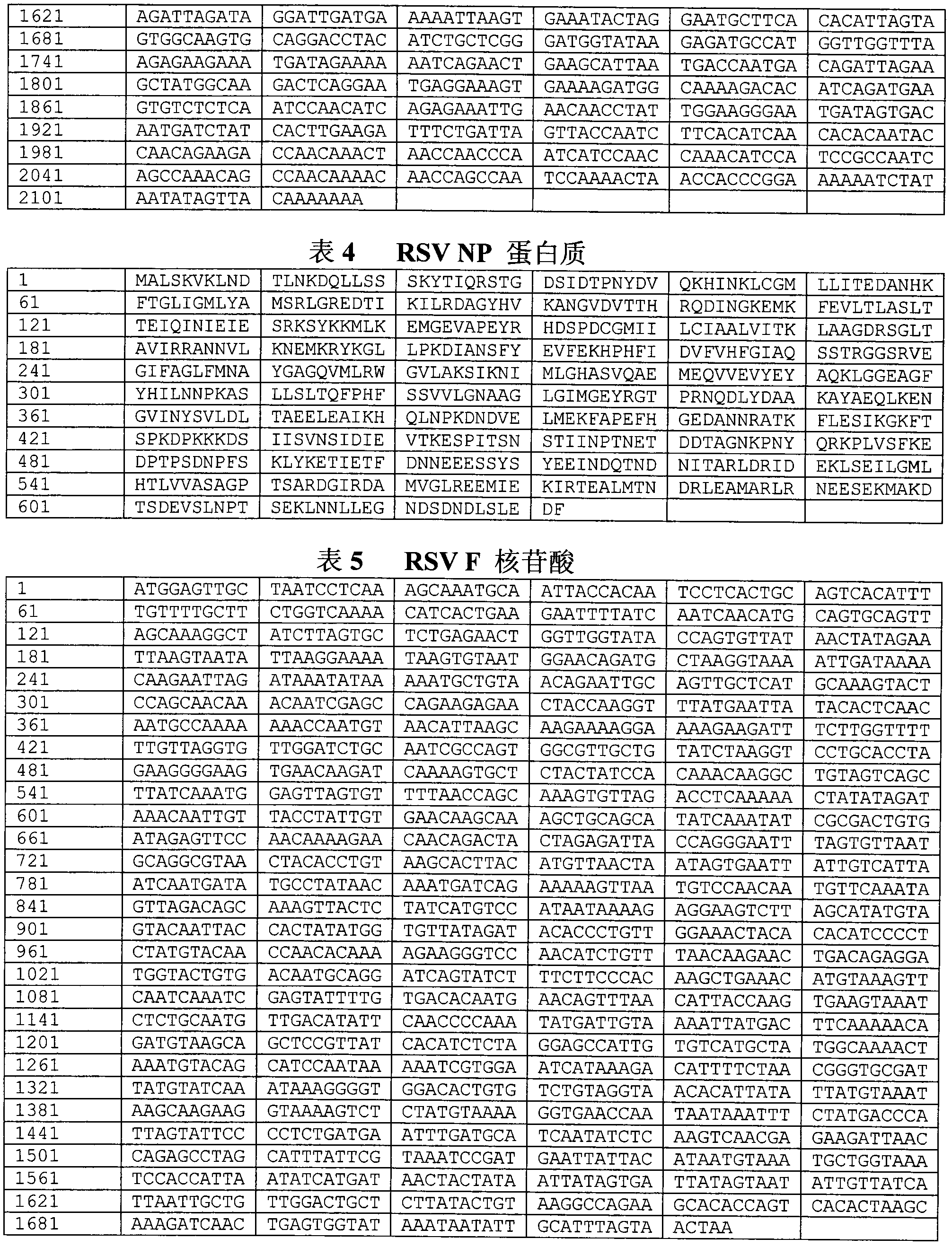
Patents
Literature
45 results about "HN Protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glycoprotein from Sendai, para-influenza, Newcastle Disease, and other viruses that participates in binding the virus to cell-surface receptors. The HN protein possesses both hemagglutinin and neuraminidase activity.
