Patents
Literature
53 results about "Sheep serum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Purity/Specifity. Normal Sheep Serum (NSS) was prepared from normal serum by a multi-step process which includes delipidation and selective precipitation. Normal Sheep Serum (NSS) was assayed by immunoelectrophoresis resulted in a multiple precipitin arcs against anti-Sheep Serum.
Competitive ELISA kit for peste-des-petits-ruminants antibody detection and preparation method thereof
ActiveCN102967710ANo cross reactionStrong specificityImmunoglobulins against virusesBiological testingSerum igeElisa kit
Belonging to the field of biotechnologies, the invention discloses a competitive ELISA kit for detection of a peste-des-petits-ruminants virus antibody. The kit comprises a detection system composed of a coating antigen reaction solution and a monoclonal antibody reaction solution. The kit adopts prokaryotically expressed peste-des-petits-ruminants Nigeria 75 / 1 strain N protein as the coating antigen and employs a monoclonal antibody against N protein as the competitive antibody. The antibody against a peste-des-petits-ruminants virus in sheep serum is detected according to a competitive ELISA principle. The kit provided in the invention can rapidly and specifically detect the peste-des-petits-ruminants virus antibody in serum, and simultaneously has the advantages of large-scale production of monoclonal antibodies, good reaction specificity, high sensitivity, simple operation, low cost, stable, reliable and easily observable reaction results, thus being very suitable for import and export quarantine of sheep, food hygiene and screening of large batches of samples in livestock breeding farms, and being easy for large-scale popularization and application.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
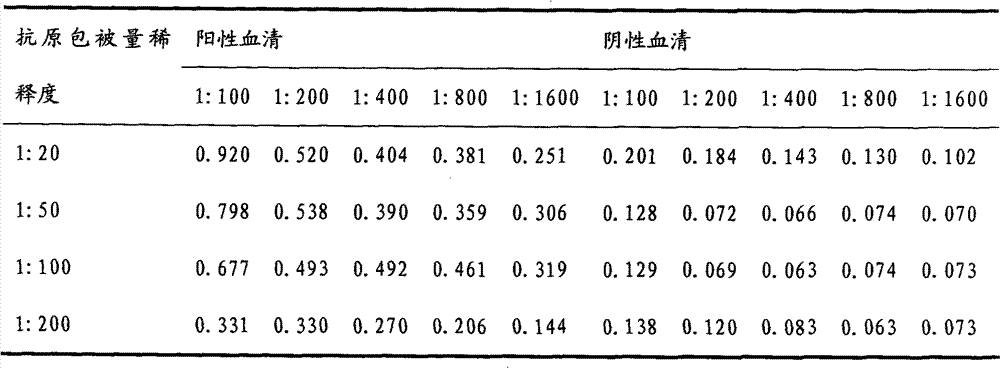
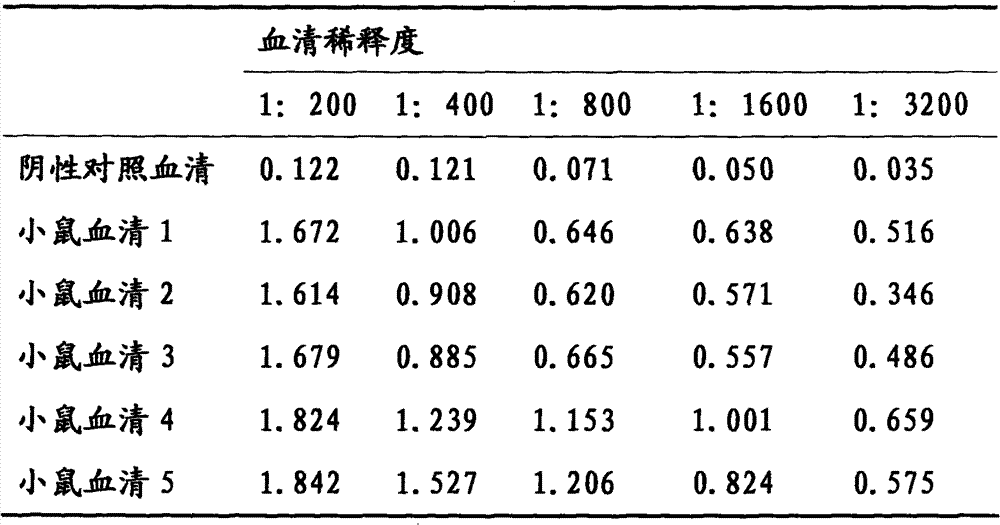
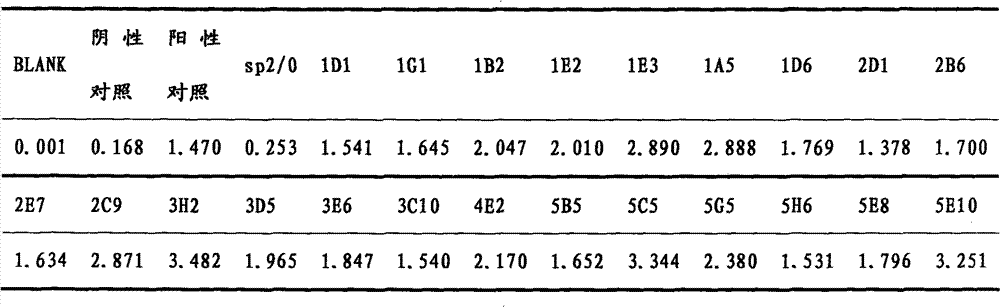
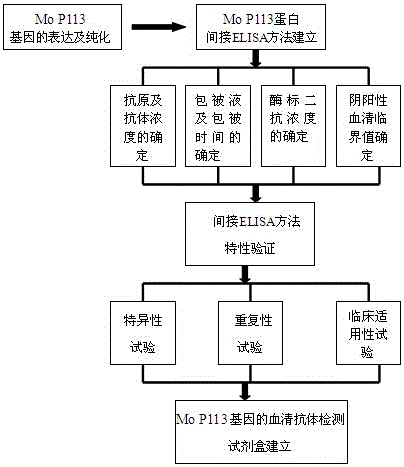
Indirect ELISA kit based on Mo P113 protein and use method
InactiveCN105717299AChange the situation of lack of commercial ELISA kitsIncreased sensitivityMaterial analysis by observing effect on chemical indicatorBiological testingEnzymeSheep serum
The invention discloses an indirect ELISA kit based on Mo P113 protein and a use method. The indirect ELISA kit based on Mo P113 protein is prepared from an elisa plate coated with Mo P113 protein, Mo standard positive serum, Mo standard negative serum, bethyl, a 50*PBST buffer solution, a substrate solution A, a substrate solution B and a stop solution. The indirect ELISA kit can detect sheep serum Mo infection antibodies and vaccine immunity antibodies, a key technology is provided for earlier monitoring of goat and sheep mycoplasmal pneumonia caused by Mo, vaccine immunity effect evaluation and correlational research work, and great significance is achieved in early finding and early prevention and control of epidemic diseases caused by the pathogeny.
