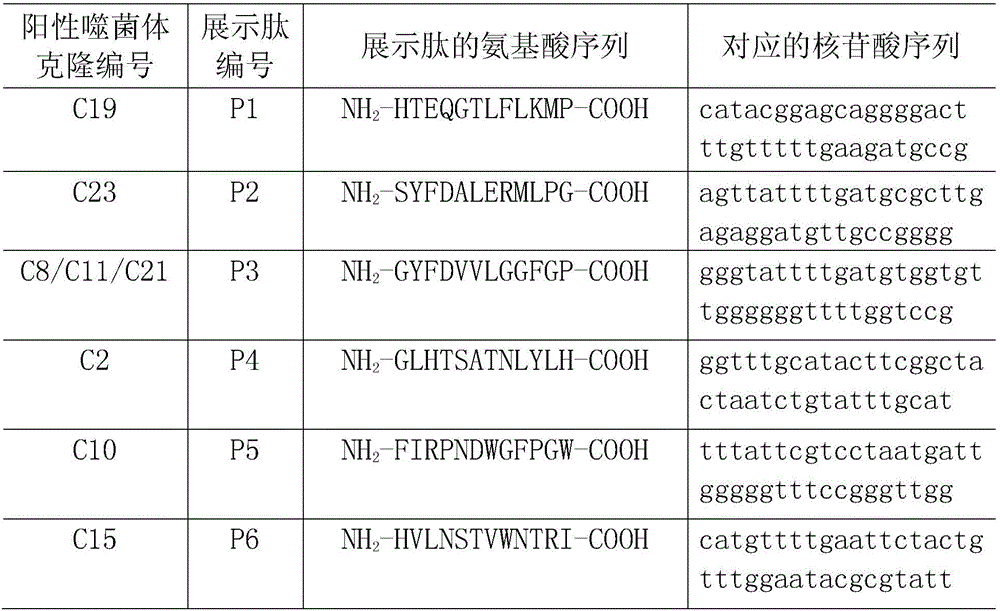
[0048] Example 2: Epitope screening and identification of FnBPA-A protein
[0049] 2.1 Panning of phage random dodecapeptide library with rabbit anti-FnBPA-A purified antibody
[0050] Phage random dodecapeptide library kit was purchased from NEB Company, and the library capacity was 2.7×10 9 transformants with a titer of 1.5 × 10 13 pfu / μL, Escherichia coli ER2537 is the host strain of the peptide library. The panning was carried out according to the kit instructions, and the brief process was as follows: use 0.1mol / L pH8.6 NaHCO 3 Solution The purified rabbit anti-FnBPA-A antibody was diluted to 100 μg / mL, coated on a 96-well plate, and kept at 4°C overnight. The next day, washed 6 times with TBST solution (TBS+0.5% Tween-20), added 5% BSA, and blocked for 1 h at 37°C. Discard the blocking solution and add 10 μL of the original peptide library (1.0×10 11 PFU), 195 μL of TBST and 195 μL of purified negative serum were mixed and added to enzyme-labeled wells, 100 μL / well,...
example 3
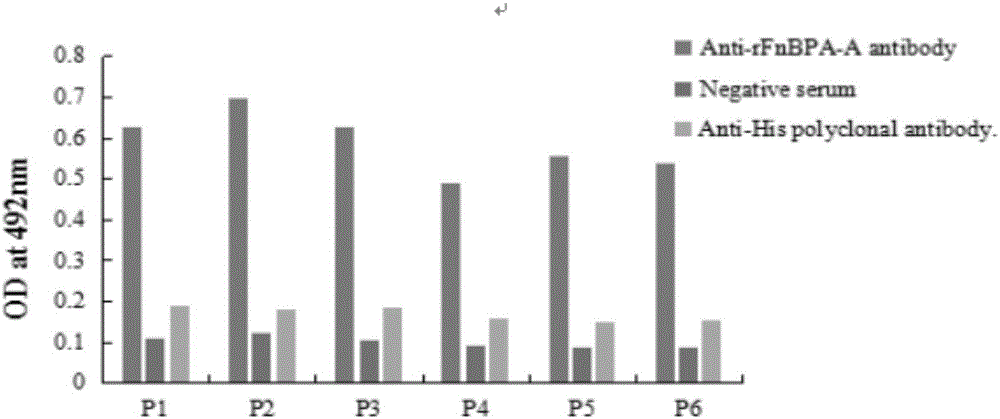
[0065] Example 3: Detection of immune activity of FnBPA-A protein mimic epitope
[0066] 3.1 Immunization of experimental mice and detection of immunogenicity of mimetope peptides
[0067] Kunming mice weighing 18-20 g were randomly divided into 12 groups, 20 in each group. The first group is the rFnBPA-A protein immunization group; the second group is the phage M13 immunization group; the 3-8 groups are the C2, C8, C10, C15, C19 and C23 positive phage clone immunization groups respectively; the 9-11 groups are the mock table Different ratios of peptides P1 and P2 were mixed group; the 12th group was the normal saline control group. The mice in the rFnBPA-A protein immunization group were injected with rFnBPA-A protein emulsified in Freund's adjuvant, 30 μg / mouse; the mice in the positive phage clone immunization group were injected with phage clone, 1×10 12 pfu / mouse; the mice in the mimotope peptide mixed group were injected with the mixed peptide emulsified in Freund's ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com