Epitope peptide H362 of HN protein in peste des petits ruminants virus (PPRV), and determination, preparation method and application thereof
ActiveCN107216372AStrong green fluorescenceGood reactogenicitySsRNA viruses negative-senseViral antigen ingredientsF proteinHN Protein
The invention relates to an epitope peptide H362 of an HN protein in PPRV, and determination, a preparation method and application thereof. The amino acid sequence of the epitope peptide is H362: <362>EANWVVPSTDVRDL<375>. The invention detects reactogenicity of a monoclonal antibody and PPRV and specificity of the monoclonal antibody; according to detection results, the monoclonal antibody has good reactogenicity to rPPRV-HN-F protein; immunoinformatic technology is cooperatively used for predicating the B cell epitope of the HN protein; an aminated ELISA plate is employed for detecting candidate epitopes and the monoclonal antibody 10E3, and the epitope peptide H362 corresponding to 10E3 is determined; and determination of the epitope peptide lays a theoretical foundation for preparation of epitope vaccine antigens and diagnostic reagent antigens for PPRV.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Respiratory syncytial virus-like particle vaccine and preparation method thereof
The invention discloses a virus-like particle vaccine (NDV / RSV VLP vaccine) of which the centre is from NVD (Newcastle disease virus) and the surface protein is from RSV (respiratory syncytial virus), and also discloses a preparation method of the virus-like particle vaccine. The RSV VLP vaccine provided by the invention comprises a VLP composed of four structural proteins of respiratory syncytial virus M, F, NP and G; and the NDV / RSV VLP vaccine comprises four structural proteins of Newcastle disease virus M, F, NP and NH and VLP, wherein the VLP is formed by two surface proteins which are a NDV F / RSV F fusion protein composed of Newcastle disease virus F protein and respiratory syncytial virus F protein and an NDV HN / RSV G fusion protein composed of Newcastle disease virus HN protein and respiratory syncytial virus G protein. The test of pesticide effectiveness shows that RSV infection can be safely and effectively prevented after various dosage forms prepared by the VLP protein antigen formed with the method by adding or not adding adjuvants are used for processing immunization for different animals or the crowd, and the vaccine for processing immune prevention for the RSV inflection is supplied for the crowd with different ages.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Poultry IL-2 and newcastle disease virus HN gene recombination fusion protein and application thereof
InactiveCN101870733AEnhance body fluidsEnhance cellular immune responseFungiViral antigen ingredientsSide effectHN Protein
The invention relates to a preparation method and application of fusion protein (chIL-2-HN fusion protein) of recombining poultry interleukin-2 (IL-2) and newcastle disease virus hemagglutinin-neuraminidase (HN), belonging to the technical field of biological engineering. The fusion protein is fused by poultry IL-2 protein and newcastle disease virus HN protein by flexible Linker peptide; a DNA sequence for coding the fusion protein is inserted into an expression vector pPICZ alpha A; saccharomycete X-33 is electrically converted to obtain a genetically engineered microorganism for efficiently expressing recombination chIL-2-HN fusion protein which is prepared by liquid culture and purification; the recombination fusion protein can serve as the novel genetic engineering vaccine and can serve as a novel immunologic adjuvant for newcastle disease common vaccine. The chIL-2-HN fusion protein of the invention has favourable safety and no toxic or side effect, which is verified by experiments of animal immunoassay. In addition, the chIL-2-HN fusion protein can effectively strengthen the level of organism cellular immunity and humoral immunity and has wide application prospect.
Owner:HENAN UNIV OF SCI & TECH
Newcastle disease virus-like particles, preparation method and application thereof
ActiveCN106754765AClose to morphological structureClose to immunogenicitySsRNA viruses negative-senseViral antigen ingredientsWAS PROTEINHN Protein
Owner:JILIN UNIV