Owner:GUIZHOU UNIV
Screening culture method for sheep oocytes in vitro
The invention provides a screening culture method for sheep oocytes in vitro, which comprises the following steps: step 1: ovaries which were killed less than half an hour ago are put into saline water at the temperature of 30DEG C to 40DEG C and containing penicillin and streptomycin, washed for 3 to 4 times within 3 hours, and oocytes are picked out; 25 to 30mu mol / L brilliant cresyl blue is put into 35DEG C to 39DEG C water bath to be dyed for 85 to 92min, the cytoplasm is blue and is washed for 3 to 4 times by in vitro maturation fluid, is put into 55 to 78mul / drip in vitro maturation culture fluid by 25 to 30m / drip, and is cultured in 5 percent CO2 by 95 percent; step 2: cumulus cells are removed from oocytes which are maturated in vitro for 25 to 28 hours; IVF washing liquid is used to wash for 2 to 4 times, and is dripped into 50 to 70mu l fertilization fluid by 25 to 30m / drop; semen is unfrozen, the fertilization fluid is moved in, supernatant fluid is centrifugurated for 4 to 5min and removed, the precipitated sperms are added into fertilization fluid drips by the density of 2 to 4*106 / ml and incubated; eggs which are fertilized for 12 to 18 hours are treated in the above step and then moved into a four-hole culture plate to be cultured; and step 3: reagent egg absorption liquid, brilliant cresyl blue maturation liquid, dyeing liquor SOF, the fertilization fluid, the culture fluid and sheep serum are prepared.
Owner:INNER MONGOLIA SAINUO GRASSLAND SHEEP IND
ELISA kit for rapidly detecting sheep mycoplasma pneumoniae antibody
The invention discloses an ELISA kit for rapidly detecting sheep mycoplasma pneumoniae antibody. The ELISA kit comprises a coated enzyme-labeled plate, negative standard serum, positive standard serum, an enzyme-labeled second antibody, a washing liquid, a diluents, a substrate liquid and an ending liquid. Recombination sheep mycoplasma pneumoniae in-vitro membrane protein MOL with relatively highimmunogenicity is acquired by building an expression carrier and is used as a coated antigen, and sheep mycoplasma pneumoniae antibody ELISA detection method and kit which are high in specificity andhigh sensitivity are built, the kit is used for sheep serum clinical sample detection, immunoprophylaxis and immunovalue study of sheep mycoplasma pneumoniae are favorably deepen, and a necessary technical means is provided for rapid detection of the sheep mycoplasma pneumoniae antibody.
Owner:GUANGXI VETERINARY RES INST
Indirect ELISA detection kit for B-type clostridium novyi
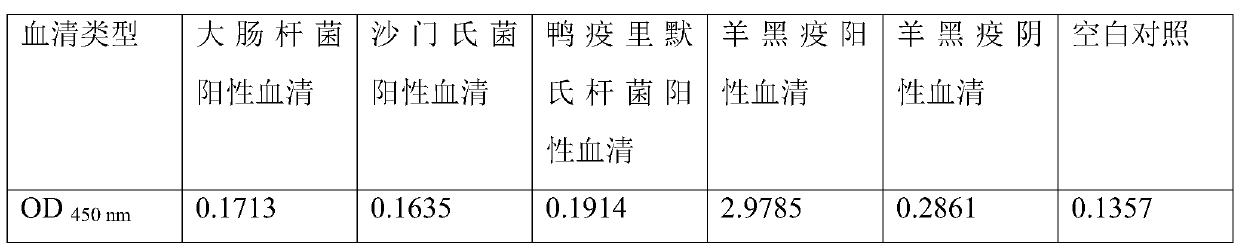
The invention discloses an indirect ELISA detection kit for B-type clostridium novyi. The indirect ELISA detection kit takes B-type clostridium novyi bacterium suspension as an envelope antigen, wherein the B-type clostridium novyi bacterium suspension is prepared by the following methods of inoculating B-type clostridium novyi into a culture medium to carry out bacterium-enrichment culture; centrifuging the cultured B-type clostridium novyi culture; and collecting bacterial cells, washing and resuspending the bacterial cells by using a carbonate buffer solution to prepare the B-type clostridium novyi bacterium suspension. The kit disclosed by the invention takes complete clostridium novyi bacterial cells as the envelope antigen, so that whether sheep serum contains an antibody to clostridium novyi or not can be detected at high sensitivity and high specificity and whether sheep are infected with a black disease or not can be judged accordingly. The indirect ELISA detection kit has the advantages that the detection result is high in specificity and good in repeatability, and a diagnosis can be quickly made and emergency treatment measures can be taken after the sheep catch the disease.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Sheep echinococcosis ELISA antibody detection kit and application thereof
PendingCN110954690AIncreased sensitivityImprove featuresBiological material analysisSheep serumEpidemiologic survey
The invention discloses a sheep echinococcosis ELISA antibody detection kit and a preparation method and application thereof. In the detection kit, an elisa plate is pre-coated with an echinococcus granulosus cocktail antigen; the echinococcus granulosus cocktail antigen is composed of an EPC1 recombinant antigen, an EgAgB1 recombinant antigen, an EgAgB2 recombinant antigen and an EgAgB4 recombinant antigen. The echinococcus granulosus cocktail antigen is composed of an EPC1 recombinant antigen, an EgAgB1 recombinant antigen, an EgAgB2 recombinant antigen and an EgAgB4 recombinant antigen. Thedetection kit provided by the invention can be used for rapidly detecting the echinococcosis antibody in the sheep serum, is simple to operate, short in consumed time, high in sensitivity and good inspecificity, and is convenient for simultaneously detecting a large number of samples. The sheep echinococcosis ELISA antibody detection kit provided by the invention has important application valuein echinococcosis antibody level detection and epidemiological investigation.
Owner:北京明日达科技发展有限责任公司
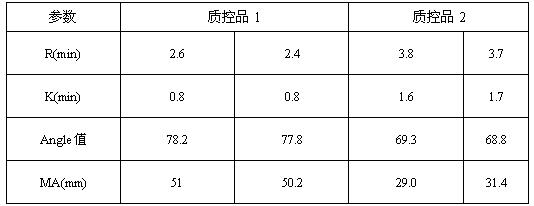
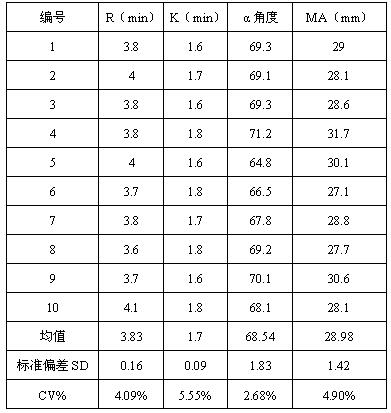
Thromboelastography instrument quality control product and preparation method thereof
ActiveCN107588998AReduce use costSimple preparation stepsPreparing sample for investigationBiological testingBiochemical engineeringQuality control
The invention discloses a quality control product which comprises a quality control product 1 and a quality control product 2, wherein the quality control product 1 comprises the following components:2-6 parts by weight of factor-rich goat plasma, 2-5 parts by weight of sheep serum and 1-4 parts by weight of fibrinogen mother liquor; the quality control product 2 comprises the following components: 1 to 4 parts by weight of factor-deficient sheep plasma, 5 to 10 parts by weight of sheep serum and 0. 3 to 2 parts by weight of the fibrinogen mother liquor, the fibrinogen mother liquor is prepared by dissolving fibrinogen in the sheep serum, wherein the mass percentage of the fibrinogen is 10%; clotting factor extraction is not needed in the preparation process of the thromboelastography instrument quality control product, preparation steps are simplified, operation is easy, cost is low, results are accurate, and stability is good; test results of the thromboelastography instrument quality control product basically meet that of a quality control product produced by already listed US Haemoscope company, the thromboelastography instrument quality control product can replace the qualitycontrol product produced by the already listed US Haemoscope company, and customer use cost is reduced.
