Polypeptide and ELISA detection reagent kit for detecting Newcastle disease virus antibody
A Newcastle disease virus and detection kit technology, applied in the field of agricultural biology, can solve the problems of poor specificity and sensitivity that cannot meet clinical requirements, and achieve the effects of easy standardization, good sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1 detects the polypeptide analysis of chicken Newcastle disease virus antibody
[0050] According to the amino acid sequence of the chicken Newcastle disease virus HN protein, the relative conserved sites were analyzed by blast software, and the B of the protein was comprehensively analyzed in combination with the hydrophilicity analysis (hydrophilicity), flexibility (flexibility), and non-shielding (accessibility) of the protein sequence. For cell epitopes (http: / / crdd.osdd.net / raghava / bcepred / ), four sequences were selected as candidate polypeptide sequences, namely: NDV-H1, NDV-H2, NDV-H3 and NDV-H4.
[0051] According to the amino acid sequence of the F protein of chicken Newcastle disease virus, its relative conserved sites were analyzed by blast software, and the B of the protein was comprehensively analyzed in combination with the hydrophilicity analysis (hydrophilicity), flexibility (flexibility), and non-shielding (accessibility) of the protein sequen...
Embodiment 2
[0059] Embodiment 2 detects the screening of the polypeptide of chicken Newcastle disease virus antibody