Establishment of hybridoma cell strain for secreting duck NDV (newcastle disease virus)-resisting isolate monoclonal antibody
InactiveCN103773736AImmunoglobulins against virusesMicroorganism based processesMolecular ImmunologyHN Protein
The invention relates to establishment of a hybridoma cell strain for secreting duck NDV (newcastle disease virus)-resisting isolate monoclonal antibody, which belongs to the technical field of molecular immunology and virology. The purified HN protein expressed by duck NDV isolate SDO3 pronucleus is used as immunogen, one hybridoma cell strain capable of secreting duck NDV-resisting isolate monoclonal antibody is researched, and the preservation number is CCTCC C2013173. The NDV specificity monoclonal antibody secreted by the hybridoma cell strain cannot be specially combined with the NDV strain but can be specifically combined with NDV clinical wild strain, so that the NDV specificity monoclonal antibody can be used as an identification reagent to be used for identifying the NDV vaccine virus and clinical wild strain, and the specificity is strong; the sensitivity is 1: 212 HAU; the hybridoma cell strain has the advantage of high sensitivity.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Human parainfluenza virus quantum dot immunochromatography typing detection card, preparation method and applications
ActiveCN105277693AHigh sensitivityStrong specificityMaterial analysisNitrocelluloseHuman Parainfluenza Virus
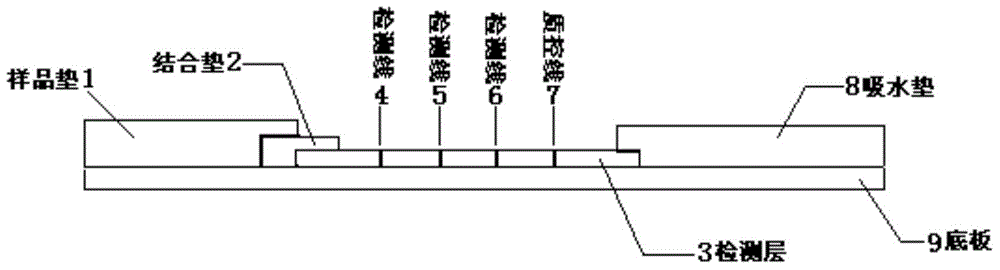
The invention provides a human parainfluenza virus quantum dot immunochromatography typing detection card, a preparation method and applications. The detection card comprises a base plate, a sample pad, a water absorption pad, a combination pad and a detection layer. The combination pad is coated with a mixture of rabit-anti I-type, II-type and III-type human parainfluenza virus HN protein polyclonal antibodies labeled with quantum dots respectively. The detection layer is composed of a solid phase nitrocellulose membrane with three detection lines and a quality control line. The three detection lines are coated with rabit-anti I-type, II-type and III-type human parainfluenza virus polyclonal antibodies respectively. The quality control line is coated with anti-rabit IgG. The detection layer is pasted on the base plate. The combination pad and the water absorption pad are arranged above two ends of the detection layer respectively, overlap with part of the detection layer and are pasted with the detection layer and the base plate respectively. The sample pad is arranged on the combination pad, overlaps with part of the combination pad and is pasted with the combination pad and the base plate. The provided detection card has advantages of simple operation, rapid detection, quantification, high sensitivity and the like.
Owner:湖北诺美华抗体药物技术有限公司
Aptamer realizing specific binding with newcastle disease virus as well as screening method and applications of aptamer
ActiveCN107058329AImprove featuresHigh affinityDNA preparationMaterial analysisHN ProteinScreening method
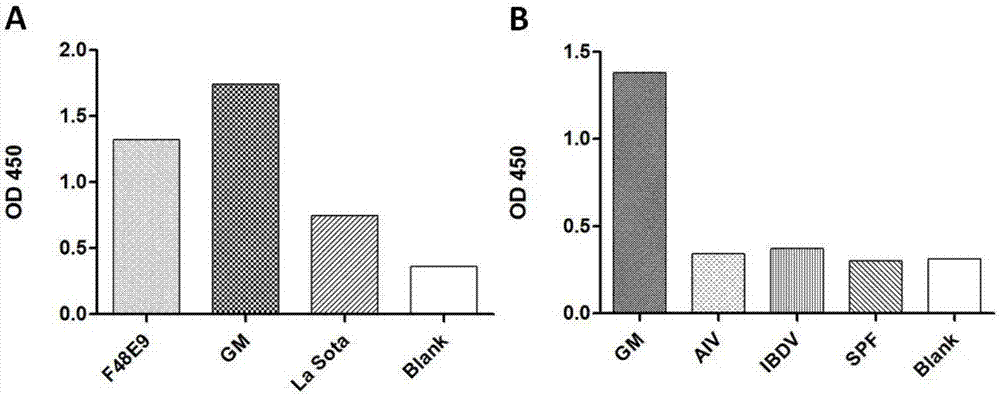
The invention discloses an aptamer realizing specific binding with the newcastle disease virus as well as a screening method and applications of the aptamer. The aptamer is specific aptamer B53 obtained through screening by adopting the SELEX technology. Specifically, the random single stranded DNA library and primers are constructed and synthesized in vitro, the newcastle disease HN protein and the newcastle disease virus GM strain realizing prokaryotic expression are adopted as target molecules, after screening, PCR amplification, positive screening and inverse screening, the screened single stranded DNA library realizing specific binding with the newcastle disease virus GM strain is subjected to clone sequencing, and thus the specific aptamer B53 is obtained. The aptamer B53 has the capacity of inhibiting virus replication, virus blood clotting activity and plaque formation, can specifically recognize the newcastle disease virus HN protein and the newcastle disease virus GM strain, has no reaction to avian influenza virus, infectious bursal disease virus and SPF chick embryo allantoic fluid, and can be used for detecting the newcastle disease virus.