Owner:上海原科实业发展有限公司
Method for detecting residual quantity of Pichia pastoris host protein in recombinant human lysozyme
ActiveCN109799335ALow cross-reactivityNo cross reactionMaterial analysisPichia pastorisTotal protein
The invention discloses a method for detecting residual quantity of Pichia pastoris host protein in a recombinant human lysozyme raw material. The method includes the steps of preparing recombinant human lysozyme; preparing Pichia pastoris host protein; detecting total protein content of the Pichia pastoris host protein; preparing antiserum of the Pichia pastoris host protein; eliminating a crossreaction between the recombinant human lysozyme and immune rabbit serum of the Pichia pastoris host protein and immune sheep serum of the Pichia pastoris host protein; preforming two-dimensional electrophoresis on the Pichia pastoris host protein; detecting antibody coverage rate of the Pichia pastoris host protein; and preforming enzyme-linked immunosorbent assay. By adopting the detection method, the Pichia pastoris host protein in the recombinant human lysozyme is continuously detected for 3 batches, and the content of the Pichia pastoris host protein is all less than 0.05%, and meets the standard of less than 0.1% specified in the Pharmacopoeia of the People's Republic of China. The detection method has the advantages of strong specificity, complete antibody coverage rate, no cross reaction and the like, and can be used for detecting the content of the Pichia pastoris host protein.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
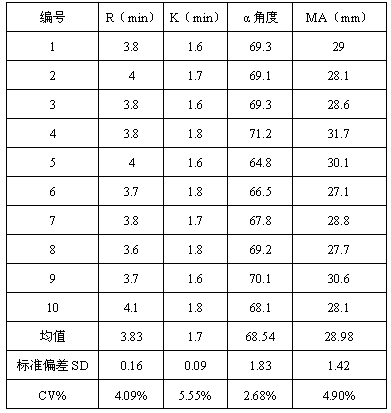
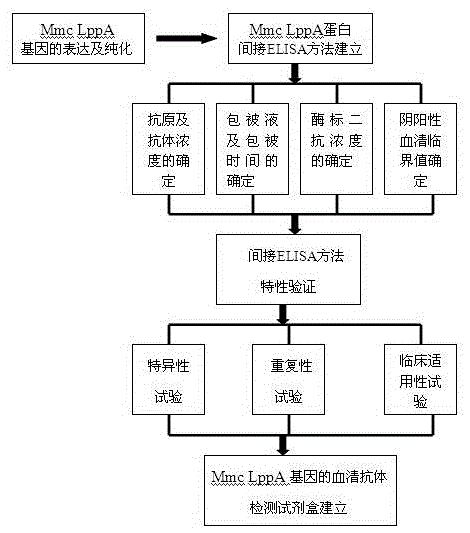
Mmc LppA protein-based indirect ELISA kit and use method
InactiveCN105606832AChange the situation of lack of commercial ELISA kitsIncreased sensitivityBiological material analysisBiological testingSheep serumBuffer solution
The invention discloses an Mmc LppA protein-based indirect ELISA kit and a use method. The Mmc LppA protein-based indirect ELISA kit comprises the following raw materials according to the formula: an elisa plate coated with Mmc LppA protein, Mmc standard positive and standard negative serum, bethyl, a 50*PBST buffer solution, a closed buffer solution, a substrate fluid A, a substrate fluid B and a stop solution. The Mmc LppA protein-based indirect ELISA kit can be used for detecting sheep serum Mmc infection antibody and vaccine immunity antibody and supplying key techniques for early monitoring, vaccine immunity effect evaluation and related research work about goat and sheep mycoplasma pneumonia caused by Mmc, and has important significance in early finding, preventing and controlling for epidemic disease caused by the pathogeny.
Owner:GUIZHOU UNIV
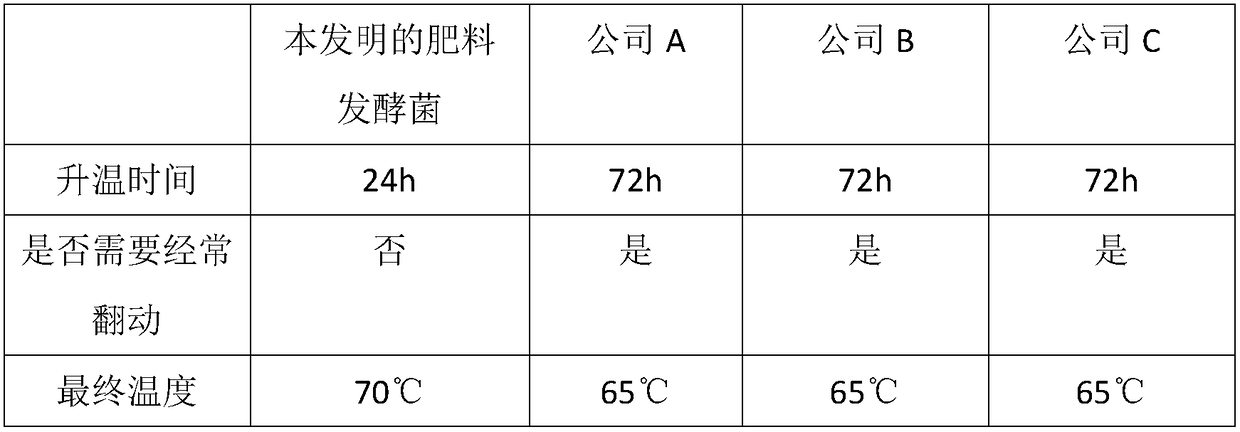
Bacterium agent for fermentation treatment of waste organic matters and preparation method
InactiveCN108192840AReduced manual turning and increased mixing volumeIncrease the amount of mixingFungiBacteriaMetaboliteOxygen
The invention belongs to the technical field of treatment of waste organic matters. Aiming at solving the technical problems in the prior art that fermentation is low, manual overturning is needed andthe waste organic matters are easy to corrupt in a fermentation process, the invention provides a bacterium agent for fermentation treatment of the waste organic matters and a preparation method. Thebacterium agent comprises a liquid bacterium agent; the liquid bacterium agent is prepared from the following components in parts by weight: 0.005 to 0.015 part of beef extract, 0.02 to 0.04 part ofpeptone, 0.04 to 0.06 part of glucose, 0.002 to 0.006 part of sodium chloride, 101.2 to 101.8 parts of distilled water, 2 to 4 parts of egg white, 0.05 to 0.2 part of sheep serum, 2 to 4 parts of brown sugar, 0.02 to 0.06 part of metabolite of photosynthetic bacteria, 0.02 to 0.06 part of metabolite of saccharomycetes and 0.2 to 0.6 part of an EM bacterium; the bacterium agent further comprises acarrier; the weight ratio of the carrier to the liquid bacterium agent is (20 to 30) to 1; the carrier is composed of organic matter particles with the mesh quantity of 10 to 20 meshes. The bacteriumagent provided by the invention has the characteristics of manpower saving and capabilities of prolonging the fermentation time and improving the fermentation efficiency, and is suitable for the wasteorganic matters with high aerobic fermentation difficulty.
