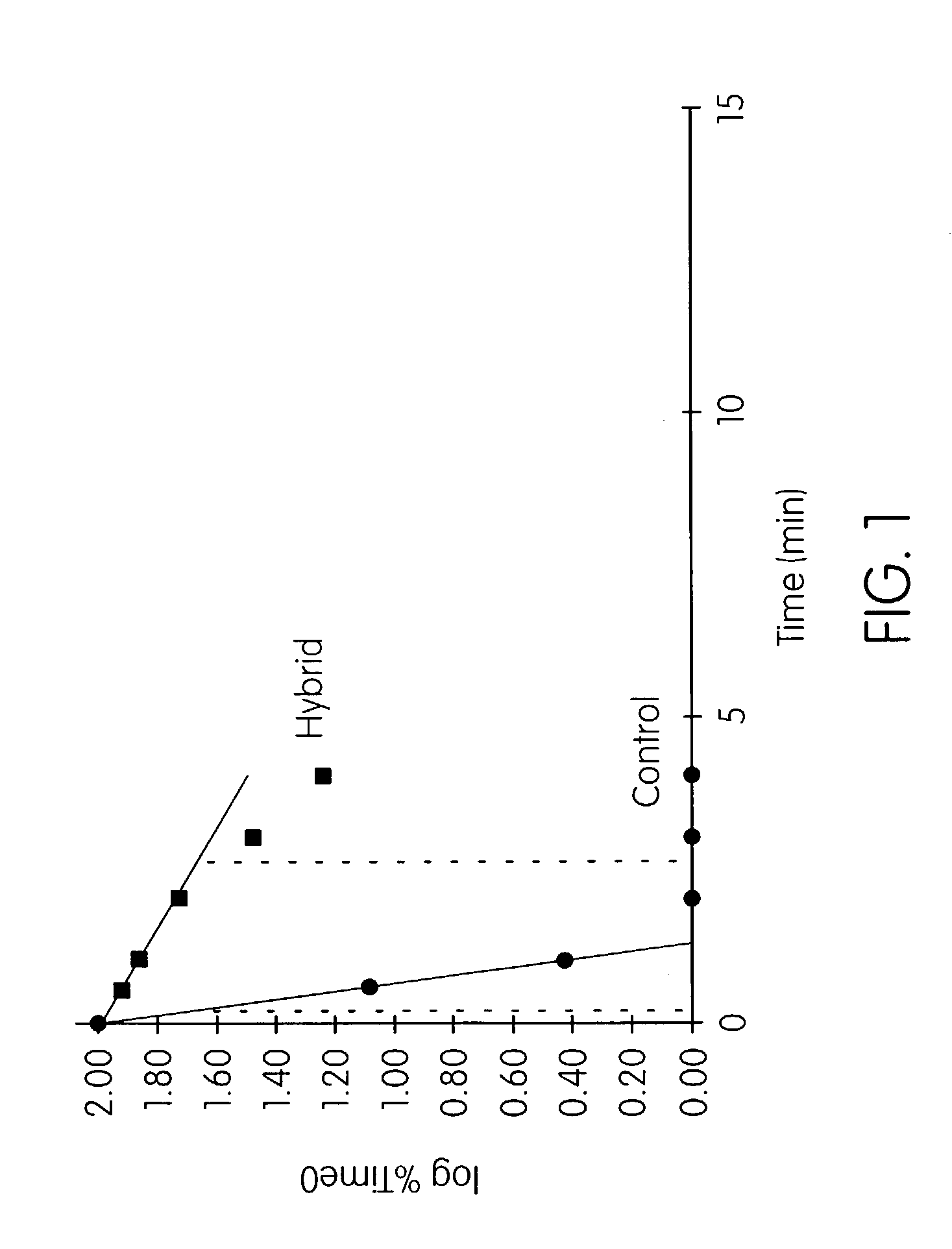
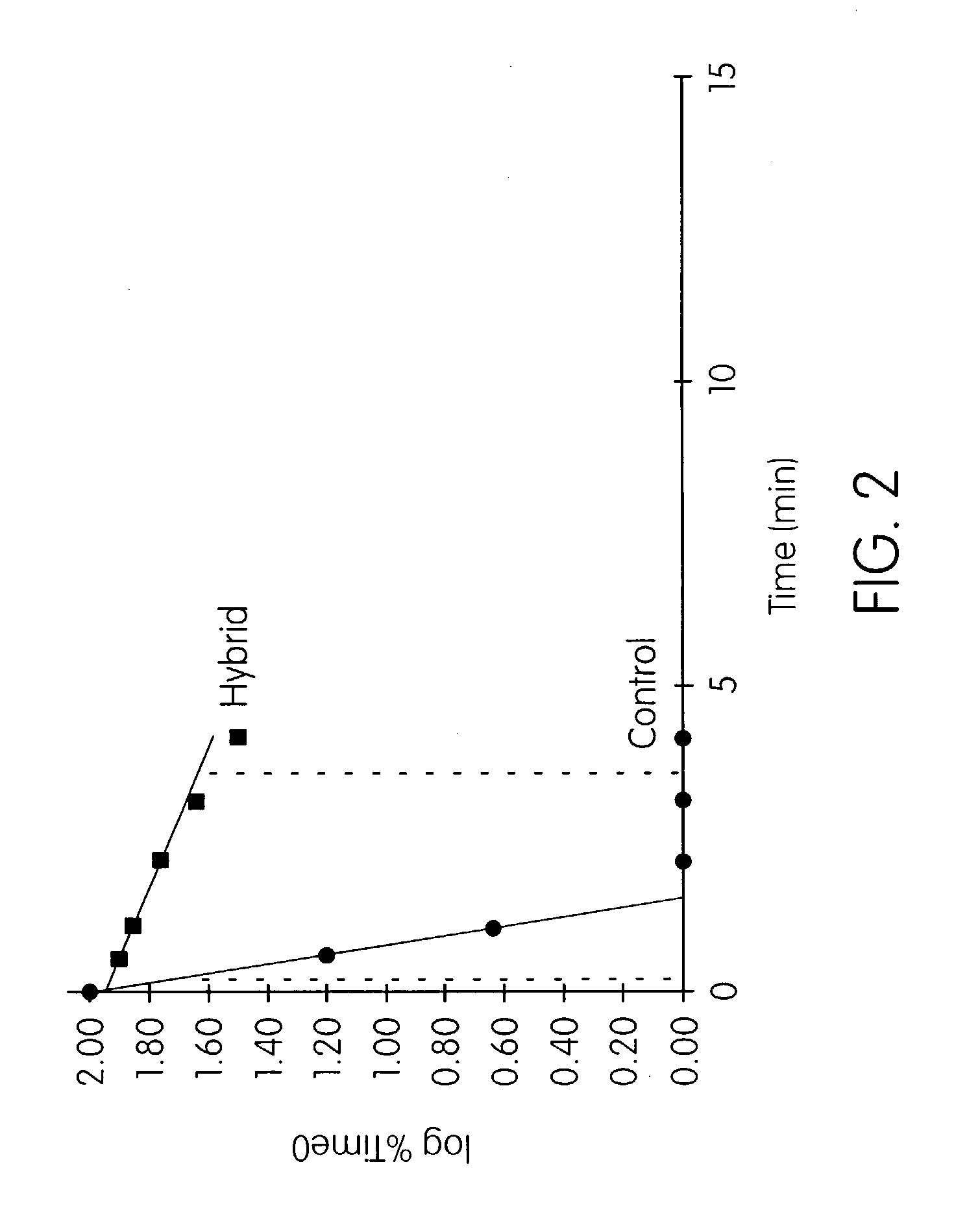
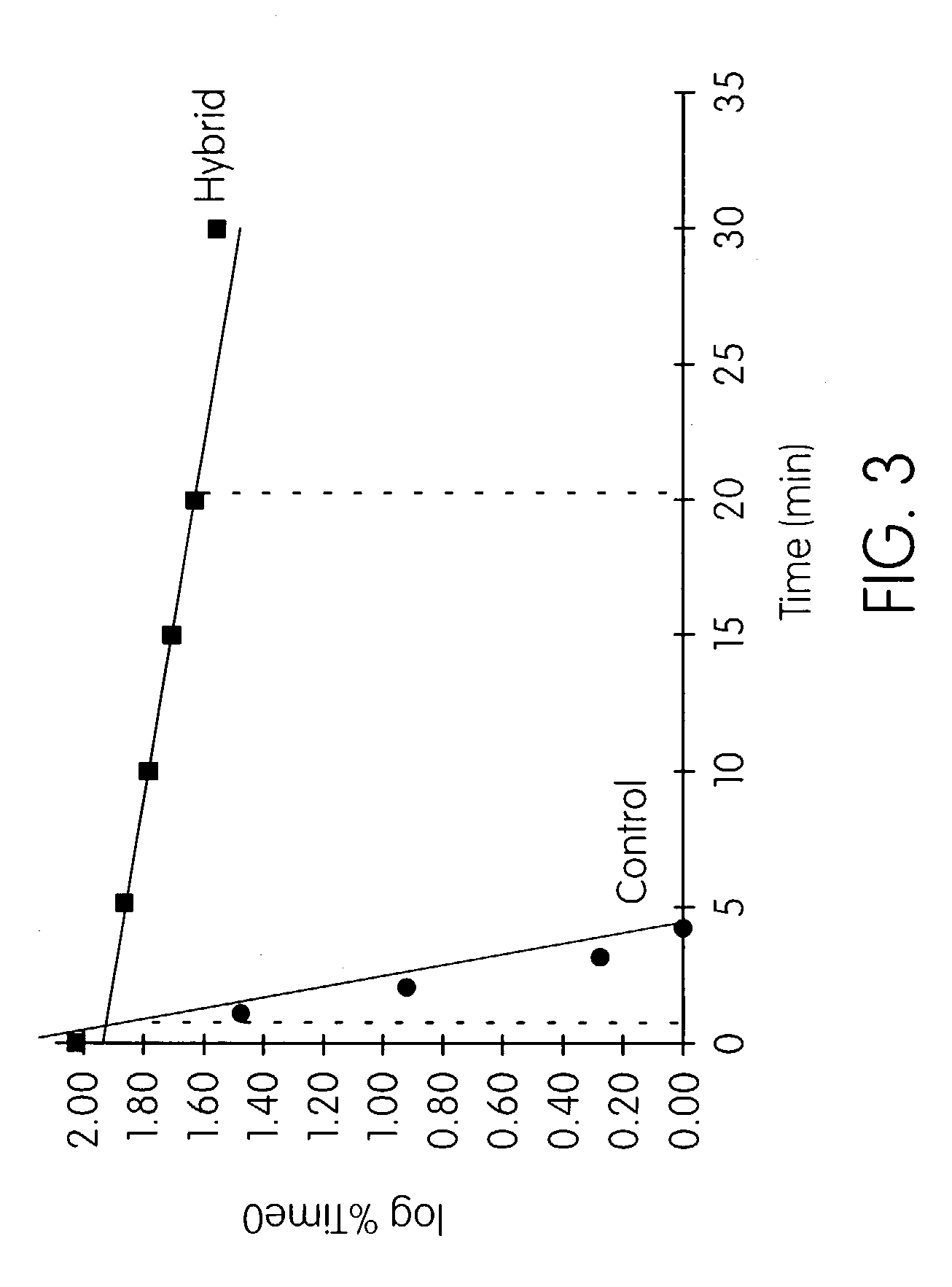
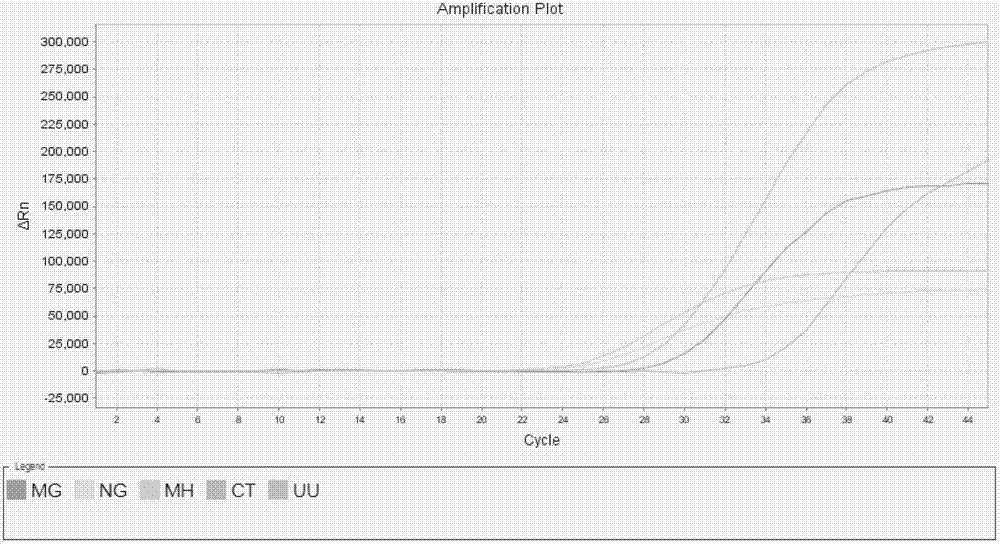
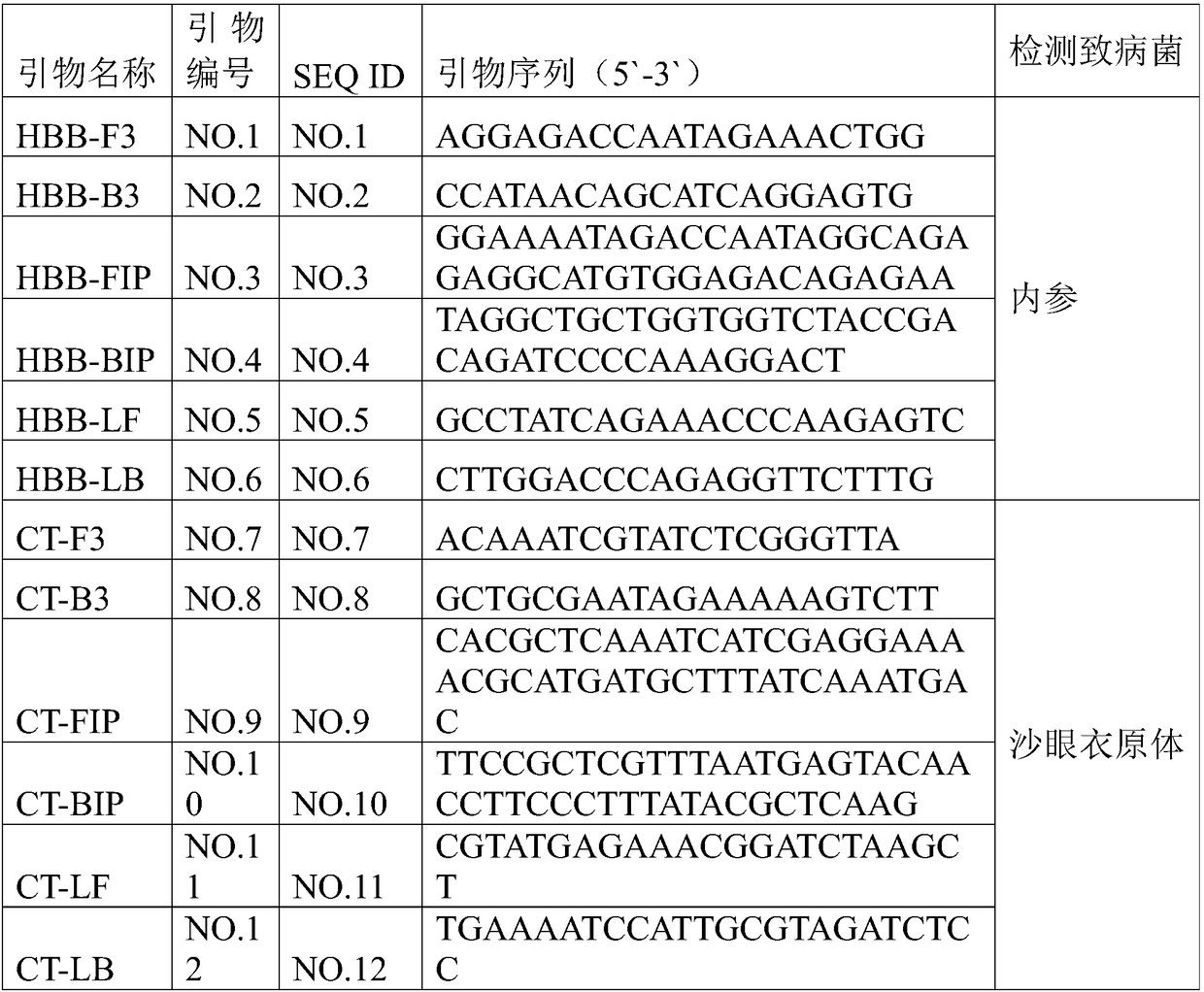
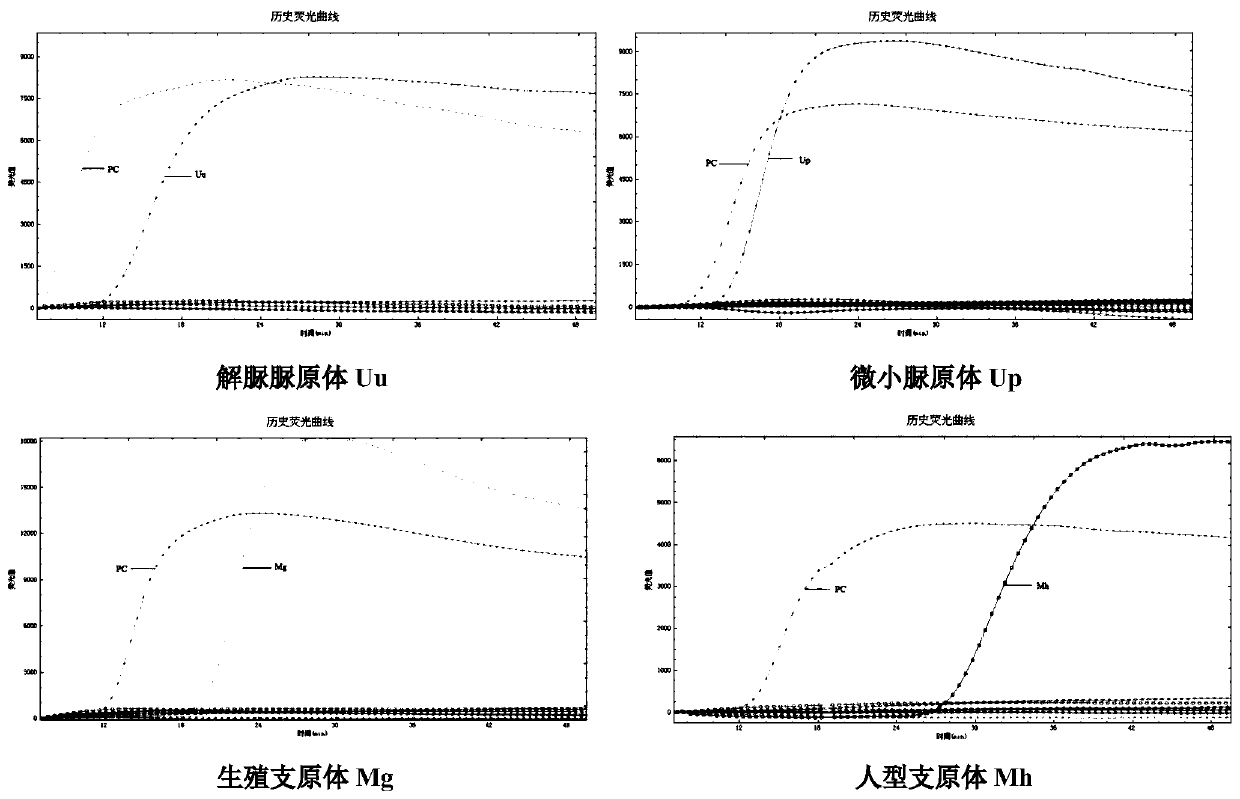
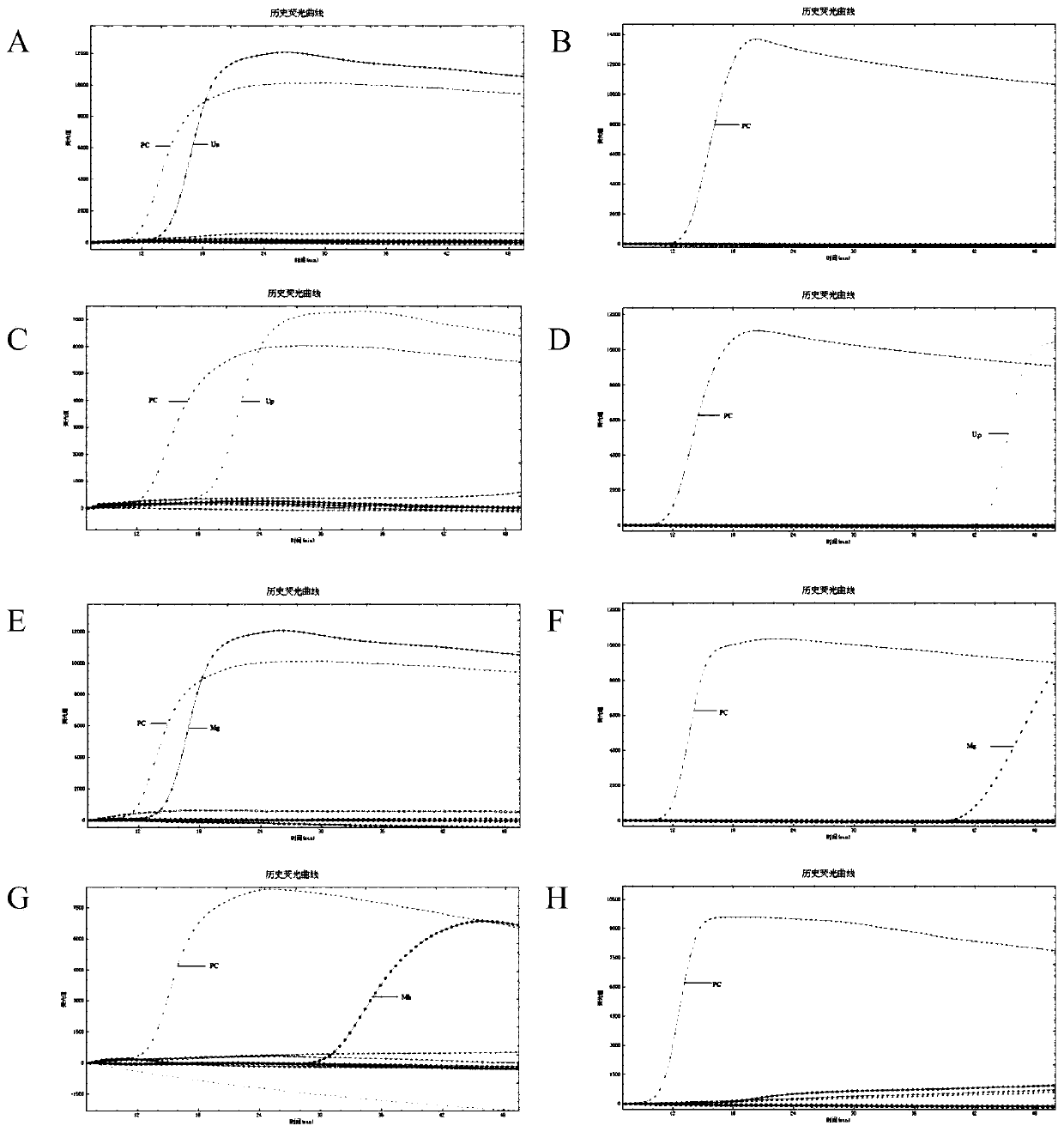
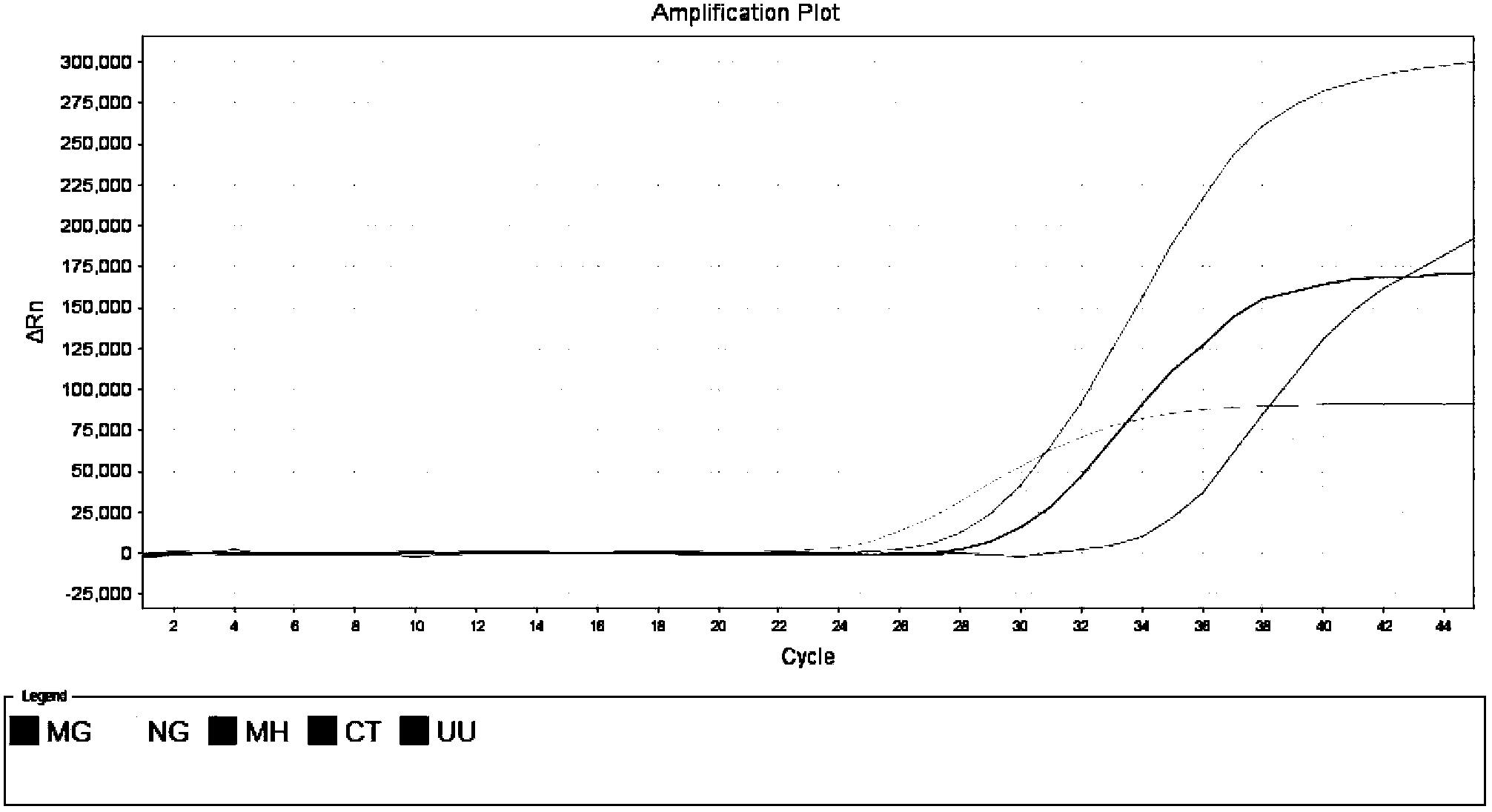
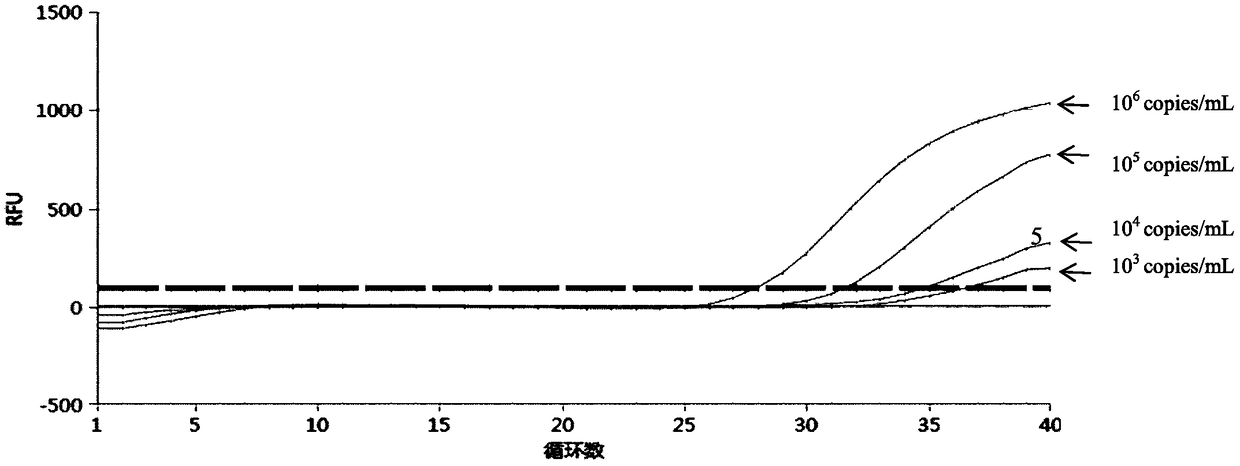
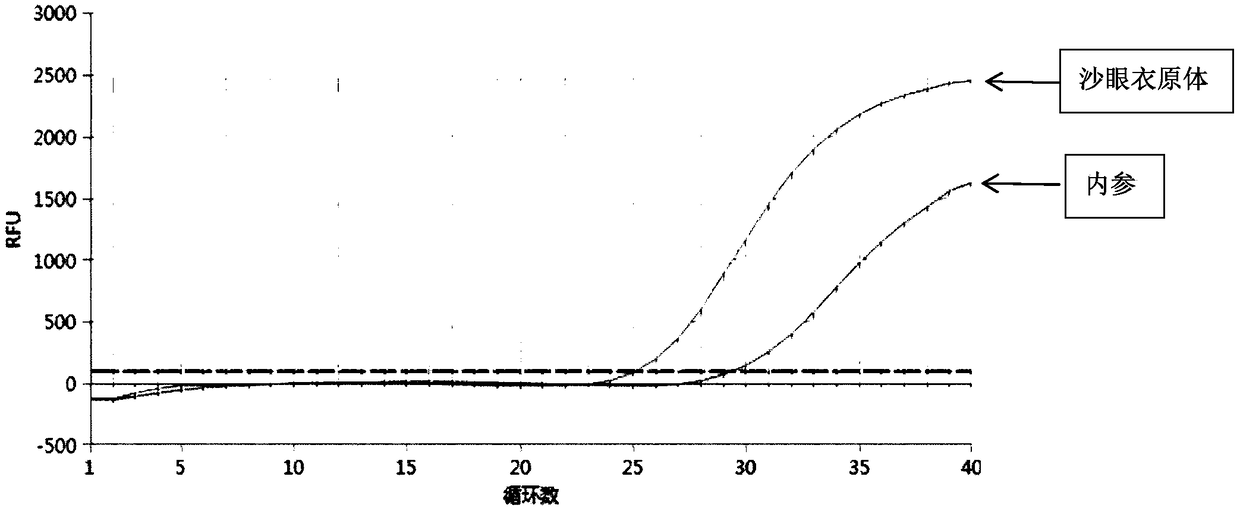
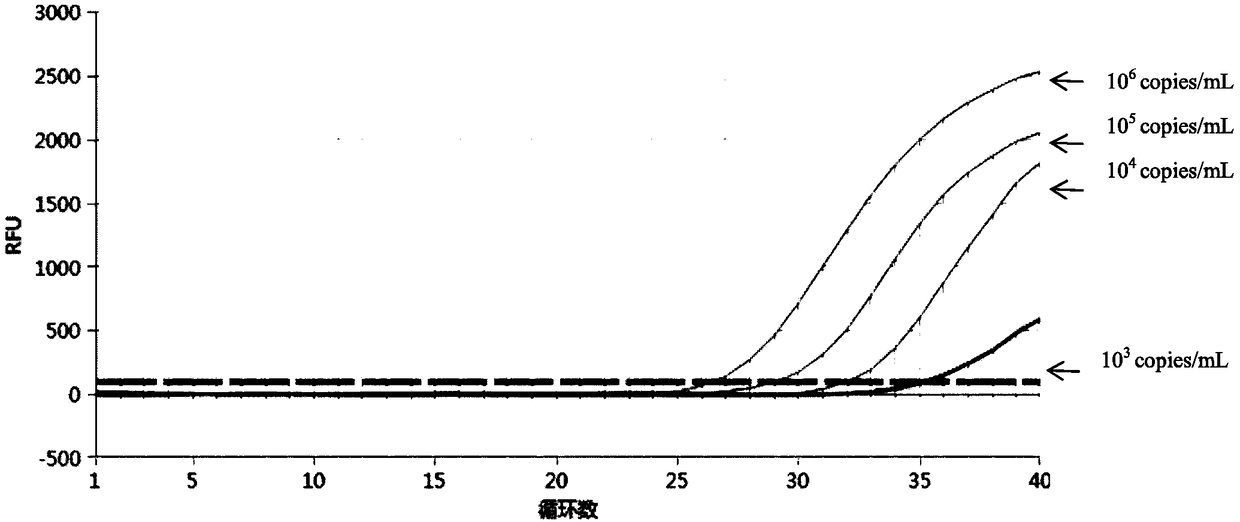
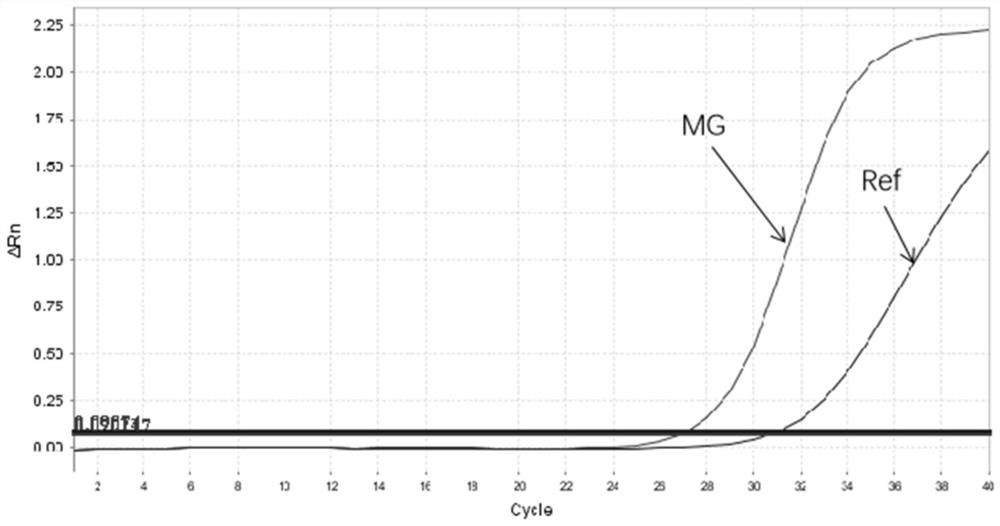
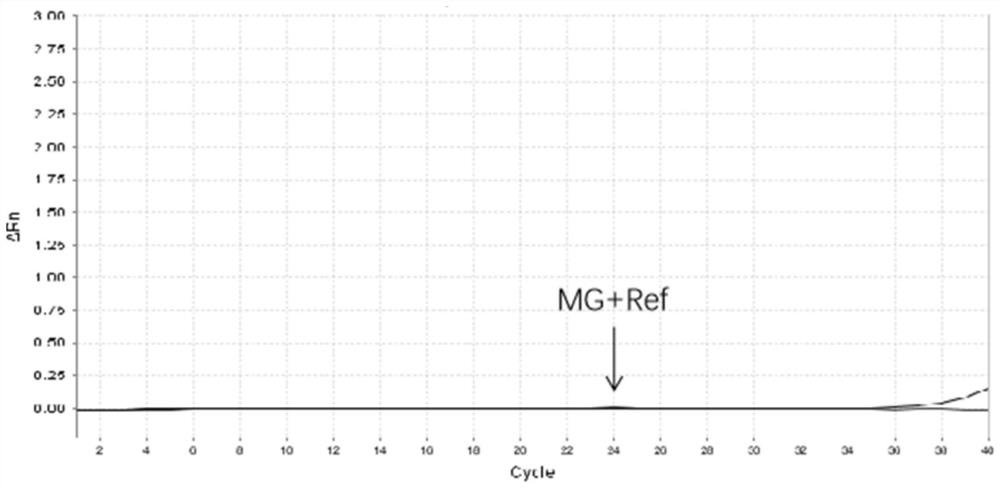
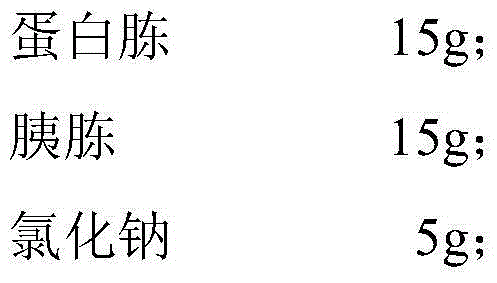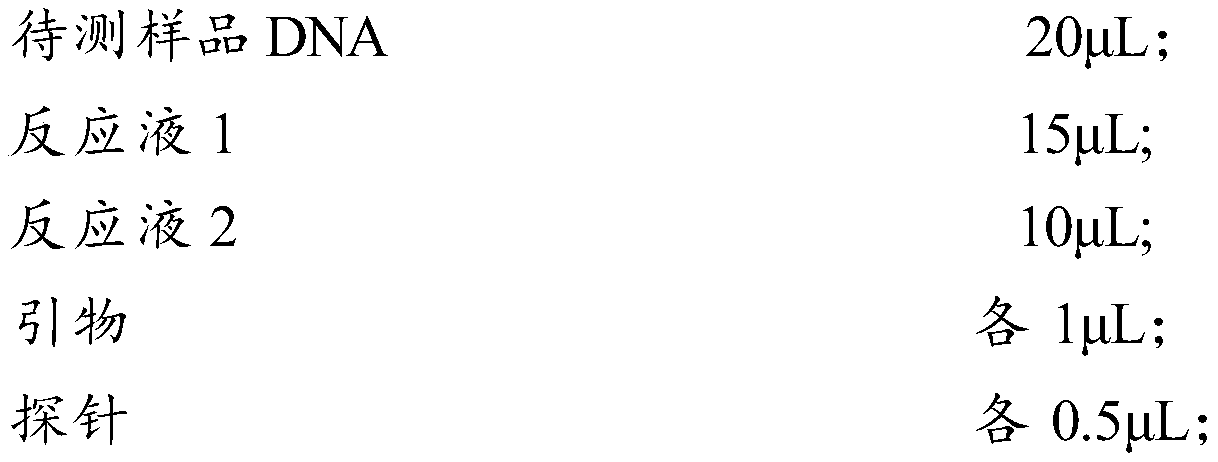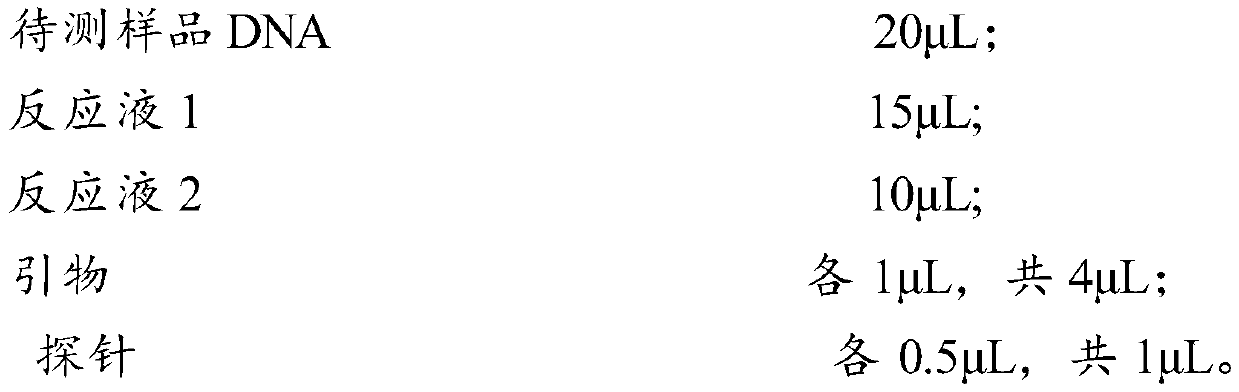Patents
Literature
45 results about "Mycoplasma genitalium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mycoplasma genitalium (MG, commonly known as Mgen), is a sexually transmitted, small and pathogenic bacterium that lives on the skin cells of the urinary and genital tracts in humans. Mgen is becoming increasingly common. Resistance to multiple antibiotics is occurring, including azithromycin which until recently was the most reliable treatment. The bacteria was first isolated from urogenital tract of humans in 1981, and was eventually identified as a new species of Mycoplasma in 1983. It can cause negative health effects in men and women. It also increases the risk factor for HIV spread with higher occurrences in homosexual men and those previously treated with the azithromycin antibiotics.