Owner:SOUTH CHINA AGRI UNIV
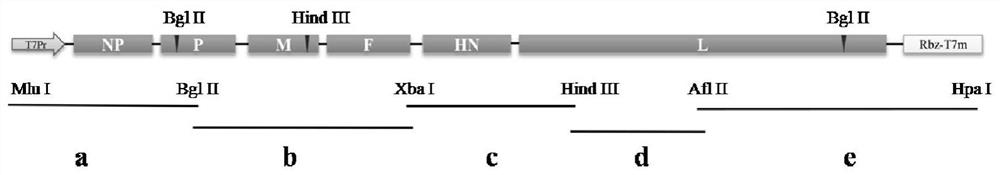
Pigeon-derived newcastle disease virus recombinant vaccine strain as well as construction method and application thereof
ActiveCN113736800AGood immune protectionHigh growth titerSsRNA viruses negative-senseViral antigen ingredientsHN ProteinF protein
The invention provides a pigeon-derived newcastle disease virus recombinant vaccine strain. A gene segment used in a process of constructing the pigeon-derived newcastle disease virus recombinant vaccine strain contains envelope glycoprotein F protein genes and HN protein genes of a pigeon-derived newcastle disease virus with attenuated toxicity. A preservation number of the specific pigeon-derived newcastle disease virus recombinant attenuated vaccine strain is CGMCC No. 21900. The pigeon-derived newcastle disease virus recombinant vaccine strain provided by the invention has the biological characteristics of high growth titer and low pathogenicity in a chick embryo, is stable in heredity, has a good immune protection effect on the pigeon-derived newcastle disease virus, can effectively inhibit and expel toxins and can be used for preventing and controlling the pigeon-derived newcastle disease virus which is popular at present, and the blank of lack of special vaccine products for prevention and control of the pigeon-derived newcastle disease in our country is filled.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
Hn epitope recognized by avian immune system and antigenic variant newcastle disease viruses carrying changes in the epitope
The present invention relates to an epitope of HN protein in Newcastle disease virus which can be recognized by an avian immune system and an antibody against the epitope, a method for detecting a Newcastle disease virus by using the antibody, and an antigenic variant of Newcastle disease virus carrying changes in the epitope. The epitope of HN protein and the antigenic variant of Newcastle disease virus can be used for developing efficient vaccines, and further, in diagnosing the Newcastle disease virus rapidly and exactly.
Owner:KBNP +1
Peste des petits ruminant virus HN protein epitope peptide as well as determination and preparation method and application thereof
PendingCN107033225AStrong green fluorescenceImprove responseSsRNA viruses negative-senseViral antigen ingredientsProtein targetHN Protein
The invention relates to peste des petits ruminant virus HN protein epitope peptide. The amino acid sequences of the epitope peptide are as follows: H123: <123>KFLNPDREYDFRDLR<137>, or / and H185: <185>GTGCLGRTVTRA<196>, or / and H487: <487>IRGPRGRCH<495>, or / and H569: <569>ECFPWYHKVWCYHDCLI<585>. B cell epitopes of a target protein are predicted by virtue of multiple immunoinformatic software, the different predicted epitopes are respectively artificially synthesized, the reactogenicity of the predicted epitopes is verified by virtue of an indirect ELISA method, an aminated ELISA Plate is coated with different polypeptides, and the reactogenicity with an antibody of HN protein is detected, and therefore, the B cell epitopes of the PPRV HN protein are authenticated.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Novel genetic engineering subunit vaccine for avian Newcastle disease viruses
ActiveCN111154778AGood immune effectProcess safetySsRNA viruses negative-senseViral antigen ingredientsAdjuvantF protein
Owner:苏州沃美生物有限公司
Newcastle disease diad vaccine and preparation method thereof
InactiveCN103495182AHigh purityImprove efficiencyPeptide/protein ingredientsViral antigen ingredientsDiseaseAdjuvant
The invention belongs to the technical field of animal vaccine preparation, and particularly relates to a diad vaccine regarding HN protein and chicken alpha interferon as effective components, and a preparation method thereof. The diad vaccine is prepared by transfection of BHK-21 cells and fully expressing obtained target protein by using HN genes and chicken alpha interferon genes through the utilization of a genetic engineering technology, and mixing the target protein with an adjuvant. When the diad vaccine prepared through the utilization of the expression proteins of the HN genes and the chicken alpha interferon genes is injected in a chicken body, the HN proteins and the alpha interferon proteins play respective biological roles in the chicken body, so that the effects of positively preventing and treating other virus infections are achieved while the virus infection of the newcastle disease is prevented. The vaccine is totally safe and reliable, is capable of inducing an organism to generate specific antibodies continuously for a long time and improving gallinaceous immunity, and well overcomes the disadvantage that the traditional vaccine only can prevent one disease.