Owner:熊万国
Culture medium for in-vitro culture of osteoblasts
InactiveCN105567629APromote growthReduce usageCulture processArtificial cell constructsSodium bicarbonateHorse serum
The invention discloses a culture medium for in-vitro culture of osteoblasts. The culture medium is characterized by being prepared from 10.0-15.0 g / L of a basal culture medium, 100-300 mg / L of tremella polysaccharide, 8-10 mL of serum, a proper amount of sodium bicarbonate for regulating the pH value to be 7.2-7.6, and the balance water added for achieving the constant volume of 1 L, wherein the basal culture medium is a DMEM or an alpha-MEM or RPMI-1640 or F12 or DMEM / F12, the purity of the tremella polysaccharide is larger than 75%, and the serum is fetal calf serum or calf serum or human serum or horse serum or sheep serum. The culture medium is used for in-vitro culture of osteoblasts. The culture medium has the advantages that growth of osteoblasts can be accelerated, and the use amount of the serum can be reduced.
Owner:SUZHOU PULUODA BIOLOGICAL SCI & TECH
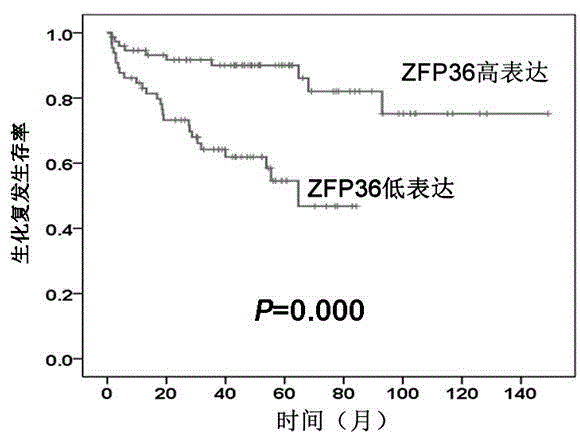
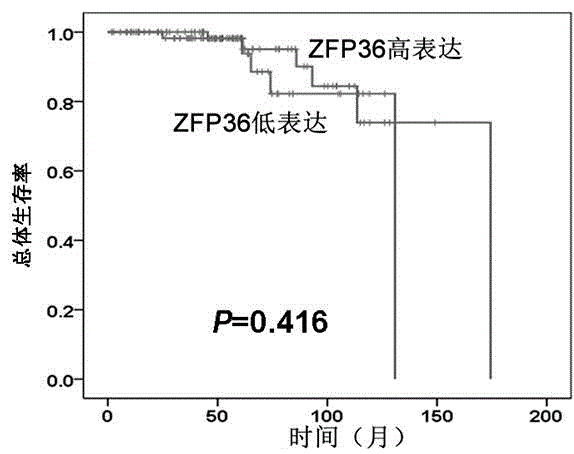
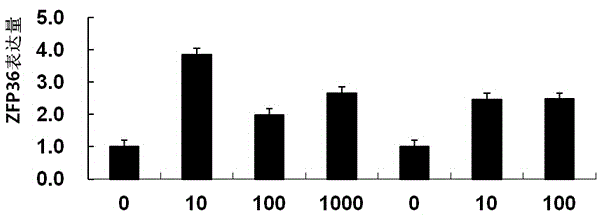
Detection reagent for prognosis of ZFP36 prostatic cancer and kit of detection reagent
InactiveCN104880565AMake up for the shortcomings of insufficient diagnostic specificityBiological testingAntiendomysial antibodiesPeroxidase
The invention discloses a detection reagent for prognosis of ZFP36 prostatic cancer and a kit of the detection reagent. The reagent is prepared from the following components (for 20 people): 1-1.5ml of H2O2, 1-1.5ml of nonimmune sheep serum working solution, 2.5-20mul of primary antibodies, namely rabbit-derived anti-ZFP36 polyclonal antibodies, 1ml of secondary antibodies, namely biotin labeled sheep anti-rabbit IgG working solution, 1ml of streptomycete antibiotin-peroxidase solution, and 2-3ml of DAB color developing agent. The detection kit for the prognosis of the prostatic cancer contains the detection reagent for detecting the inflammatory status of the tumor. The detection reagent is capable of detecting the inflammatory status of the tumor; a feasible method is provided for the diagnosis of the prostatic cancer and the prediction of the postoperative recurrence transfer rate, the postoperative recurrence transfer time and the life time of a prostatic cancer patient.
Owner:GUIZHOU PROVINCIAL PEOPLES HOSPITAL
Immunofluorescence kit and method for E-Cadherin mutation of peripheral blood circulating tumor cells of non-small cell lung cancer patient
InactiveCN111638357AReal-time detectionReduce lossesBiological material analysisBiological testingOncologyTumor cells
The invention provides an immunofluorescence kit and method for E-Cadherin mutation of peripheral blood circulating tumor cells of a non-small cell lung cancer patient, and belongs to the technical field of molecular biology. The kit comprises 45 mL of a diluent, 1 mL of a decolorizing solution, 0.5 mL of a staining solution A, 1 mL of a staining solution B, 200 [mu] l of methanol, 200 [mu] l of 2% PFA, 100 [mu] l of 10% goat serum, 100 [mu] l of a primary antibody working solution, 100 [mu] l of a secondary antibody working solution and a DAPI mounting medium. According to the detection method provided by the invention, the E-Cadherin expression condition of the patient suffering from advanced or recurrent non-small cell lung cancer can be detected without obtaining a tissue specimen through needle biopsy. The technology belongs to minimally invasive technology and can realize real-time detection. According to the method provided by the invention, a false positive result caused by anedge effect possibly generated in the dyeing process can be avoided, the stability is high, the cell loss is reduced, and the detection accuracy is improved.
Owner:山东凯歌智能机器有限公司
Immunofluorescence kit and detection method for detecting PD-L1 gene mutation of peripheral blood circulating tumor cells of small cell lung cancer patient
InactiveCN111638359AReal-time detectionReduce lossesBiological material analysisBiological testingOncologyTumor cells
The invention provides an immunofluorescence kit and a detection method for detecting PD-L1 gene mutation of peripheral blood circulating tumor cells of a small cell lung cancer patient, and belongs to the technical field of molecular biology. The kit comprises 45 mL of a diluent, 1 mL of a decolorizing solution, 0.5 mL of a staining solution A, 1 mL of a staining solution B, 200 [mu] l of methanol, 200 [mu] l of 2% PFA, 100 [mu] l of 10% goat serum, 100 [mu] l of a primary antibody working solution, 100 [mu] l of a secondary antibody working solution and a DAPI mounting medium. According to the detection method provided by the invention, the PD-L1 expression condition of a patient suffering from advanced or recurrent small cell lung cancer can be detected without obtaining a tissue specimen through needle biopsy. The technology belongs to minimally invasive technology and can realize real-time detection. According to the method provided by the invention, a false positive result causedby an edge effect possibly generated in the dyeing process can be avoided, the stability is high, the cell loss is reduced, and the detection accuracy is improved.