Probes, compositions and kits for determining the presence of Mycoplasma pneuomoniae in a test sample
The present invention relates to oligonucleotides useful for determining the presence of Mycoplasma pneumoniae and / or Mycoplasma genitalium in a test sample. The oligonucleotides of the present invention may be incorporated into hybridization assay probes, capture probes and amplification primers, and used in various combinations thereof.
Owner:GEN PROBE INC
Quintuple fluorescent PCR (polymerase chain reaction) quick and hypersensitive detection kit and application thereof
ActiveCN102888464AGuaranteed accuracyGuaranteed reliabilityMicrobiological testing/measurementFluorescence/phosphorescenceMycoplasma hominisPcr method
The invention relates to a real-time PCR (polymerase chain reaction) method for quintuply detecting target nucleic acid in a nucleic acid extracting solution in a single PCR reaction vessel, which is used for detecting Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis and Mycoplasma genitalium in a sample.
Owner:苏州华益美生物科技有限公司
Probes, compositions and kits for determining the presence of Mycoplasma genitalium in a test sample
The present invention relates to oligonucleotides useful for determining the presence of Mycoplasma pneumoniae and / or Mycoplasma genitalium in a test sample. The oligonucleotides of the present invention may be incorporated into hybridization assay probes, capture probes and amplification primers, and used in various combinations thereof.
Owner:GEN PROBE INC
PCR method of multiple sex propagate pathogene synchronous detection and kit
InactiveCN101935686AThe detection process is fastSimple stepsMicrobiological testing/measurementMicroorganism based processesDiseaseMycoplasma hominis
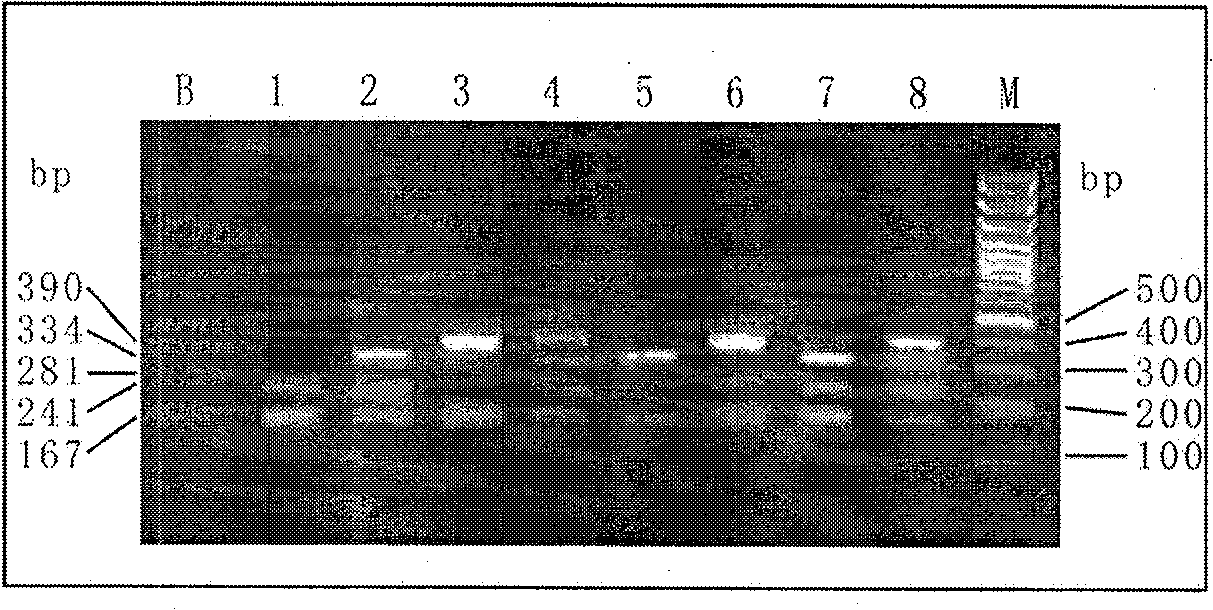
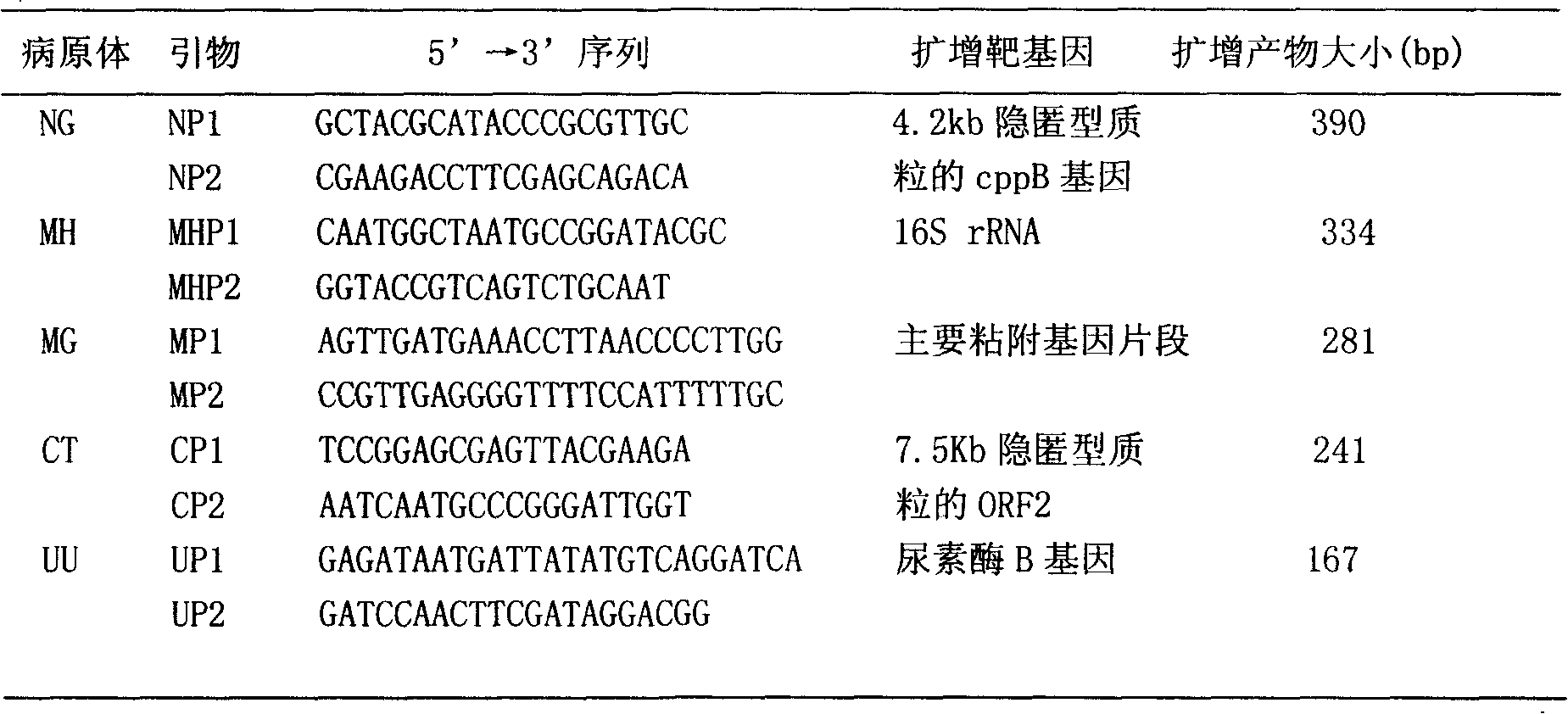
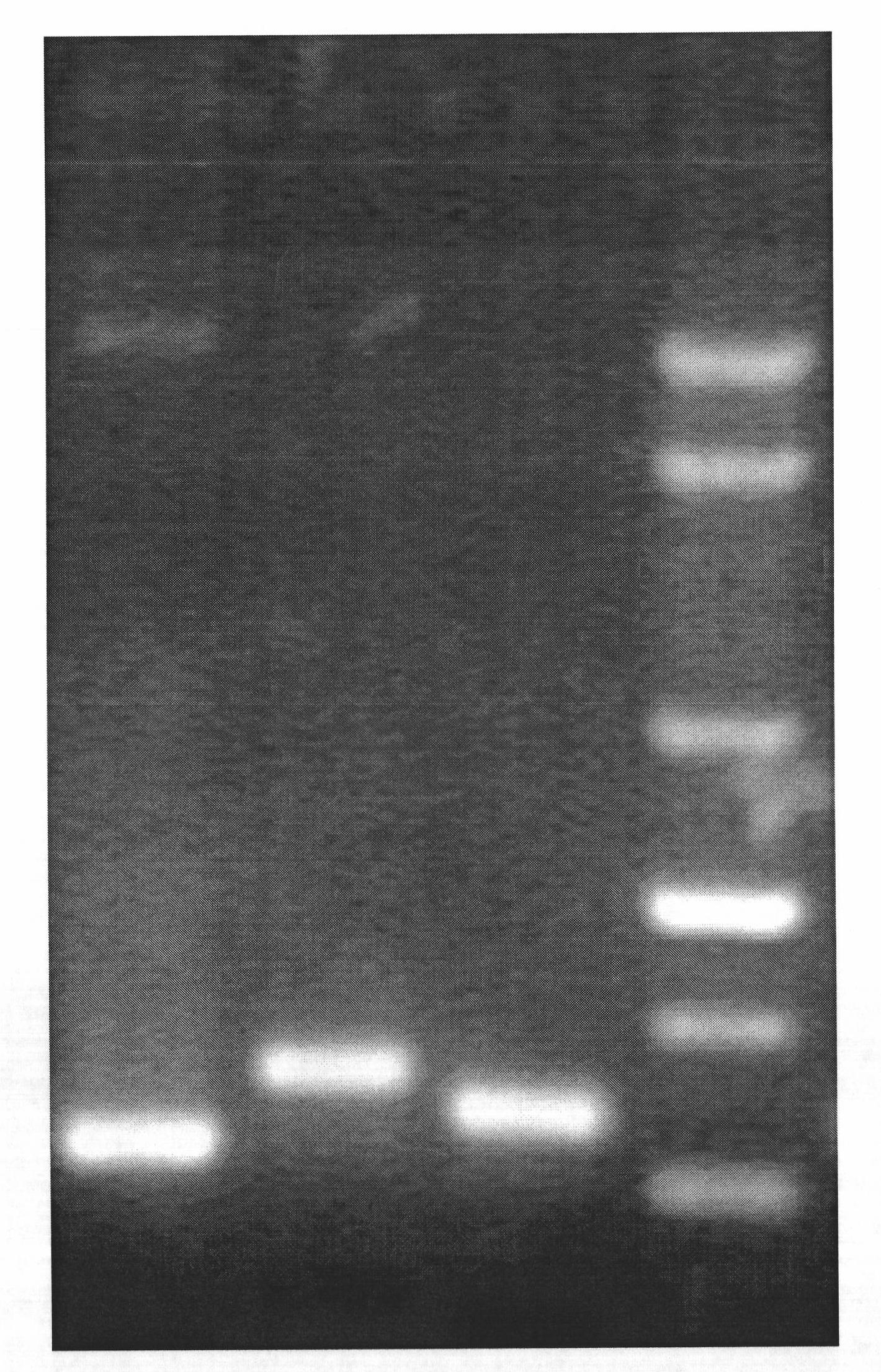
The invention discloses a single-tube multiple PCR method for synchronous rapid detection of five sex propagate pathogenes (neisseria gonorrhoeae (NG), mycoplasma hominis (MH), mycoplasma genitalium (MG), chlamydia trachomatis (CT) and ureaplasma urealyticum (UU)) and a kit. The method is characterized by amplifying the five pathogenes and performing gel electrophoresis separation and detection by reasonably designing a primer and optimizing the primer concentration combination and PCR condition in the same reaction tube and under the same heat cycle condition. The invention has the characteristics of sensitivity, rapidness, convenience, and the like of the classic PCR, mainly realizes synchronous detection of the five pathogenes, has lower cost, can be used for developing relative kits and is used for clinical diagnosis and epidemiological survey and control.