Owner:河南亚卫动物药业有限公司
Chicken IL-18 (Interleukin-18) and Newcastle disease virus HN (Hemagglutinin-neuraminidase) gene recombinant fusion protein and application
InactiveCN102676471AEnhance immune responseStimulate differentiationFungiHydrolasesSide effectWhite blood cell
The invention relates to a preparation method and application of a recombinant chicken interleukin-18 (IL-18) and Newcastle disease virus hemagglutinin-neuraminidase (HN) fusion protein (chIL-18-HN fusion protein). The fusion protein is formed by fusing chicken IL-18 (chiIL-18) protein and Newcastle disease virus HN protein through a flexible Linker peptide; a DNA (Deoxyribonucleic Acid) sequence for coding the fusion protein is inserted into an expression vector pPICZalphaA and yeast X-33 is electrically converted to obtain genetic engineering bacteria for efficiently expressing the recombinant chIL-18-HN fusion protein; the recombinant chIL-18-HN fusion protein is prepared through liquid culture and purification; and the recombinant fusion protein can be used as a Newcastle disease novel genetic engineering vaccine and can also be used as a novel immunologic adjuvant for the Newcastle disease conventional vaccine. Animal immunologic tests prove that the chIL-18-HN fusion protein provided by the invention has the advantages of high safety, no toxic or side effect, capability of effectively enhancing cellular immunity and humoral immunity level of organisms and broad application prospect.
Owner:HENAN UNIV OF SCI & TECH
Newcastle disease virus gene VI type vaccine strain and application thereof
PendingCN114164184AReduced risk of reversion to virulenceImproving immunogenicitySsRNA viruses negative-senseViral antigen ingredientsArginineHN Protein
The invention provides a Newcastle disease virus gene type VI vaccine strain which is constructed by carrying out attenuated mutation on an F gene of a pigeon-derived gene type VI Newcastle disease virus, replacing the F gene of a Newcastle disease virus strain with the preservation number of CCTCC NO: V201968 and then carrying out genetic rescue. The amino acids at the 340 site, the 342 site, the 347 site and the 353 site of the HN protein of the Newcastle disease virus strain with the preservation number of CCTCC NO: V201968 are mutated into histidine, asparagine, lysine and arginine respectively. The invention also provides an application of the Newcastle disease virus gene VI type attenuated strain NDV-VIb in preparation of vaccines. The skeleton virus of the constructed gene VI type Newcastle disease virus attenuated strain comes from the modified gene VII type Newcastle disease virus attenuated strain, is safe and reliable, and reduces the risk of virulence reversion; the constructed gene VI type recombinant Newcastle disease virus attenuated strain is mainly used for preventing and controlling the Newcastle disease virus of pigeons, and the F gene is derived from host pigeons and has better immunogenicity than the gene VI type Newcastle disease virus strain derived from chicken.
Owner:YEBIO BIOENG OF QINGDAO
Vaccine composition, kit, preparation methods of vaccine composition and kit, and applications of vaccine composition and kit
InactiveCN107029230ANo side effectsImprove immunityAntibacterial agentsBacterial antigen ingredientsBordetella bronchiseptica antigenParainfluenza virus antigen
The invention provides a vaccine composition. The vaccine composition comprises a dog parainfluenza virus antigen with the immunity effective dose, a dog bordetella bronchiseptica antigen with the immunity effective dose, and an adjuvant, wherein the dog parainfluenza virus antigen is selected from an inactivated dog parainfluenza virus antigen or at least one of F protein, HN protein, M protein, NP protein, P protein and L protein, and the bordetella bronchiseptica antigen is selected from inactivated dog bordetella bronchiseptica antigen or p68 protein. Compared with the vaccines in the prior art, the vaccine composition provided by the invention has the better immune effect, and has no side effects, and thus the risk that a veterinarian worker or a contactor is infected is reduced. The invention further relates to a kit. The kit contains the inactivated dog parainfluenza virus antigen or the subunit antigen, the attenuated live dog bordetella bronchiseptica antigen or the attenuated live dog parainfluenza virus antigen, and the inactivated dog bordetella bronchiseptica antigen or p68 protein.
Owner:PU LIKE BIO ENG
Human parainfluenza virus type 3 (HPIV-3) wild strain and application thereof
ActiveCN110628724AAvoid infectionHigh titerSsRNA viruses negative-senseViral antigen ingredientsHN ProteinF protein
The invention relates to a human parainfluenza virus type 3 (HPIV-3) wild strain and an application thereof. The preservation number of the wild strain is CCTCC NO:V201911, the wild strain has HN protein and F protein of an HPIV-3 virus, and the wild strain can be applied to an HPIV-3 virus infected mouse model and an HPIV-3 neutralizing antibody detection experiment, and can be applied to preparation of vaccines for preventing parainfluenza type 3. The human parainfluenza virus type 3 wild strain disclosed by the invention is a cloned purified high-titer HPIV3LZ1728C19 virus strain free fromspecific exogenous factor plaque formation, and a human parainfluenza virus type 3 HN protein subunit vaccine prepared from the virus can effectively prevent parainfluenza virus type 3 infection.