Owner:山东凯歌智能机器有限公司
Immunofluorescence kit for detecting CEA gene mutation of peripheral blood circulating tumor cells of non-small cell lung cancer patient and detection method
InactiveCN111521793AReal-time detectionReduce lossesCell dissociation methodsTumor/cancer cellsOncologyBiology
The invention provides an immunofluorescence kit for detecting CEA gene mutation of peripheral blood circulating tumor cells of a non-small cell lung cancer patient and a detection method, and belongsto the technical field of molecular biology. The kit comprises 45mL of a diluent, 1mL of a destaining solution, 0.5mL of a staining solution A, 1mL of a staining solution B, 200[mu]l of methanol, 200[mu]l of 2% PFA, 100[mu]l of 10% goat serum, 100[mu]l of a primary antibody working solution, 100[mu]l of a secondary antibody working solution and a DAPI mounting medium. According to the detection method provided by the invention, the CEA expression condition of a patient suffering from advanced or recurrent non-small cell lung cancer can be detected without obtaining a tissue specimen through needle biopsy. The technology belongs to a minimally invasive technology and can realize real-time detection. According to the method provided by the invention, a false positive result caused by an edge effect possibly generated in the dyeing process can be avoided, the stability is good, the cell loss is reduced, and the detection accuracy is improved.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
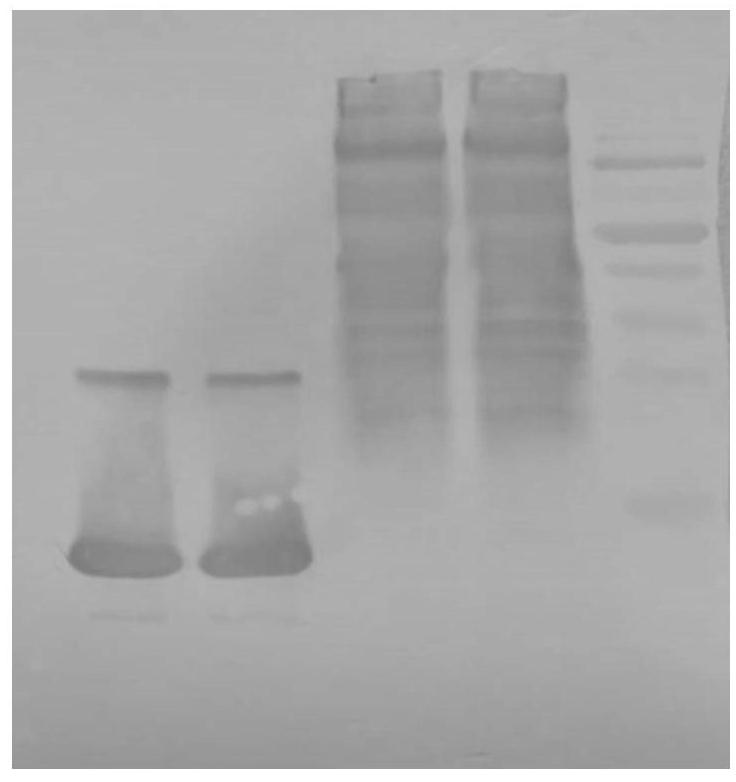
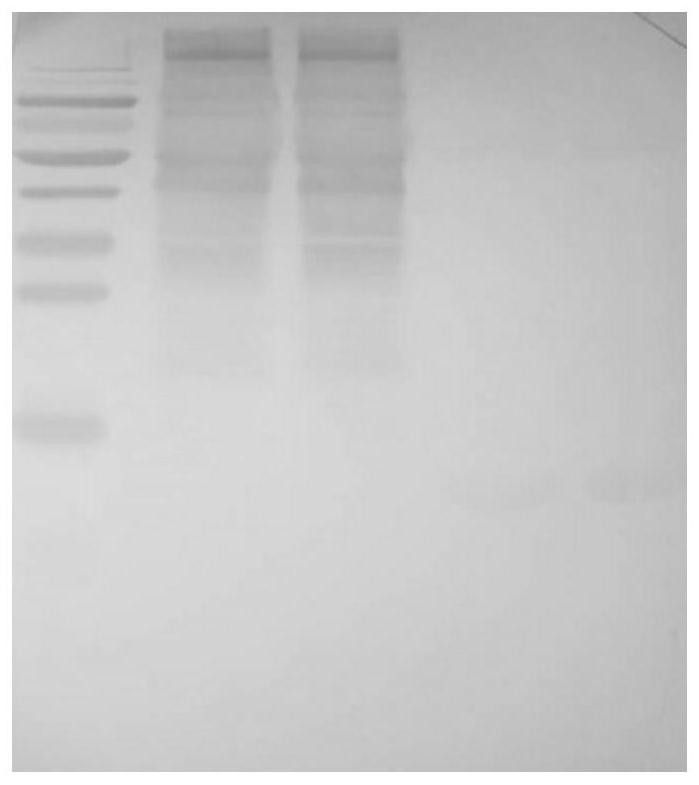
Extraction method for sheep serum albumin
InactiveCN108822205AAvoid gatheringAvoid sex changeSerum albuminPeptide preparation methodsCell AggregationsProtein aggregation
The invention provides an extraction method for sheep serum albumin. The method comprises the following steps: (1) fresh sheep blood is taken, an anticoagulant accounting for 12% of the original serumvolume is added, after qualified inspection and quarantine, centrifugation is performed at 4 DEG C and 2500 rpm for 15 min, a supernatant is taken and frozen at subzero 20 DEG C, frozen serum is taken, thaws sufficiently at room temperature and is centrifuged at 10000 rpm for 12 min, and a supernatant is taken and is the sheep serum. The extraction method for the sheep serum albumin is short in flow, simple to operate and high in production efficiency, no organic solvent is used in the method, protein aggregation or denaturation is avoided, meanwhile, environmental pollution and unsafe factors in the production process can be reduced, product purity is improved, and the method can be widely applied to the fields of medical treatment, biochemistry and the like.
Owner:天津市正江现代生物技术有限公司
Antigen for detecting sheep echinococcus antibody and preparation method of agar diffusion plate
PendingCN111875690AImprove general performanceAvoid Biosafety ConcernsBiological material analysisPeptide preparation methodsEscherichia coliAntigen
The invention discloses an antigen for detecting a sheep echinococcosis antibody and a preparation method of an agar diffusion plate, and belongs to the technical field of biological detection. The preparation method of the antigen comprises the following steps: performing enzyme digestion connection on a sheep echinococcosis EG95 protein nucleic acid sequence, and constructing the sheep echinococcosis EG95 protein nucleic acid sequence on a plasmid vector; then performing converting into escherichia coli, constructing an expression strain, performing IPTG induced expression, and performing crushing to obtain an inclusion body; and performing washing, cracking, centrifuging, purifying, dialyzing, concentrating, freeze-drying, and diluting with PBS. The preparation method of the agar diffusion plate comprises the following steps: adding water and PBS into agarose, performing heating, adding polyethylene glycol 6000, MES and sodium chloride, performing cooling, performing punching, and sealing the bottom. The antigen and the agar diffusion plate provided by the invention have the advantages of high sensitivity, strong specificity and strong universality, have good biosafety, are easyfor large-scale production, are especially suitable for clinical detection of sheep serum by grassroots veterinarians, and have wide popularization and application value.
Owner:CHONGQING AULEON BIOLOGICALS
Preparation process of perfusion agent for treating dairy cow mastitis
ActiveCN103285079AGood treatment effectHigh activityAntibacterial agentsPharmaceutical delivery mechanismAnaphylaxisIrritation
The invention relates to a veterinary drug, and in particular relates to a preparation process of a perfusion agent for treating dairy cow mastitis. The process comprises the steps of extracting active ingredients of scutellaria baicalensis, flos lonicerae and leonurus through methods of water extraction, concentration, acid eduction and the like. The traditional Chinese medicine perfusion agent has no toxicity, irritation or anaphylaxis; the perfusion agent has better treatment effect on clinical mastitis models of milk goats and the best perfusion dose is 10ml per mammary area; the perfusion agent is capable of remarkably enhancing the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in ill goat serum and reducing the contents of nitric oxide (NO) and malonaldehyde (MDA).
