American-type PRRSV N protein antigenic epitope and applications thereof
A protein antigen, American-type technology, applied in the application, virus antigen components, anti-virus immunoglobulin and other directions, can solve the problems of insufficient research on the PRRSVN protein cell epitope, and achieve the effect of good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Selection and preparation of immunogen
[0044] 1. Selection of Immunogen
[0045] The inventor finds through long-term, a large amount of research, practice, experiment:
[0046] First, among the proteins encoded by the PRRSV genome, the nucleocapsid protein encoded by ORF7, namely the N protein, is highly conserved;
[0047] Secondly, the immune reactivity induced by N protein in the body is much higher than that of other proteins, so it is easy to obtain high-affinity monoclonal antibodies;
[0048] Thirdly, the content of N protein is the highest after PRRSV infection, accounting for 40% to 60% of the total structural protein, which is the dominant target for PRRSV antigen detection.
[0049] Based on the above three points, N protein becomes the preferred target protein.
[0050] 2. Preparation of Immunogen
[0051] The accession number of the nucleotide sequence of N protein on Genebank is: EF112445.
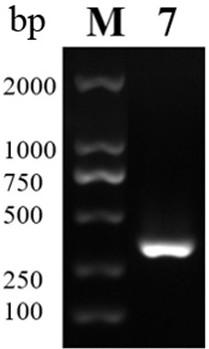
[0052] (1) Construct the pET-28a-N recombinant...
Embodiment 2
[0059] Example 2: Preparation and Identification of Monoclonal Antibody
[0060] 1. Animal Immunization
[0061] (1) Add the immunogen N protein to Freund's complete adjuvant for the first immunization, and emulsify it to make the immune antigen;
[0062] (2) Three 4- to 8-week-old female BALB / c mice were immunized by multi-point subcutaneous injection on the back, with an immune dose of 50 μg / mouse;
[0063] (3) BALB / c mice were boosted with the same method and dose after emulsification with Freund's incomplete adjuvant and immune antigen every 3 weeks;
[0064] (4) After the fourth immunization, 3 to 4 days before cell fusion, BALB / c mice were super-immunized with immunogen without adjuvant by tail vein injection, and the immunization dose was 100 μg / mouse;
[0065] (5) Determination of titer and sensitivity of multiple antiserum:
[0066] One week after the last booster immunization, tail-cut blood was collected from 3 mice respectively, and then the potency and sensitiv...
Embodiment 3
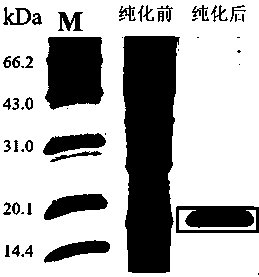
[0087] Example 3: Purification of Antibodies
[0088] Caprylic acid-ammonium sulfate method for antibody purification, the operation method is as follows:
[0089] (1) Take out the frozen monoclonal antibody ascites or antiserum, and centrifuge at 6000 r / min for 30 minutes to obtain the supernatant, which is diluted 5 times with sodium acetate buffer (0.06mol / L pH4.8).
[0090] (2) Adjust the pH to 4.5 with NaOH (5 mol / L), stir slowly at room temperature for 0.5 h, and add n-octanoic acid to a final concentration of 25 μL / mL.
[0091] (3) Centrifuge at 6000 r / min for 30 minutes at 4°C, and keep the supernatant.
[0092] (4) Filter the obtained supernatant with medium-speed filter paper, add 10×PBS buffer solution (pH7.4), the volume is 1 / 10, mix well and adjust the pH to 7.4.
[0093] (5) Add solid ammonium sulfate to the mixture at 4°C, the addition ratio is 0.2778 g / mL, and can be added several times, and the addition is completed within 30 minutes.
[0094] (6) Centrifug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com