Epitope delivery system with Escherichia coli heat labile enterotoxin B subunits serving as carriers
A heat-resistant enterotoxin and delivery system technology, applied in the direction of introducing foreign genetic material, antiviral agents, and microbial-based methods using carriers, can solve problems such as the inability to maintain the quality uniformity of the complex and the high price of chemical cross-linking agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1, construction of pMD18T-LTB-Linker engineering bacteria.
[0085] Using pMD18T-LT as a template, pMD18T-LTB-Seq1, pMD18T-LTB-Seq2, pMD18T-LTB-Seq3, pMD18T-LTB-Seq4, pMD18T-LTB-Seq4-G, pMD18T-LTB-Seq4 were constructed by PCR method -Seq2, pMD18T-LTB-Seq4-Seq3 recombinant plasmids, each plasmid was transformed into E.coli TOP10, and pMD18T-LTB-Linker engineering bacteria were constructed after enzyme digestion and sequencing identification.
[0086] Linker is therefore specifically: Seq1, Seq2, Seq3, Seq4, Seq4-G, Seq4-Seq2 and Seq4-Seq3.
Embodiment 2
[0087] Example 2, construction of engineering bacteria expressing LTB-Linker-Pep1 and LTB-Linker-Pep2.
[0088] Using the pMD18T-LTB-Linker in Example 1 as a template, connect the tested Pep1 and Pep2 fragments to pMD18T-LTB-Linker by PCR to construct pMD18T-LTB-Linker-Pep1 and pMD18T-LTB-Linker-Pep2 recombinant plasmids , using endonucleases NcoI and BamHI to perform double digestion to obtain the target fragment, which was ligated with the same double digestion plasmid pET22b to construct and express recombinant plasmids pET22b-LTB-Linker-Pep1 and pET22b-LTB-Linker-Pep2.
[0089] LTB-Linker-Pep1 includes: LTB-Seq1-Pep1(L1P1), LTB-Seq2-Pep1(L2P1), LTB-Seq3-Pep1(L3P1), LTB-Seq4-Pep1(L4P1), LTB-Seq4-G-Pep1 (L4GP1) and LTB-Seq4-Seq2-Pep1 (L42P1).
[0090] LTB-Linker-Pep2 includes: LTB-Seq1-Pep2(L1P2), LTB-Seq2-Pep2(L2P2), LTB-Seq3-Pep2(L3P2), LTB-Seq4-Pep2(L4P2), LTB-Seq4-Seq2-Pep2 (L42P2) and LTB-Seq4-Seq3-Pep2 (L43P2).
[0091] Transform Escherichia coli E.coli BL21(DE3) wi...
Embodiment 3
[0092] Example 3, the expression of LTB-Linker-Pep1 and LTB-Linker-Pep2.
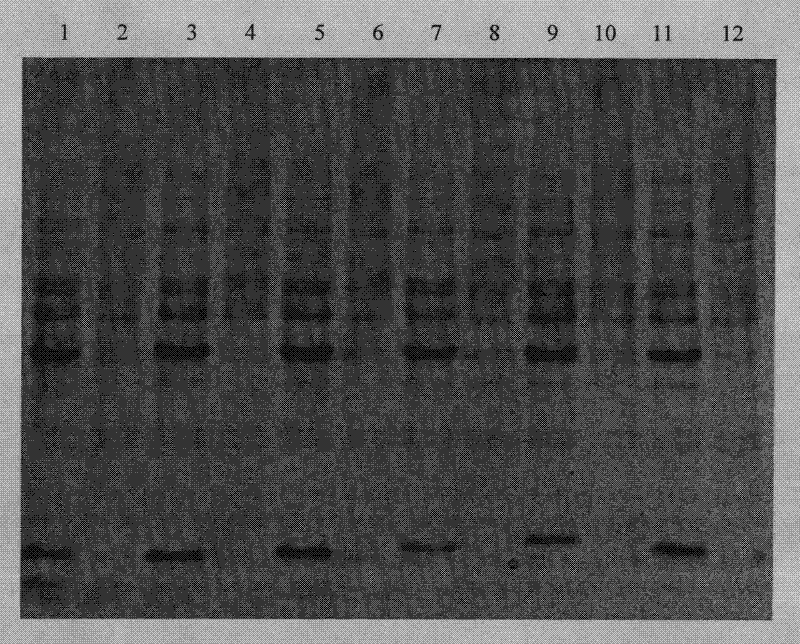
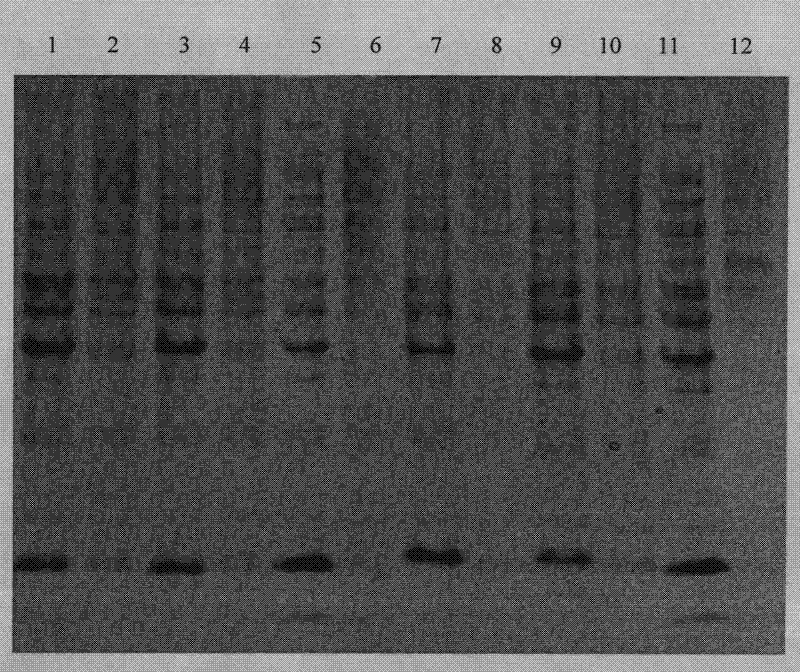
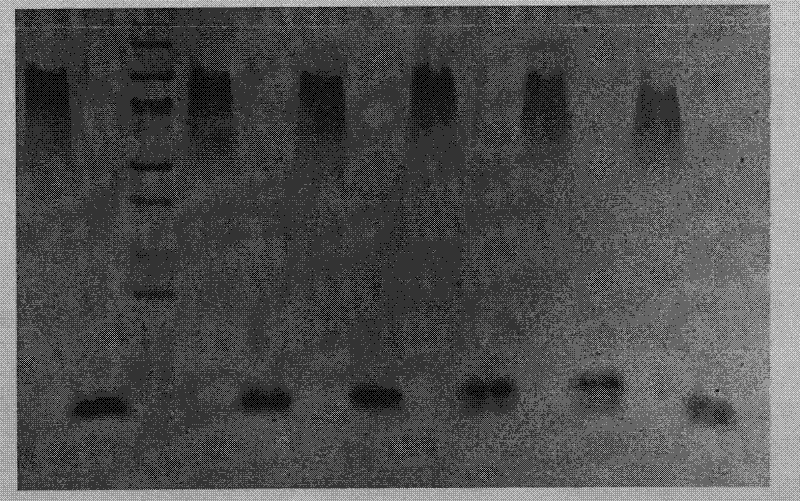
[0093]Recombinant engineered bacteria pET22b-LTB-Linker-Pep1 / E.coli BL21(DE3) and pET22b-LTB-Linker-Pep2 / E.coliBL21(DE3) were expressed by 1L fermentation respectively, and IPTG was added to the final concentration of 0.1mmol / L, Expression was induced overnight at 18°C. The expression results are shown in the appendix figure 1 and attached figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com