Owner:湖南圣雅凯生物科技有限公司 +1
Indirect ELISA detection kit for sheep clostridium putrificum
The invention discloses an indirect ELISA detection kit for sheep clostridium putrificum. The indirect ELISA detection kit is characterized in that a clostridium putrificum suspension is used as a coating antigen. The clostridium putrificum suspension is prepared by the following steps: inoculating a clostridium putrificum colony into a culture medium, and performing static culture for 12-24 hours in an anaerobic environment at the temperature of 37 DEG C; and centrifuging a clostridium putrificum culture obtained by culture, collecting the clostridium putrificum, performing washing, and re-suspending the clostridium putrificum by using a carbonate buffer solution to prepare the clostridium putrificum suspension. The kit disclosed by the invention can be used for detecting whether sheep serum contains an antibody of the clostridium putrificum in a high-sensitivity and high-specificity manner, and judging whether a sheep is infected with the clostridium putrificum or not according to the detection, so that after the sheep is sick, the diagnosis can be rapidly carried out and emergency treatment measures can be taken.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
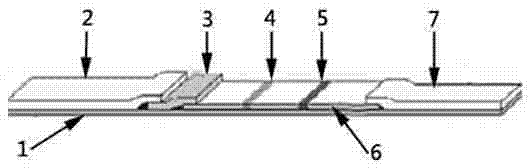
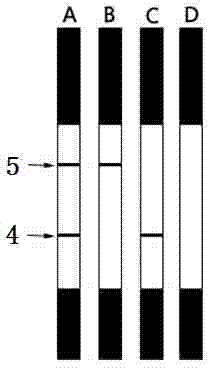
Ovinetheileriasis immuno colloidal gold detection test paper strip and preparation method thereof
InactiveCN104730239AEasy to operateEasy to makeBiological material analysisTheileria uilenbergiSmall sample
The invention discloses an ovinetheileriasis immuno colloidal gold detection test paper strip for rapidly detecting theileria uilenbergi and theileria luwenshuni antibodies in sheep serum. The ovinetheileriasis immuno colloidal gold detection test paper strip comprises a PVC pad, wherein a sample pad for feeding of a sample to be detected, a colloidal gold pad coated with gold marked theileria uilenbergi TuIP recombinant protein antigen, a nitrocellulose membrane and a water absorbing pad are sequentially arranged on the upper surface of the PVC pad; one end of the sample pad is arranged above one end of the colloidal gold pad; the other end of the colloidal gold pad is arranged above one end of the nitrocellulose membrane; one end of the water absorbing pad is arranged above the other end of the nitrocellulose membrane; the nitrocellulose membrane is coated with a detection line of the theileria uilenbergi TuIP recombinant protein antigen and a quality control line of a polyclonal antibody. The invention further provides a preparation method of the detection test paper strip. The detection test paper strip prepared by using the preparation method has the characteristics of small sample amount and simplicity in operation and is simple, convenient and rapid in detection, low in cost and applicable to use for basic level veterinarian laboratories and veterinarian operators, no complex instrument is needed, and the result can be directly judged by naked eyes.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
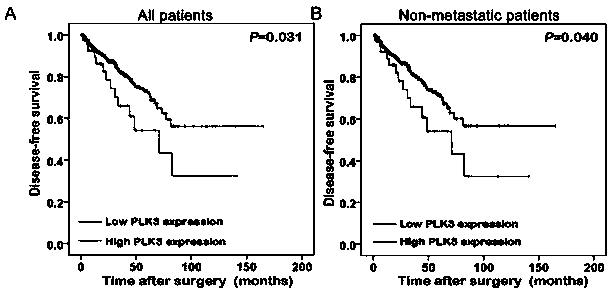
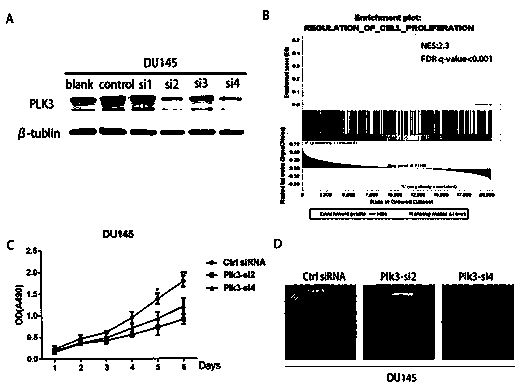
Plk3 prostatic cancer prognosis detection reagent and kit
The invention discloses a Plk3 prostatic cancer prognosis detection reagent and kit. The reagent comprises (for 20 people) 1-1.5ml of H2O2, 1-1.5ml of non-immune sheep serum working solution, 2.5-20mul of rabbit Plk3 polyclonal antibodies serving as primary antibodies, 1ml of biotin-labeled goat anti-rabbit IgG working solution serving as secondary antibodies, 1ml of streptomycete antibiotin-peroxidase solution and 2-3ml of DAB color developing agents. The Plk3 prostatic cancer prognosis detection kit comprises the Plk3 detection reagent. Prostatic cancer recurrence can be forecasted.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
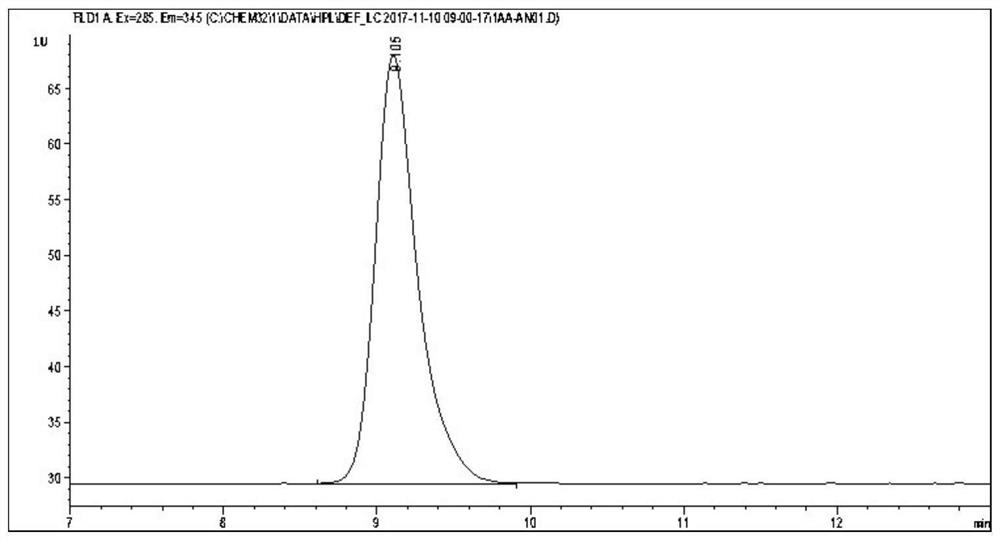
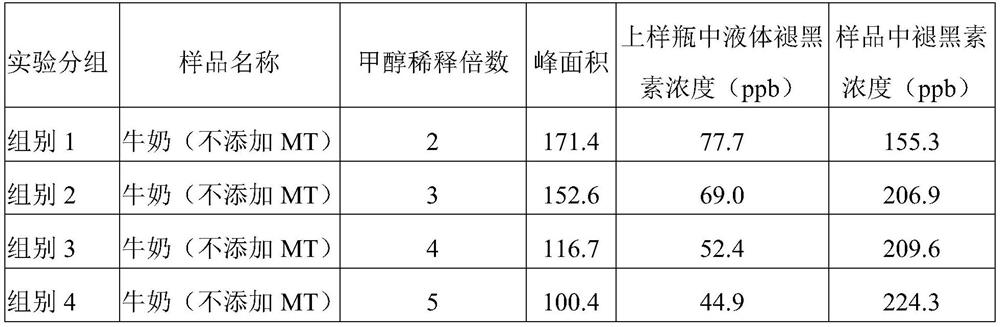
Sample rapid pretreatment method for determining melatonin in milk, serum and products thereof
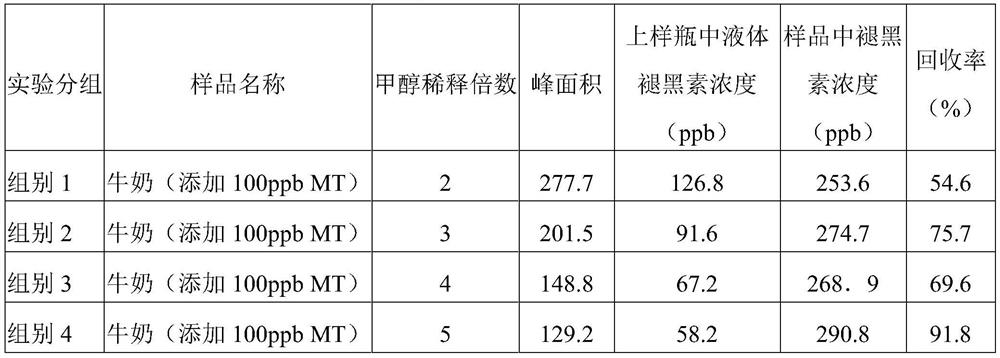
The invention provides a rapid sample pretreatment method for determining melatonin in milk, serum and products thereof. According to the method, a sample is diluted by using methanol, and the melatonin content is determined by using high performance liquid chromatography, so that the purpose of rapidly and accurately determining melatonin in milk, serum and products thereof is achieved. Accordingto the method disclosed by the invention, the effect is optimal when milk is diluted to 5 times of the volume of an original sample, and the melatonin recovery rate is 91.5-134.7%; better recovery rate can be obtained when the sheep serum is diluted to 2 times, 3 times, 4 times and 5 times of the original volume, and the recovery rate is 81.02%-97.44%. The method has the advantages of low cost, simple steps and time saving, and provides a beneficial reference for further development of research related to melatonin in milk and serum.
Owner:CHINA AGRI UNIV
Method for producing transgenic sheep by injecting lentivirus into perivitelline space