Owner:唐文志
Method of detecting infection with urogenital mycoplasmas in humans and a kit for diagnosing same
A method and a kit are provided for detecting an infection by mycoplasma, particularly an urogenital infection in humans. The presence of specific anti-mycoplasma antibodies in a biological sample ofa diagnosed subject is detected by reaction with an immobilized mixture of various antigenic determinants associated with a variety of pathological states.
Owner:MOR RES APPL LTD
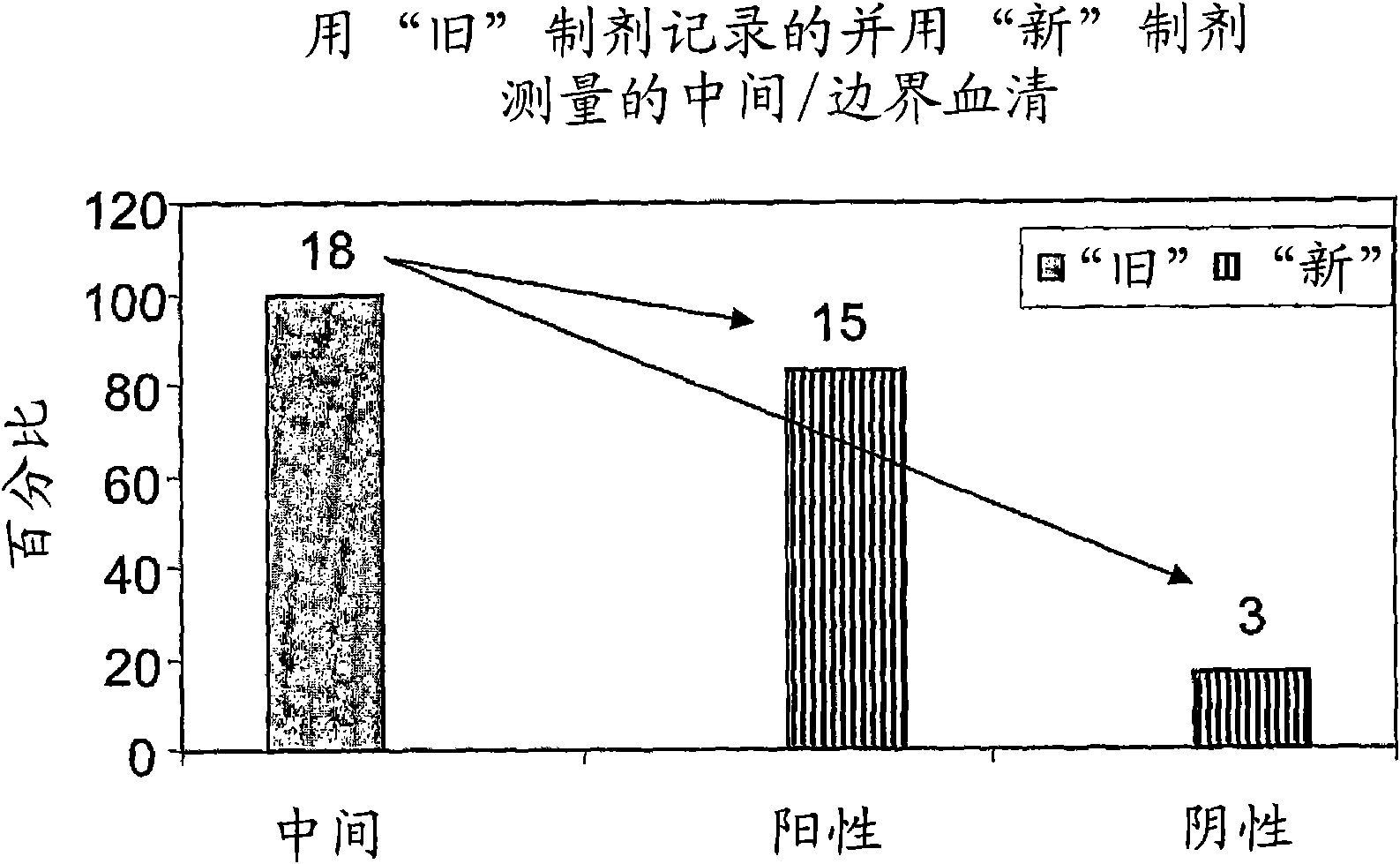
DNA chip, kit for detecting or genotyping bacteria causing sexually transmitted diseases, genotyping antibacterial drug resistance and detecting or genotyping method using the same
InactiveUS20120004113A1Quickly and accurately detectEasy to explainNucleotide librariesMicrobiological testing/measurementDiseaseEscherichia coli
Disclosed are a DNA chip and a kit capable of quickly and accurately detecting or genotyping the highly prevalent and important eleven microbes causing sexually transmitted diseases (STD) Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma genitalium, Mycoplasma hominis, syphilis-causing treponema, pallidum, chancroid-causing Haemophilus ducreyi, genital herpes-causing herpes simplex virus 1 and 2, human papillomavirus (HPV) and Trichomonas vaginalis and three related organisms Candida albicans, Gardnerella vaginalis and coliform bacteria and analyzing antibiotic resistance against tetracycline and lactam antibiotics, and a method for detecting or genotyping using the same. According to the present invention, the presence, genotype and antibiotic resistance of the fourteen organisms can be analyzed quickly and accurately from a DNA sample. With excellent sensitivity, specificity, reproducibility and accuracy of the 14 STD-causing and related microorganisms may be automatically identified quickly and accurately from multiple samples, and selection of antibiotics may be aided.
Owner:GOODGENE
Real-Time Multiplex Detection of Three Bacterial Species Responsible for Sexually Transmitted Diseases
The invention relates to the detection of three different bacterial species which are responsible for sexually-transmitted diseases, i.e., Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG) and Mycoplasma genitalium (MG). The invention more particularly relates to the detection of these three species in real-time PCR, in multiplex PCR and in real-time multiplex PCR. The invention provides reference templates sequences, which are especially adapted to the design of primers and probes, which can be used together in the same tube to detect CT and / or MG and / or NG by real-time multiplex amplification.