Owner:LANZHOU INST OF BIOLOGICAL PROD
Novel genetically engineered subunit vaccine for newcastle disease virus
InactiveCN109735552AReduce purification timeSimplify production stepsSsRNA viruses negative-senseViral antigen ingredientsNucleotideHN Protein
Owner:苏州世诺生物技术有限公司
Mumps virus HN antigen and application of antigen in detection of mumps-resisting virus antibody
The invention discloses a mumps virus HN antigen capable of detecting a mumps-resisting virus antibody, especially a mumps-resisting virus neutralized antibody and further discloses application of the antigen in preparation of detection agent for detecting the mumps-resisting virus neutralized antibody and a corresponding detection kit.
Owner:北京市华信行生物科技有限公司
Polypeptide and ELISA detection reagent kit for detecting Newcastle disease virus antibody
ActiveCN110240635AImprove standardizationIncreased sensitivitySsRNA viruses negative-senseVirus peptidesDiseaseHN Protein
The invention discloses polypeptide and ELISA detection reagent kit for detecting a Newcastle disease virus antibody. The reagent kit is used for coating an elisa plate through screened Newcastle disease HN protein polypeptide. Through detection of combining capacity of a serum antibody and polypeptide in samples, and through assistance with the antibody of goat anti-chicken Ig gamma labelled with horseradish peroxidase, the level of Newcastle disease virus antibody is detected. The reagent kit is used for detecting avian influenza virus H5, H7, H9 subtype antibodies, chicken infectious Fabricius' bursal disease virus antibodies, and chicken infectious bronchitis virus antibodies to be negative. The reagent kit can detect that the hemagglutination inhibition (HI) titer is greater than or equal to 4log<2> serum, and can meet clinical serum sensitivity detection requirements. When the reagent kit and a hemagglutination inhibition method are both used for detecting clinical serum, the coincidence rate is as high as 98.7%. The reagent kit is simple to operate, free from special material requirements, good in result judgment objectivity, free from influence by subjective judgment by operators, and suitable for being used as a tool for detecting and monitoring the level of the antibodies after immunization with a Newcastle disease vaccine.
Owner:广纳达康(广州)生物科技有限公司
Recombinant baculovirus for expressing heat-resistant HN protein of Newcastle disease virus as well as preparation method and application of recombinant baculovirus
ActiveCN112239752AImprove thermal stabilityHigh heat resistanceSsRNA viruses negative-senseViral antigen ingredientsHN ProteinTGE VACCINE
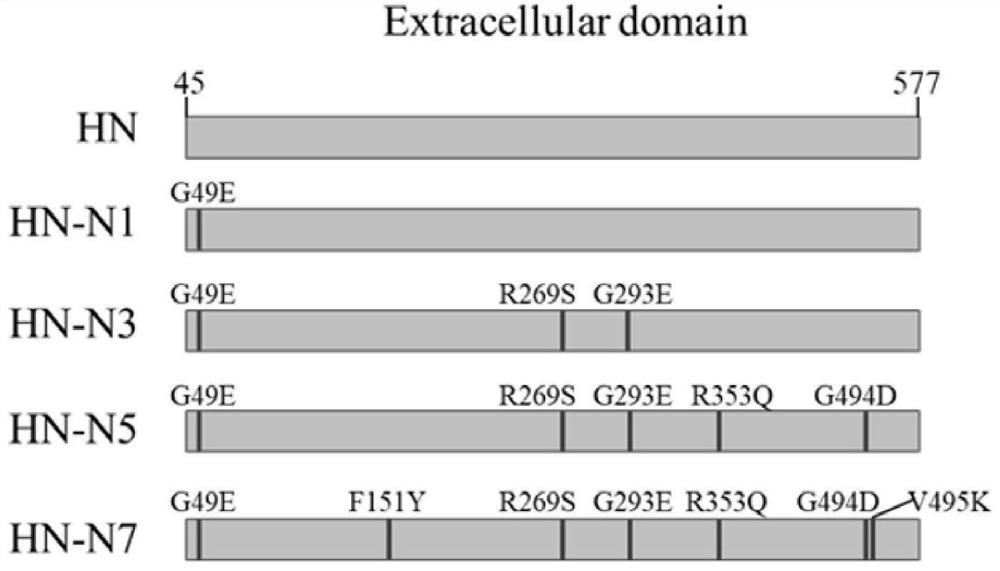
The invention discloses a recombinant baculovirus for expressing heat-resistant HN protein of the Newcastle disease virus as well as a preparation method and application of the recombinant baculovirus. The virus strain is preserved in China Center for Type Culture Collection, the preservation address is Wuhan University, Wuhan, China, and the preservation number is CCTCC NO: V202044. The preparation method of the ecombinant baculovirus comprises the following steps of carrying out point mutation on an HN gene of a Newcastle disease virus non-heat-resistant LaSota strain, including G49E, R269S,G293E, R353Q and G494D, inserting the mutated HN gene into a baculovirus genome, and carrying out virus rescue to obtain the recombinant baculovirus rAC-HN-N5 strain. The virus strain can efficientlyexpress mutant HN protein. Compared with wild-type HN protein of the LaSota strain, the mutant HN protein has the advantage that the thermal stability is obviously enhanced. The mutant HN protein expressed and purified by utilizing the recombinant baculovirus rAC-HN-N5 strain can be used for preparing a heat-resistant subunit vaccine for the Newcastle disease, so that the dependence of the vaccine on a cold chain is reduced, and a new thought is provided for the development of the subunit vaccine with high heat stability.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Vaccine composition, preparation method and application thereof