The invention provides a method for producing transgenic sheep by injecting lentivirus into perivitelline space; lentiviral vector and virus packaging plasmid are cotransfected into 293T cells and then centrifugated after 48 hours to prepare the concrete of the lentiviral; the concrete of the lentiviral is injected into oocyte perivitelline space which is 24 to 25 hours mature, is integrated with sperms to implement in vitro fertilization, is put into culture fluid after 12 hours to be cultured, and the cleavage rate and the transgenic rate are calculated after 48 hours. Step 1: an oocyte is washed by in vitro maturation fluid for 3 to 4 times, and the 75 to 78um l / drop in vitro maturation fluid which is dripped by 25 to 30m / drop is cultured in 5 percent CO2 by 95 percent; step 2: a cumulus cell is removed from the in vitro matured oocytes, the lentiviral is injected into the cell perivitelline space and is washed by IVF washing liquid for 2 to 4 times, and fertilization fluid is dripped by 25 to 30m / drop; semen is unfrozen, the fertilization fluid is moved in, supernatant fluid is centrifugurated and removed, the sperms are precipitated and added into fertilization fluid drips and incubated; eggs which are fertilized for 12 to 18 hours are treated in the above step and then put into a four-hole culture plate to be cultured; and step 3: medicine lentiviral, egg pumping liquid, the maturation fluid, micromanipulation liquid, SOF, the fertilization fluid, the culture fluid and sheep serum are prepared.
Owner:新疆维吾尔自治区畜牧科学院中国-澳大利亚绵羊育种研究中心
Method for preparing dodecyl-pectin microspheres carrying sheep serum protein
ActiveCN105030695AFacilitate cross-linkingEffective embeddingPeptide/protein ingredientsPharmaceutical non-active ingredientsMicrosphereSheep serum
The invention provides a method for preparing dodecyl-pectin microspheres carrying sheep serum protein. According to the method, pectin is modified through bromododecane, and the obtained dodecyl-pectin is used for embedding sheep serum protein to prepare microspheres carrying medicine. The prepared dodecyl-pectin has good performance of crosslinking with calcium ions and is capable of embedding the sheep serum protein effectively to form a dense structure; the sheep serum protein can be kept in the whole form in the stomach liquid, the protein medicine can be released under the colon PH, and the purpose of releasing the medicine by the colon in a fixed-point mode is achieved.
Owner:NANCHANG UNIV
Immunofluorescence kit and method for detecting PD-L1 gene mutation of peripheral blood circulating tumor cells of non-small cell lung cancer patient
InactiveCN111521792AReal-time detectionReduce lossesCell dissociation methodsBiological material analysisOncologyTumor cells
The invention provides an immunofluorescence kit and method for detecting PD-L1 gene mutation of peripheral blood circulating tumor cells of a non-small cell lung cancer patient, and belongs to the technical field of molecular biology. The kit comprises 45mL of a diluent, 1mL of a destaining solution, 0.5mL of a staining solution A, 1mL of a staining solution B, 200[mu]l of methanol, 200[mu]l of 2% PFA, 100[mu]l of 10% goat serum, 100[mu]l of a primary antibody working solution, 100[mu]l of a secondary antibody working solution and a DAPI mounting medium. According to the detection method provided by the invention, the PD-L1 expression condition of a patient suffering from advanced or recurrent non-small cell lung cancer can be detected without obtaining a tissue specimen through needle biopsy. The technology belongs to a minimally invasive technology and can realize real-time detection. According to the method provided by the invention, a false positive result caused by an edge effectpossibly generated in the dyeing process can be avoided, the stability is good, the cell loss is reduced, and the detection accuracy is improved.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Hematopoietic stem cell cryopreservation solution and hematopoietic stem cell cryopreservation method
InactiveCN110896946AReduces Ice Crystal DamageHigh recovery rateDead animal preservationHydroxyethyl starchPyrrolidinones
The invention discloses a hematopoietic stem cell cryopreservation solution and a hematopoietic stem cell cryopreservation method. The cryopreservation solution is composed of a cryoprotectant, a culture medium and human serum albumin, and the cryoprotectant comprises 25 ml of dimethyl sulfoxide, 5 g of hydroxyethyl starch, 15 ml of normal saline and 5 g of polyvinylpyrrolidone; and the culture medium comprises 1.5 g of carrot sugar, 25 g of amino acids and 20 g of fetal sheep serum. The cryopreservation method has the advantages of simplicity in operation, low technical requirements, timely cryopreservation, solving of the problem that the traditional hematopoietic stem cells can be cryopreserved after several days of culture and observation, and provision of reliable guarantee for storage, application and transplantation of the hematopoietic stem cells.