Owner:BIO RAD EURO GMBH
Genitourinary tract mycoplasma culture medium and detect method thereof
InactiveCN101555457APromote growthExtended period of timeBacteriaMicrobiological testing/measurementPenicillinArginine
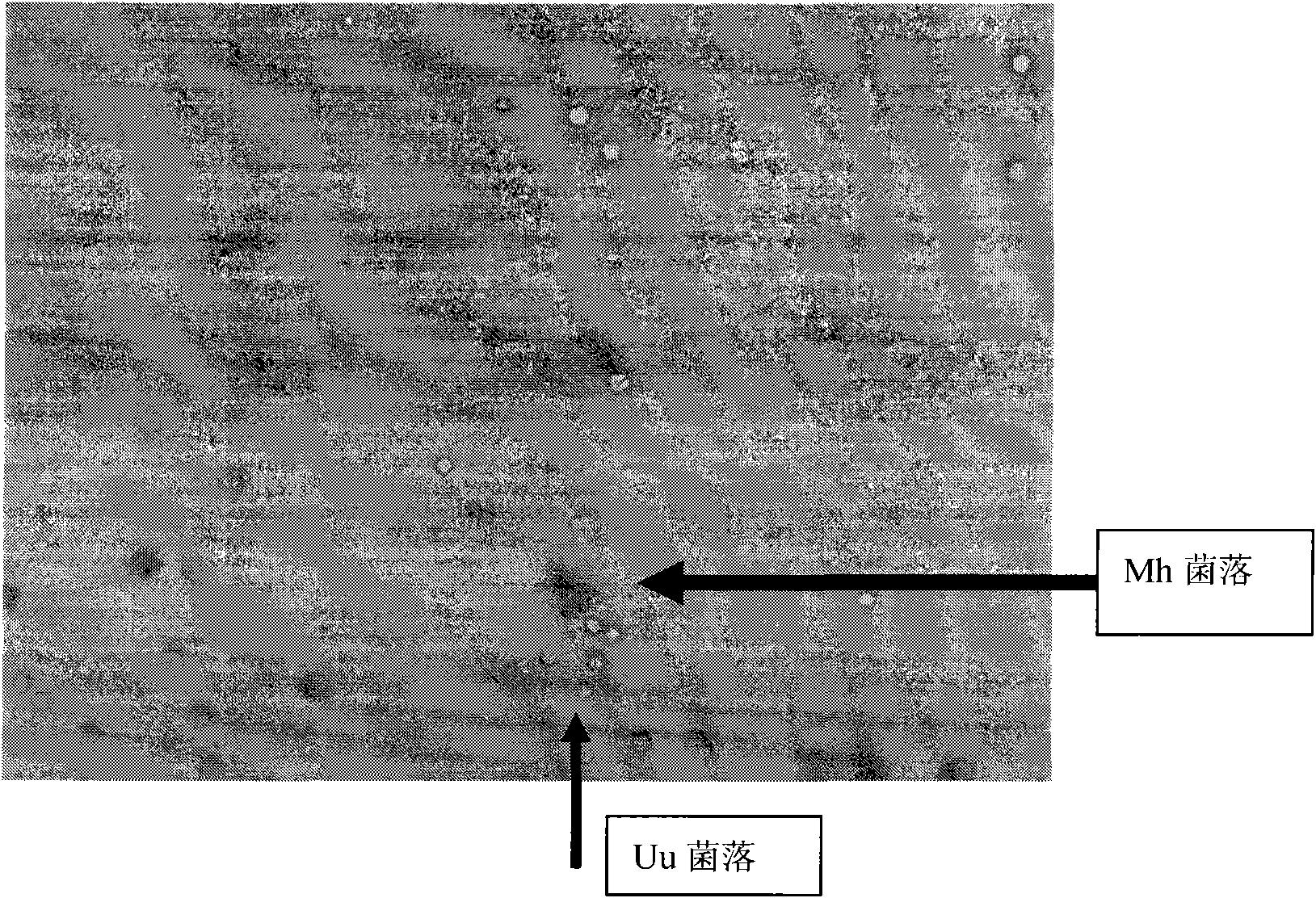
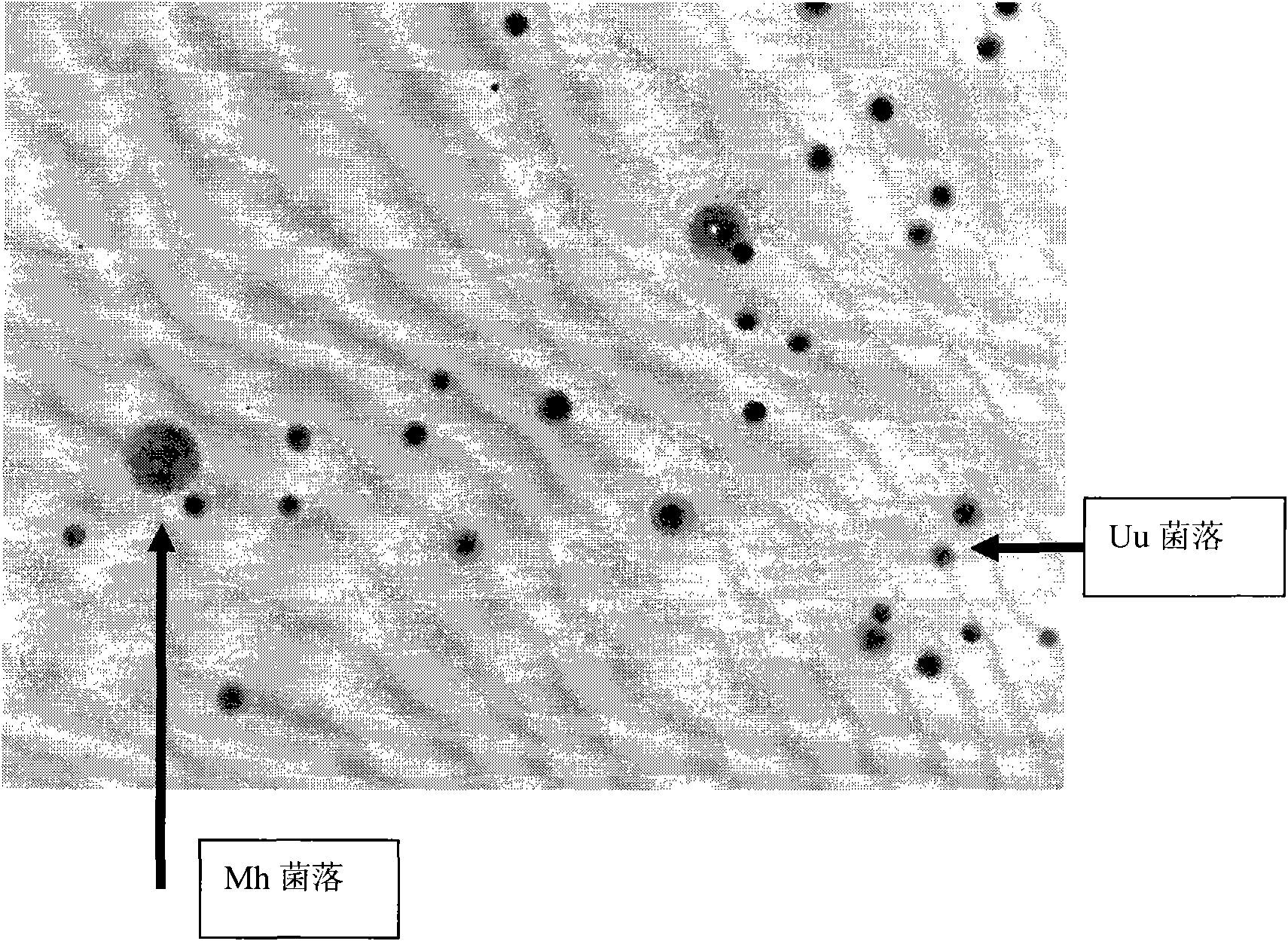
The invention relates to genitourinary tract microorganism cultivation and detect method thereof, in particular to a genitourinary tract ureaplasma urealyticum (Uu) and mycoplasma hominis (Mh) culture medium and a detect method thereof. The culture medium comprises a fluid medium and a solid medium which are combined when the medium is used for culturing, separating and differentiating mycoplasma. The formulation comprises essential elements such as tryptic soy broth, yeast extract, urea, arginine, L-cysteine, phenol red, HEPES liquid, calf (fetal calf) serum, manganese sulfate, vancomycin, amphotericin B, trimethoprim, polymyxin, penicillin, and the like, and thereby the nutrition for mycoplasma growth and the antibiotic combination for inhibiting undesired microbe growth are optimized, no millipore filter is needed for sample inoculation and subcultivation, and the mycoplasma grows quickly. The fluid medium can maintain and prolong the logarithmic phase and indicate the growth of the mycoplasma, and the solid medium can used for observing Uu and Mh bacterial colonies, which has large background reflectance, thereby being easy for differentiation and significantly increasing the sensibility and the specificity of genitourinary tract mycoplasma detect.
Owner:上海市皮肤病性病医院
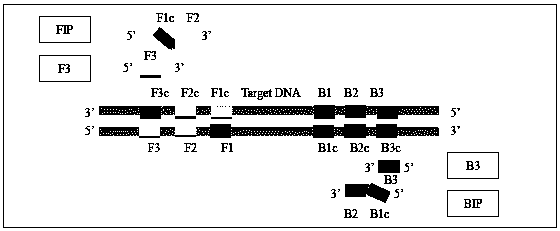
Method for detecting three kinds of urogenital canal mycoplasma by loop-mediated isothermal amplification technology
InactiveCN107686863AStrong specificityEfficient amplificationMicrobiological testing/measurementMicroorganism based processesConserved sequenceFluorescence
The invention discloses an LAMP detection method for three kinds of urogenital canal infection mycoplasma which are common for human and a dedicated primer and a kit thereof. The method is applied toperforming isothermal amplification on ureaplasma urealyticum (UU), mycoplasma genitalium (MG) and mycoplasma hominis (MH). The LAMP primer is designed according to specific conserved sequences of theUU, the MG and the MH, and each group of the primer includes four piece of oligonucleotide (shown in a table 1, a table 2 and a table 3 in the description). When being applied to the urogenital canalmycoplasma, the LAMP primer present white precipitate when being observed by the naked eyes; in positive reaction, after SYBR GREEN is added, fluorescent green is remarkably enhanced when being observed under an ultraviolet lamp. Detection results of a real-time turbidity meter show that product turbidity can be increased along with prolonging reaction time; after being detected by gel electrophoresis, the LAMP primer is in a trapezoid stripe. The LAMP detection method disclosed by the invention provides a novel technological platform for mycoplasma detection; a sample to be measured can be applied to DNA extracted by commercialized kits or purified DNA and is also suitable for coarse extracted DNA with a boiling method as a representative; the LAMP detection method is suitable for beingpopularized and applied in grassroots units, field monitoring and bedside detection.
Owner:TIANJIN MEDICAL UNIV
Mycoplasma nucleic acid isothermal amplification method
The invention discloses a mycoplasma nucleic acid isothermal amplification method. In particular, the mycoplasma nucleic acid isothermal amplification method includes the step that amplification is carried out in a reaction system, wherein the reaction system comprises specific primer pairs for amplifying mycoplasmas, and primers can be used for amplifying amplification products corresponding to mycoplasma feature sequences from a detection sample with an extra-low mycoplasma copy number. By means of the mycoplasma nucleic acid isothermal amplification method, the to-be-detected sample containing mycoplasma RNA can be rapidly amplified with high specificity, high flexibility and low pollution; and the mycoplasma nucleic acid isothermal amplification method is particularly suitable for amplification and detection of the to-be-detected sample containing mycoplasma genitalium MG and / or mycoplasma pneumoniae MP. The mycoplasma nucleic acid isothermal amplification method has the beneficial effects of being high in detection efficiency and high in accuracy.