ActiveCN104513317AEase of mass productionEasy to storeViral antigen ingredientsAntiviralsProtective antigenHN Protein
The invention provides a Newcastle disease virus fusion protein, which includes two protective antigen fragments, an F1 protein and an F2 protein, in an F protein, and includes an HN protein, wherein an amino acid sequence of the F1 protein is represented as the SEQ ID NO.2, the amino acid sequence of the F2 protein is represented as the SEQ ID NO.4, and the amino acid sequence of the HN protein is represented as the SEQ ID NO.6. The invention also discloses a vaccine composition containing the Newcastle disease virus fusion protein in an immunizing dose and an application of the vaccine composition in prevention and / or treatment of Newcastle disease virus in a gene VII type. The Newcastle disease vaccine composition can generate cell immunity and humoral immunity at the same time and also can generate protective immunity to the Newcastle disease virus in the gene VII type. The vaccine composition is broad-spectrum.
Owner:PU LIKE BIO ENG
A mutant strain of heat-resistant Newcastle disease virus and its preparation method and application
ActiveCN112126629BHigh heat resistanceImprove thermal stabilitySsRNA viruses negative-senseViral antigen ingredientsArginineHN Protein
The invention discloses a heat-resistant Newcastle disease virus mutant strain and a preparation method and application thereof. The mutant strain is deposited in the China Center for Type Culture Collection, and the deposit address is Wuhan University, Wuhan, China, and the deposit number is CCTCC NO: V202041. The preparation method uses the Newcastle disease virus TS09-C strain as the parent strain, and introduces 4 amino acid mutations into its HN protein: the 3rd arginine is mutated to serine, and the 197th arginine is mutated to isoleucine. acid, histidine at position 203 was mutated to asparagine and lysine at position 495 was mutated to valine. The heat resistance test results show that the heat resistance of the virus mutant strain is significantly higher than that of the parent strain, and is much higher than that of the commonly used vaccine strains, which proves that the present invention further improves the thermal stability of the existing heat-resistant strains.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Newcastle disease virus HN and chick anemia virus VP3 gene joined antineoplastic biologic preparation
InactiveCN1156576CAvoid side effectsSafe and reliableAntineoplastic agentsVector-based foreign material introductionHN ProteinWilms' tumor
The present invention discloses 3 antineoplastic nucleovaccines able to express newcastle disease virus HN gene and chick anemia virus VP3 gene: pIRHNVP3, pVHN and pVVP3. After transfection of eukaryotic cell, the pIRHNVP3 can express HN protein and VP3 protein at the same time. After mixing and transfection of pVHN and pVVP3, HN protein and VP3 protein can be obtained. Said nucleovaccines and specific proteins can be used to prevent and treat tumor of people and animals.
Owner:MILITARY VETERINARY INST MILITARY SUPPIES PLA
Newcastle disease virus vaccine
ActiveCN113292639BLow costGood immune effectSsRNA viruses negative-senseAntibody mimetics/scaffoldsHN ProteinF protein
The invention relates to the field of biotechnology, in particular, it provides a Newcastle disease virus vaccine. Prepare the protein of Newcastle disease virus vaccine, including the F protein of Newcastle disease virus and the HN protein of Newcastle disease virus, wherein, the nucleotide sequence of encoding F protein is as SEQ ID NO.1 (gene type VII) or SEQ ID NO.3 ( Gene Type II), the nucleotide sequence encoding HN protein is shown in SEQ ID NO.2 (Gene Type II) or SEQ ID NO.4 (Gene Type VII). The protein is suitable for the eukaryotic cell expression system, simplifies the process for preparing the active ingredient of the Newcastle disease virus vaccine, and reduces the cost at the same time. Moreover, the protein has broad-spectrum antigenicity and can achieve a good cross-protection effect.