[0060] The above-mentioned artificially synthesized polypeptide was coupled with BSA carrier protein by glutaraldehyde method to improve the coating efficiency of the polypeptide. The specific method is: 2 mg of carrier BSA is dissolved in 2 mL of PBS buffer (0.01 mol / L, pH 7.4), dialyzed in PBS buffer at room temperature overnight; 1 mg / mL of polypeptide is added to 2 mL of carrier BSA solution, and the After stirring, 1 mL of 0.2% glutaraldehyde solution was added dropwise to the above mixture, and stirred while adding. Stir at room temperature for 4 hours, add 400 μL of 1 mol / L glycerol to the above mixture, and continue stirring for 1 hour to terminate the reaction; put the above mixture into a dialysis bag, place it in PBS buffer and dialyze overnight at 4°C; collect the peptide carrier pair Combined, store at -80°C.
[0061] The coupled polypeptides obtained above...
Embodiment 3
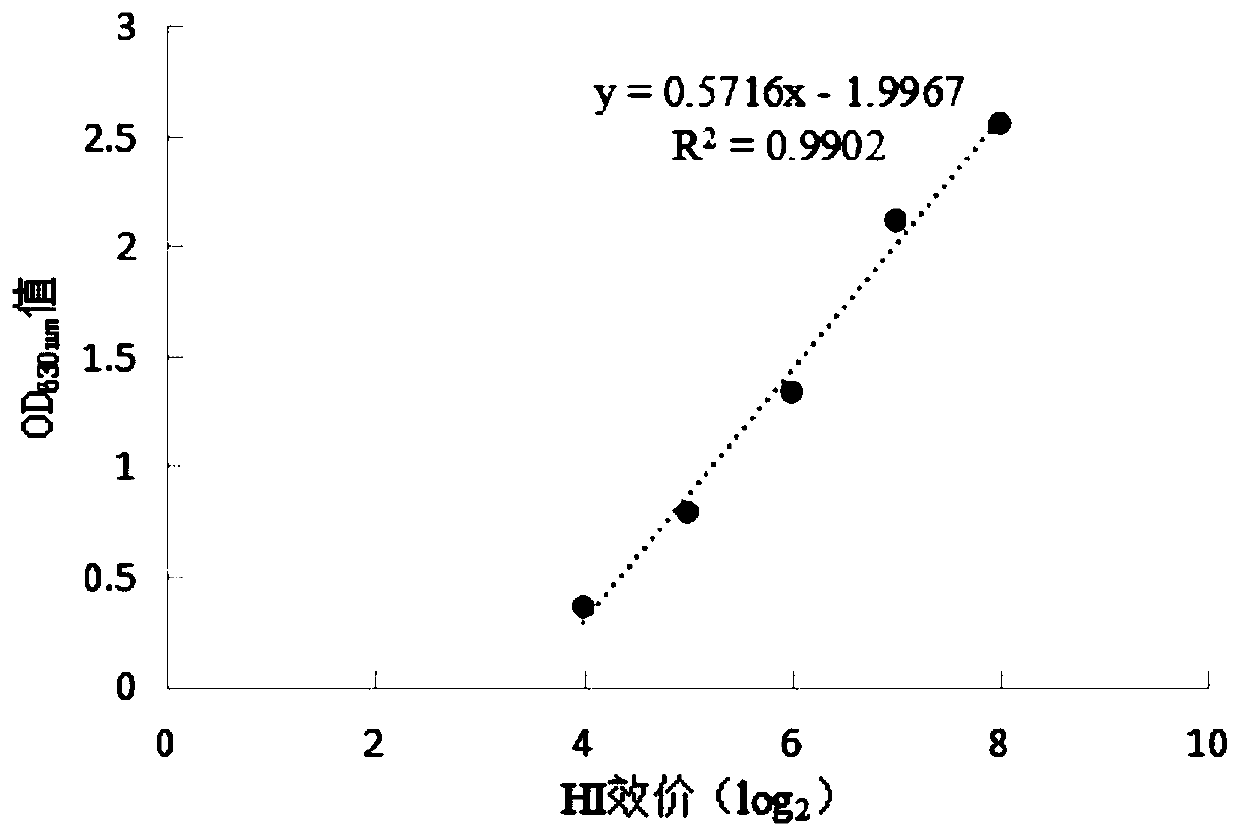
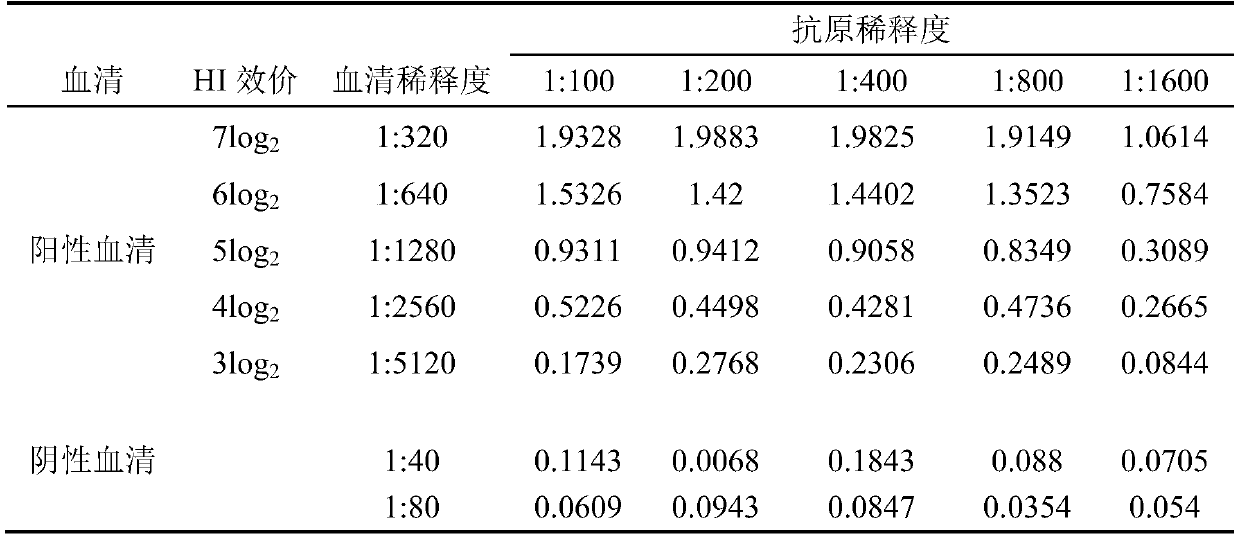
[0065] Example 3 Determination of the Optimum Coating Concentration of Antigen and Optimum Serum Dilution of Newcastle Disease Virus ELISA Antibody Detection Kit
[0066] Determine the optimal coating concentration of the antigen and the optimal dilution of the serum according to the matrix titration method, and use the coating solution to mix the NDV-H4 polypeptide and the BSA conjugate at 1:100, 1:200, 1:400, 1: After dilution of 800 and 1:1600, add them to the microtiter plate respectively, add 1 column for each dilution, 100 μL per well, and place it at 2-8°C for 15 hours. Discard the coating solution, add 200 μL of blocking solution (5% skim milk) to each well, incubate at 37° C. for 1 hour, discard the blocking solution in the well. Use the sample diluent to dilute the positive serum of Newcastle disease virus 1:320, 1:640, 1:1280, 1:2560, 1:5120, dilute the negative serum 1:40, 1:80, and dilute the positive serum and negative serum Each dilution of each dilution was ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com