Owner:SHANGHAI RENDU BIOTECH
Composition and kit for simultaneously detecting mycoplasma urealytium, mycoplasma hominis and mycoplasma genitalium
ActiveCN101824471AImprove early detection rateSave operating timeMicrobiological testing/measurementMicroorganism based processesDiseaseNucleotide
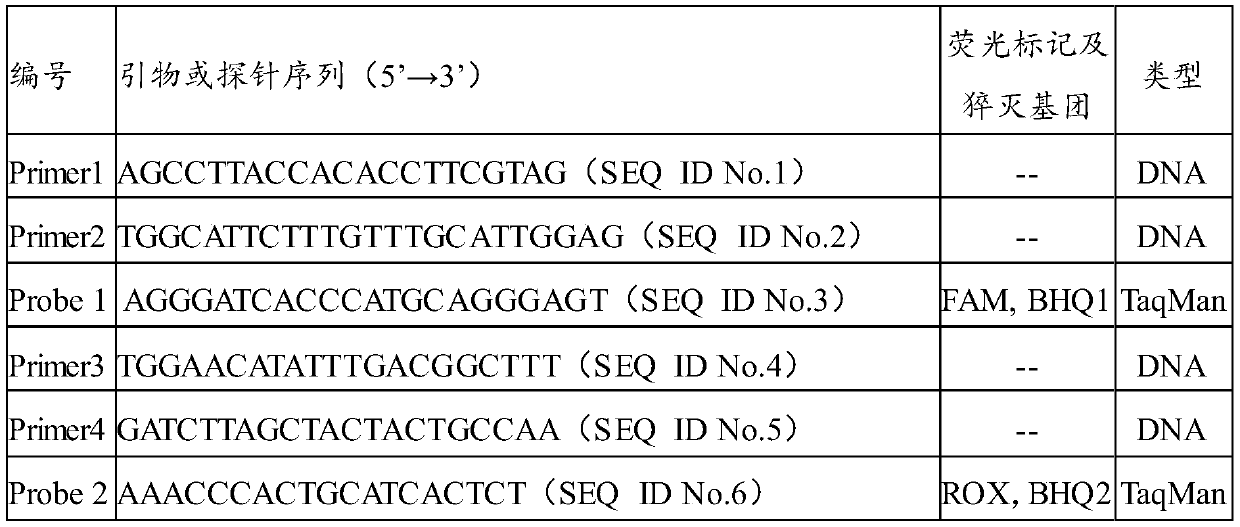
The invention discloses a composition and a kit for simultaneously detecting mycoplasma urealytium, mycoplasma hominis and mycoplasma genitalium, which comprise sequences shown by relevant SEQ ID No:1-6 or at least 15 nucleotide continuous fragments thereof, or the specific primers of sequences which have at most 5 nucleotide differences with the sequences shown by relevant SEQ ID No:1-6 or continuous fragments and sequences shown by SEQ ID No:7-9 or complementary sequences thereof or at least 15 nucleotide continuous fragments thereof or the specific probes of sequences which have at most 5 nucleotide differences with the. sequences shown by SEQ ID No:7-9 or the complementary sequences thereof or the 15 nucleotide continuous fragments The composition and the kit for simultaneously detecting the mycoplasma urealytium, the mycoplasma hominis and the mycoplasma genitalium can simultaneously detect three different mycoplasmas in one reaction system, and thereby, the early detection rate of the infectious diseases of urogenital systems is increased, the operation time of operation staffs is reduced, the detection cost is reduced and the financial burdens are relieved for patients.
Owner:北京鑫诺美迪基因检测技术有限公司
Fluorescent PCR (polymerase chain reaction) primer, probe and detecting kit for detecting mycoplasmas genitalium
ActiveCN108410957AHigh sensitivityHigh detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationRepetitive SequencesNucleotide
The invention provides a fluorescent PCR primer, probe and detecting kit for detecting mycoplasmas genitalium. According to the fluorescent PCR primer, probe and detecting kit for detecting the mycoplasmas genitalium, by comparing the genomes of all known mycoplasmas genitalium in the prior art, a multicopy repetitive sequence gene with the sequence shown as SEQ ID NO.1 is screened out, and the real-time fluorescent PCR primer and a probe of the multicopy repetitive sequence gene, which separately have nucleotide sequences shown as SEQ ID NO.2, SEQ ID NO.3 and SEQ ID NO.4, are designed. The detecting kit can reach a mycoplasma genitalium detecting sensitivity as low as 0.5 copies, have no specific amplification and present good specificity for 10 other common mycoplasmas and 7 common mycoplasmas genitalium, and have the advantages of being accurate in detection, high in sensitivity and specificity, simple and rapid, thereby achieving high specimen detecting ability.