Owner:贵州汉氏联合生物技术有限公司
Screening culture method for sheep oocytes in vitro
The invention provides a screening culture method for sheep oocytes in vitro, which comprises the following steps: step 1: ovaries which were killed less than half an hour ago are put into saline water at the temperature of 30DEG C to 40DEG C and containing penicillin and streptomycin, washed for 3 to 4 times within 3 hours, and oocytes are picked out; 25 to 30mu mol / L brilliant cresyl blue is put into 35DEG C to 39DEG C water bath to be dyed for 85 to 92min, the cytoplasm is blue and is washed for 3 to 4 times by in vitro maturation fluid, is put into 55 to 78mul / drip in vitro maturation culture fluid by 25 to 30m / drip, and is cultured in 5 percent CO2 by 95 percent; step 2: cumulus cells are removed from oocytes which are maturated in vitro for 25 to 28 hours; IVF washing liquid is used to wash for 2 to 4 times, and is dripped into 50 to 70mu l fertilization fluid by 25 to 30m / drop; semen is unfrozen, the fertilization fluid is moved in, supernatant fluid is centrifugurated for 4 to5min and removed, the precipitated sperms are added into fertilization fluid drips by the density of 2 to 4*106 / ml and incubated; eggs which are fertilized for 12 to 18 hours are treated in the above step and then moved into a four-hole culture plate to be cultured; and step 3: reagent egg absorption liquid, brilliant cresyl blue maturation liquid, dyeing liquor SOF, the fertilization fluid, the culture fluid and sheep serum are prepared.
Owner:INNER MONGOLIA SAINUO GRASSLAND SHEEP IND
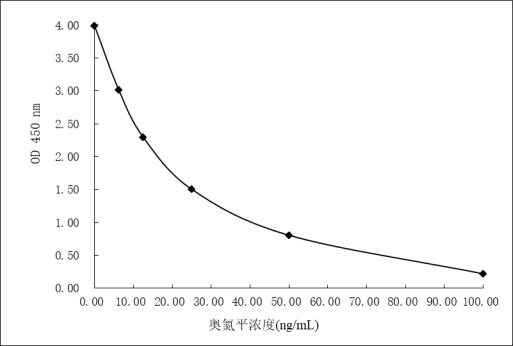
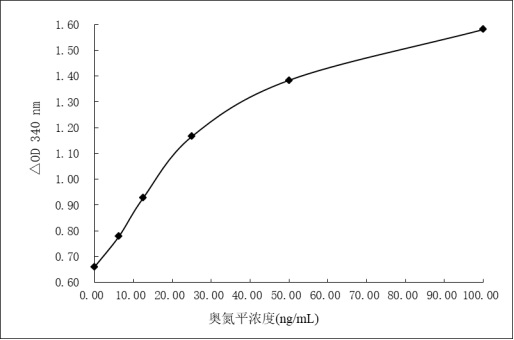
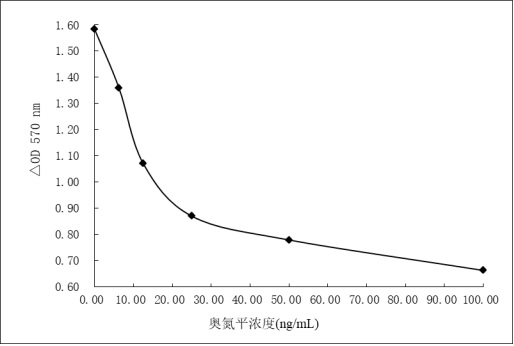
Olanzapine derivative, immunogen, anti-olanzapine specific antibody and preparation method and application thereof
PendingCN114685527AImproving immunogenicityHigh sensitivitySerum immunoglobulinsSerum albuminAntiendomysial antibodiesEnzyme immunoassays
The invention discloses an olanzapine derivative, an immunogen, an anti-olanzapine specific antibody as well as a preparation method and application of the olanzapine specific antibody. Firstly, a novel olanzapine derivative and recombinant sheep serum albumin obtained through genetic engineering modification are coupled to prepare an olanzapine artificial antigen, then the olanzapine artificial antigen is used for immunizing an experimental animal to obtain an anti-olanzapine specific antibody, and ELISA detection shows that the specific antibody is high in specificity and sensitivity and can be used for preparing the olanzapine specific antibody. An interference experiment shows that the specific antibody does not have any cross reaction with 100 common drugs; the anti-olanzapine specific antibody is applied to preparation of olanzapine detection reagents, the olanzapine detection reagents comprise an olanzapine average phase enzyme immunoassay reagent and an olanzapine latex enhanced immunoturbidimetry detection reagent, and the detection reagents can realize high-flux and rapid detection of olanzapine on a full-automatic biochemical analyzer.
Owner:长沙博源医疗科技有限公司
Detection method of Pichia pastoris host protein residues in recombinant human lysozyme
ActiveCN109799335BLow cross-reactivityNo cross reactionMaterial analysisPichia pastorisProtein total
A method for detecting the residual amount of Pichia host protein in recombinant human lysozyme raw materials, comprising preparing recombinant human lysozyme, preparing Pichia host protein, detecting the total protein content of Pichia host protein, and preparing Pichia host protein Antiserum, elimination of cross-reaction between recombinant human lysozyme and Pichia host protein immune rabbit serum and Pichia host protein immune sheep serum, two-dimensional electrophoresis of Pichia host protein, detection of antibody coverage of Pichia host protein, enzyme Composition of linked immunoassay steps. The Pichia host protein in the recombinant human lysozyme is detected in three consecutive batches by using the detection method of the present invention, and the content of the Pichia host protein is all <0.05%, meeting the <0.1% standard stipulated in the Pharmacopoeia of the People's Republic of China. The detection method of the invention has the advantages of strong specificity, full coverage of antibodies, no cross-reaction, etc., and can be used for content detection of Pichia pastoris host protein.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
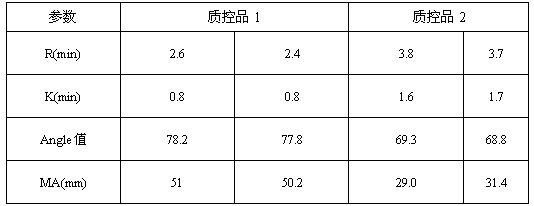
Thromboelastography quality control product and preparation method thereof
ActiveCN107588998BReduce use costSimple preparation stepsPreparing sample for investigationBiological testingBiotechnologyAnimal science
The invention discloses a quality control product, comprising a quality control product 1 and a quality control product 2, characterized in that, the quality control product 1 comprises the following components: 2-6 parts by weight of factor-rich sheep plasma, 2-5 parts by weight The goat serum in parts by weight and the fibrinogen mother liquor in 1-4 parts by weight; the quality control product 2 includes the following components: 1-4 parts by weight of factor-deficient goat plasma, 5-10 parts by weight of goat serum and 0.3- 2 parts by weight of fibrinogen mother solution, the fibrinogen mother solution is prepared by dissolving fibrinogen in goat serum, wherein the mass percentage of fibrinogen is 10%; There is no need to extract coagulation factors, the preparation steps are simplified, the operation is easy, the cost is low, the results are accurate, and the stability is good; Replacing the quality control products produced by the listed American Haemoscope company, reducing the cost of use for customers.
Owner:上海原科实业发展有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com