Owner:天康制药股份有限公司
Newcastle disease virus vaccine strain for gene VII type genetic rescue
PendingCN114164182AImprove immunityImprove protectionSsRNA viruses negative-senseViral antigen ingredientsHN ProteinArginine
The invention provides a gene VII type Newcastle disease virus vaccine strain, which is a gene VII type Newcastle disease virus attenuated strain NDV-VIIb strain, and is prepared by respectively mutating 340-site amino acid, 342-site amino acid, 347-site amino acid and 353-site amino acid of HN protein of a Newcastle disease virus strain with the preservation number of CCTCC NO: V201968 into histidine, asparagine, lysine and arginine; and then constructing through genetic rescue. The gene VII type Newcastle disease NDV-VIIb strain provided by the invention is used for preparing vaccines. The immune effect of the Newcastle disease virus gene VII type attenuated strain provided by the invention is obviously superior to that of a parent strain, and a good protection effect can be provided for the current popular gene VII type Newcastle disease virus.
Owner:YEBIO BIOENG OF QINGDAO
Vectors and cells for preparing immunoprotective compositions derived from transgenic plants
The inventions is drawn towards vectors and methods useful for preparing genetically transformed plant cells that express immunogens from pathogenic organisms which are used to produce immunoprotective particles useful in vaccine preparations. The invention includes plant optimized genes that encode the HN protein of Newcastle Disease Virus. The invention also relates to methods of producing an antigen in a transgenic plant.
Owner:BOYCE THOMPSON INST FOR PLANT RES +1
hn-vp233-221aa fusion protein and its preparation method and application
ActiveCN107245105BImprove protectionEnhance cellular immune responseSsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininDisease
The invention discloses HN-VP233-221aa fusion protein as well as a preparation method and application thereof. The HN-VP233-221aa fusion protein comprises an amino acid sequence of infectious bursal disease virus VP233-221aa, an amino acid sequence of Newcastle disease virus hemagglutinin-neuraminidase (HN protein) and a flexible Linker peptide amino acid sequence located between the amino acid sequence of the VP233-221aa and the amino acid sequence of the HN protein of Newcastle disease virus; the HN-VP233-221aa fusion protein is formed by connecting a main protective gene segment of VP2 and an HN gene of the Newcastle disease virus in series through a flexible Linker peptide (-G-S-). The recombinant HN-VP233-221aa fusion protein disclosed by the invention can be used for effectively inducing an organism to generate body fluid and cell immune response and can be used for inducing immunized chickens to generate high-level HN specific antibodies and VP2 antibodies and accelerating a proliferation and activation capability of chicken T lymphocytes; the HN-VP233-221aa fusion protein has a very good protection effect on attacks of infectious bursal disease virulent strains and Newcastle disease virulent strains so that the effect of preventing two diseases through one injection is realized.
Owner:HENAN UNIV OF SCI & TECH
Mumps virus hn antigen and its use in detecting anti-mumps virus antibodies
The invention discloses a mumps virus HN antigen capable of detecting a mumps-resisting virus antibody, especially a mumps-resisting virus neutralized antibody and further discloses application of the antigen in preparation of detection agent for detecting the mumps-resisting virus neutralized antibody and a corresponding detection kit.
Owner:北京市华信行生物科技有限公司
HN epitope recognized by avian immune system and antigenic variant newcastle disease viruses carrying changes in the epitope
The present invention relates to an epitope of HN protein in Newcastle disease virus which can be recognized by an avian immune system and an antibody against the epitope, a method for detecting a Newcastle disease virus by using the antibody, and an antigenic variant of Newcastle disease virus carrying changes in the epitope. The epitope of HN protein and the antigenic variant of Newcastle disease virus can be used for developing efficient vaccines, and further, in diagnosing the Newcastle disease virus rapidly and exactly.
Owner:KBNP +1
HN protein mutant gene VII type Newcastle disease virus recombinant vaccine strain
ActiveCN113897376AGenetic stabilityHigh growth titerSsRNA viruses negative-senseViral antigen ingredientsHN ProteinF protein
The invention provides an HN protein mutant gene VII type Newcastle disease virus recombinant vaccine strain. A gene segment used in the process of constructing the gene VII type Newcastle disease virus recombinant vaccine strain contains a envelope glycoprotein F protein gene and an HN protein gene of a gene VII.2 subtype Newcastle disease virus with weakened toxicity. The gene VII type Newcastle disease virus recombinant vaccine strain provided by the invention has the biological characteristics of high growth titer and low pathogenicity in a chicken embryo, is stable in heredity, has a good immune protection effect on the Newcastle disease virus, can effectively inhibit toxin expelling, can be used for preventing and controlling the currently popular gene VII type Newcastle disease virus, and has a wide application prospect.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com