Owner:西安博睿康宁生物医学中心有限公司
Compositions and methods for detection of mycoplasma genitalium
ActiveUS20170342468A1Effective transcriptionIncrease inclusivenessMicrobiological testing/measurementMycoplasmaGene
Methods for the rapid detection of the presence or absence of Mycoplasma genitalium (MG) in a biological or non-biological sample are described. The methods can include performing an amplifying step, a hybridizing step, and a detecting step. Furthermore, primers, probes targeting the target MG gene, along with kits are provided that are designed for the detection of MG.
Owner:ROCHE MOLECULAR SYST INC
Isolation medium of genitourinary tract mycoplasma Un and Mh and application of Un and Mh isolation medium
ActiveCN104694425AImprove performanceHigh selectivityBacteriaMicroorganism based processesArginineMycoplasma
The invention relates to an isolation medium of genitourinary tract mycoplasma Un and Mh and application of the isolation medium. Each 1,000ml of culture medium contains the following components: 15g of peptone, 15g of tryptone, 5g of sodium chloride, 5g of urea, 5g of arginine, 10ml of 0.4% phenol red, 50ml of bovine serum, 50-150ml of DMEM, 5-15ml of self-made biological factor and pure water added to a constant volume of 1,000ml. Compared with the prior art, according to the culture medium disclosed by the invention, due to the addition of an additive, the performance and the selectivity of the culture medium are improved; the nutrients are relatively abundant; the growth and reproduction requirements of Uu and Mh are easily met; and the result is observed and reported a day earlier in comparison with a traditional culture medium in general condition.
Owner:众爱生河北生物科技有限公司
Method for detecting microorganisms belonging to mycoplasma pneumoniae and/or mycoplasma genitalium
ActiveUS20120244544A1Rapidly and specifically diagnosedSugar derivativesMicrobiological testing/measurementMicroorganismMycoplasma
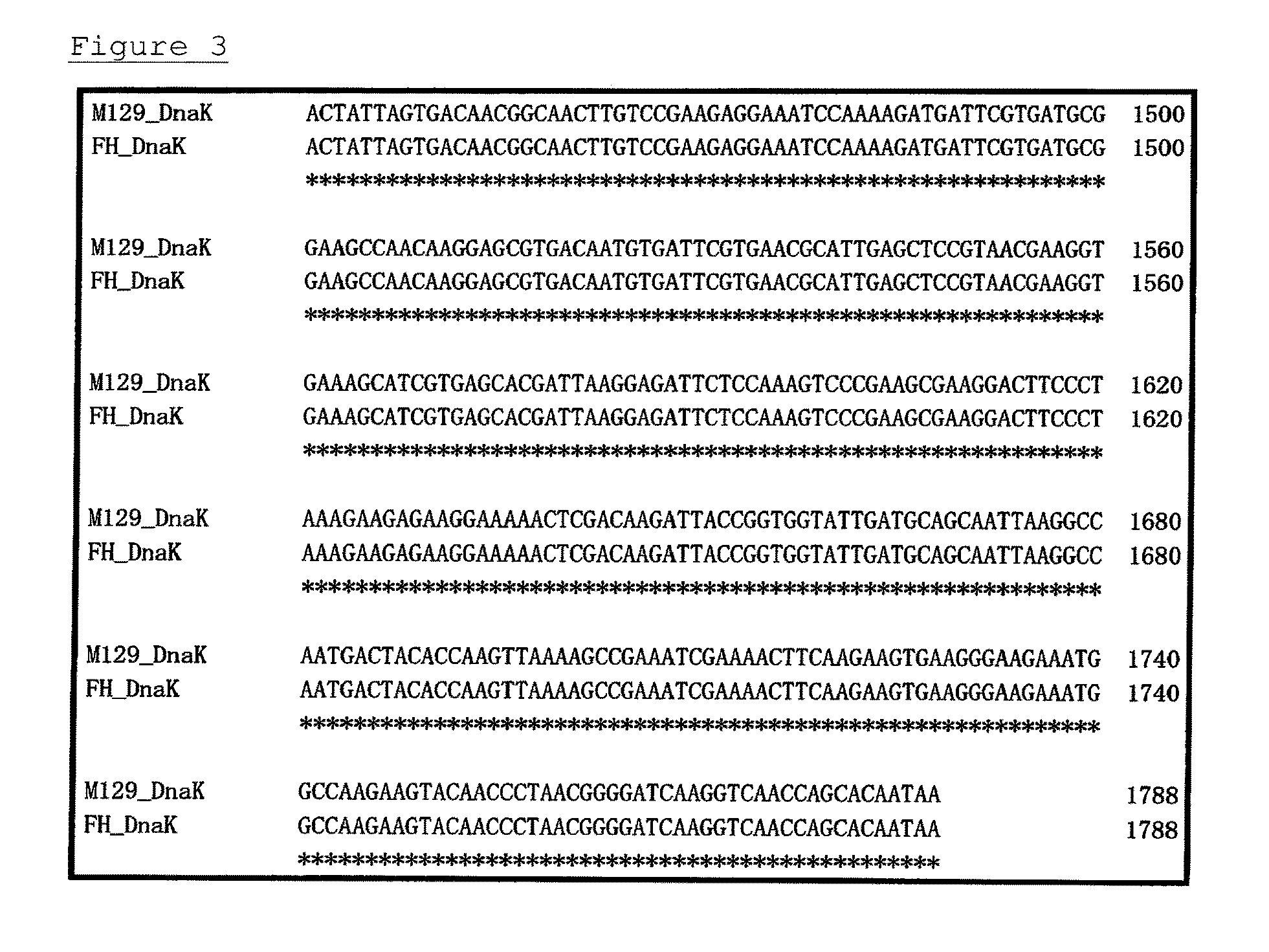
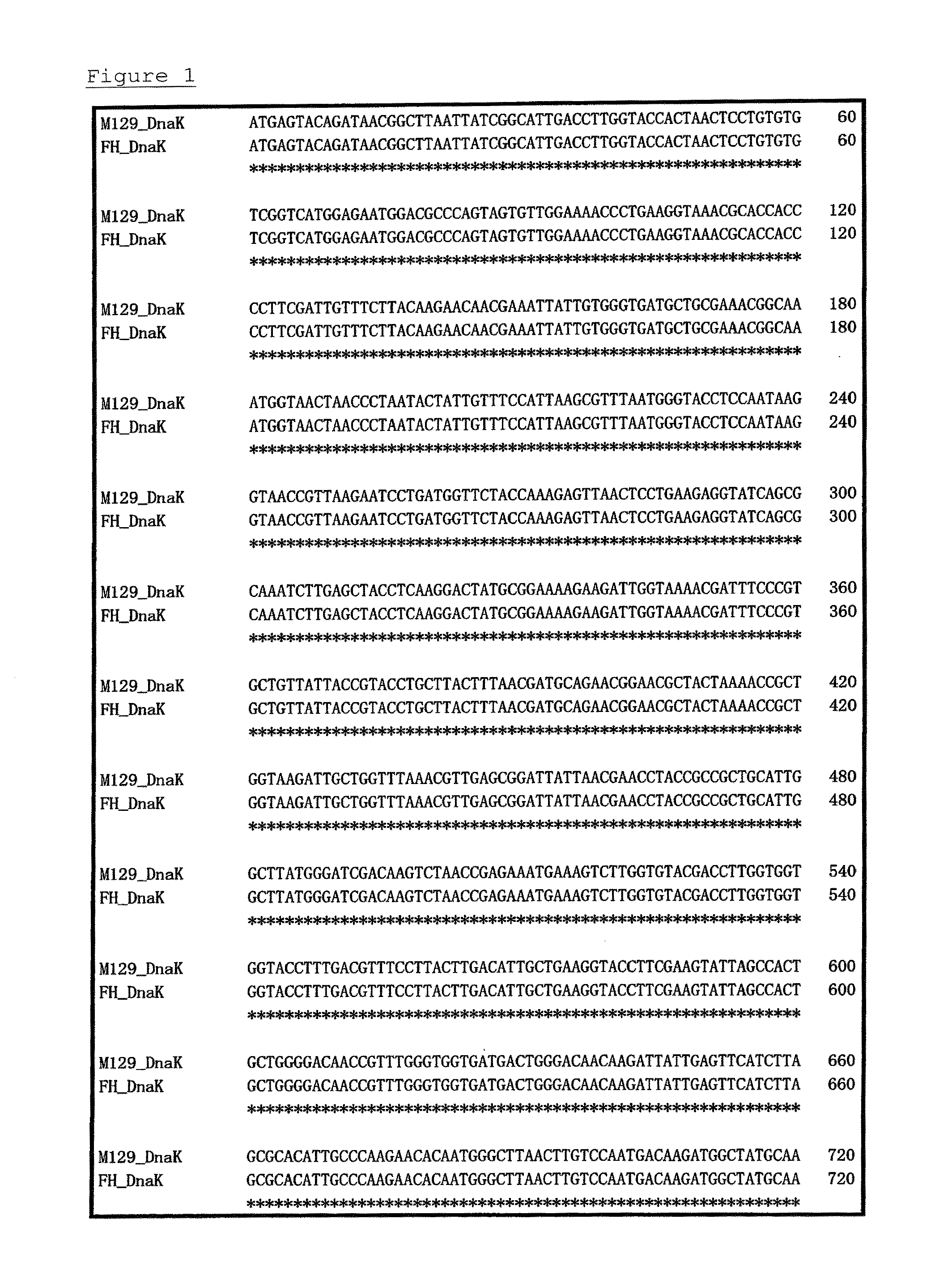
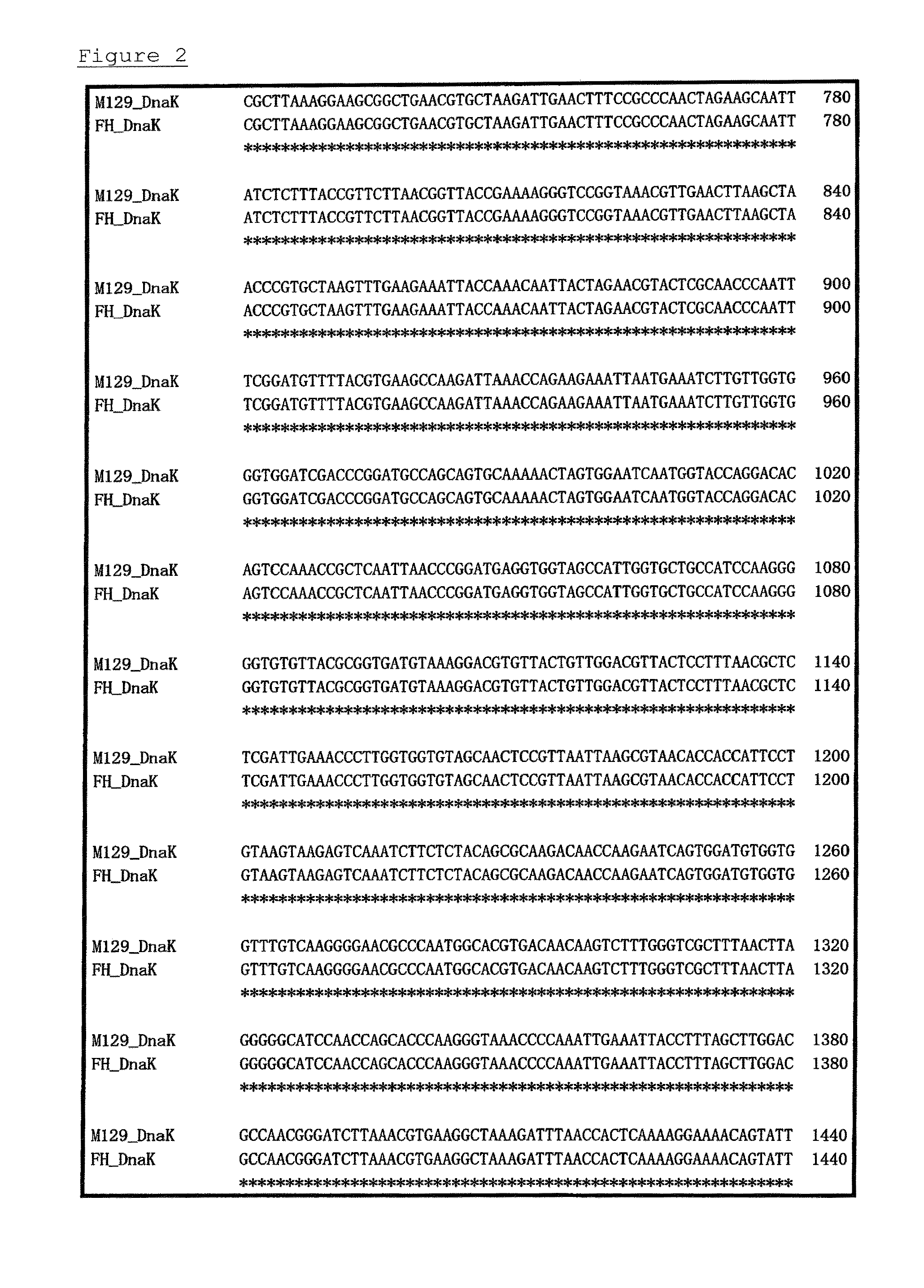
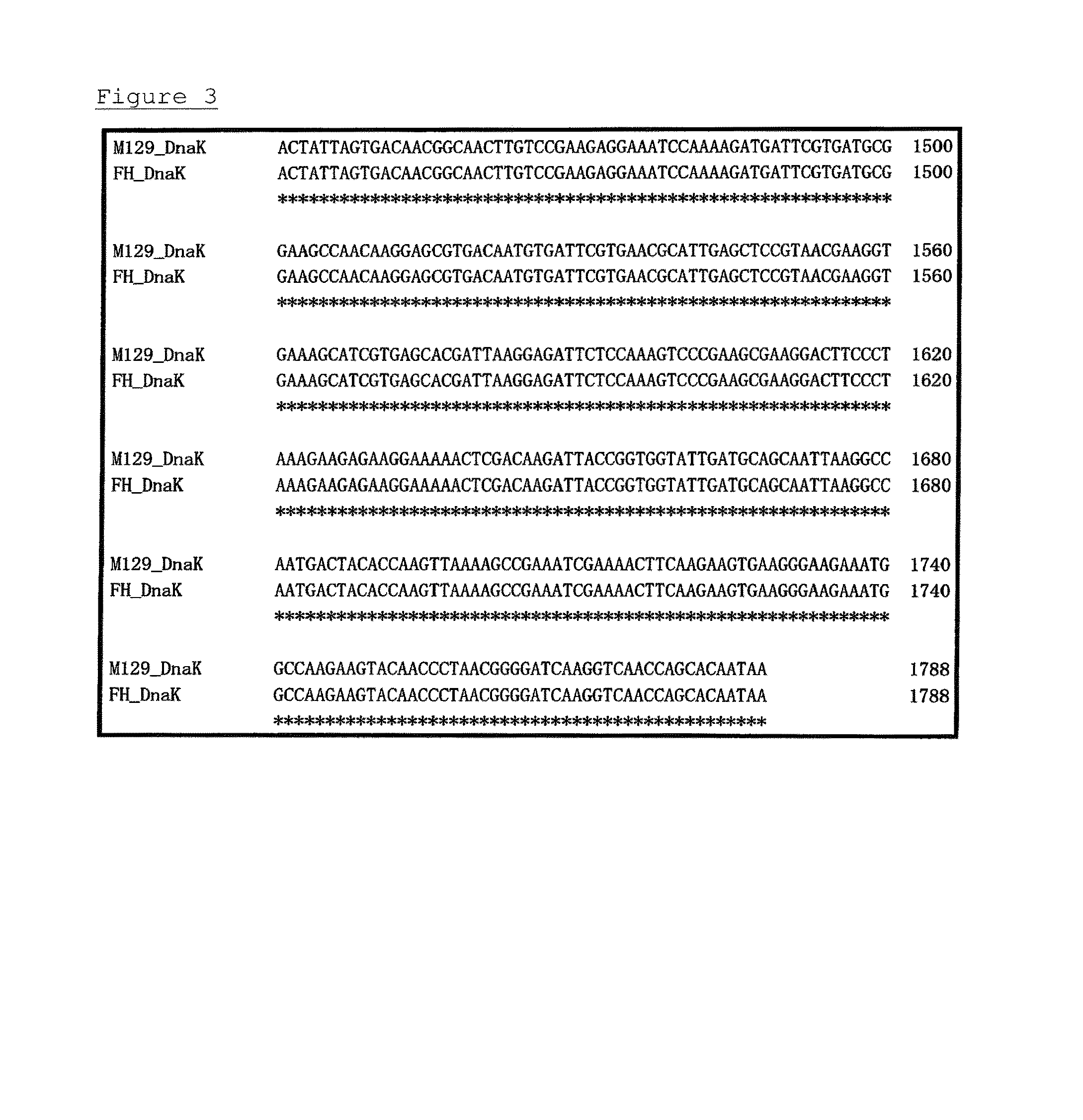
A detection method and a detection kit for rapidly and specifically diagnosing Mycoplasma pneumoniae and / or Mycoplasma genitalium infections are provided. The DnaK of Mycoplasma pneumoniae or Mycoplasma genitalium is used as an indicator.
Owner:MITSUBISHI CHEM MEDIENCE
Method for rapidly detecting urogenital tract mycoplasmas based on RPA
InactiveCN111763752AQuick checkSensitive detectionMicrobiological testing/measurementMicroorganism based processesMedicineMicrobiology
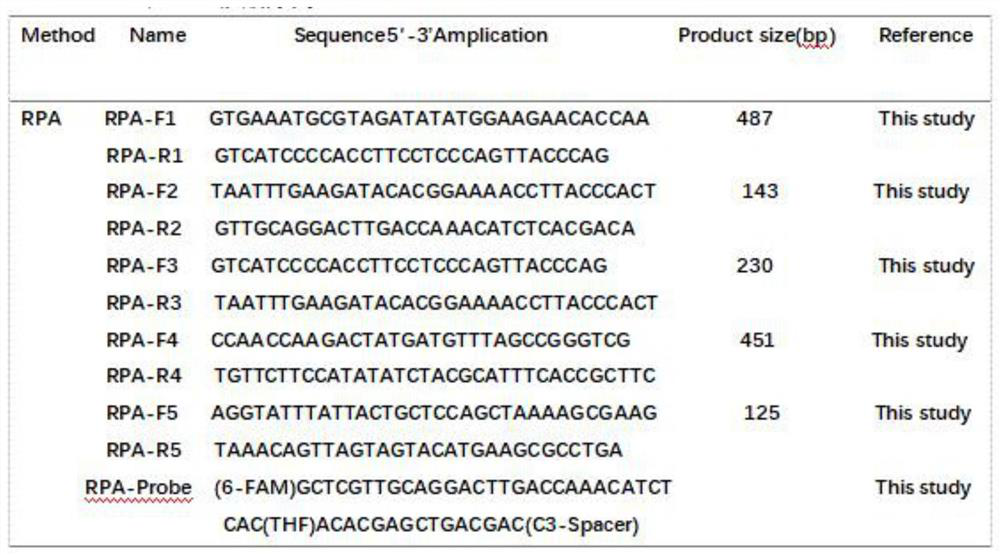
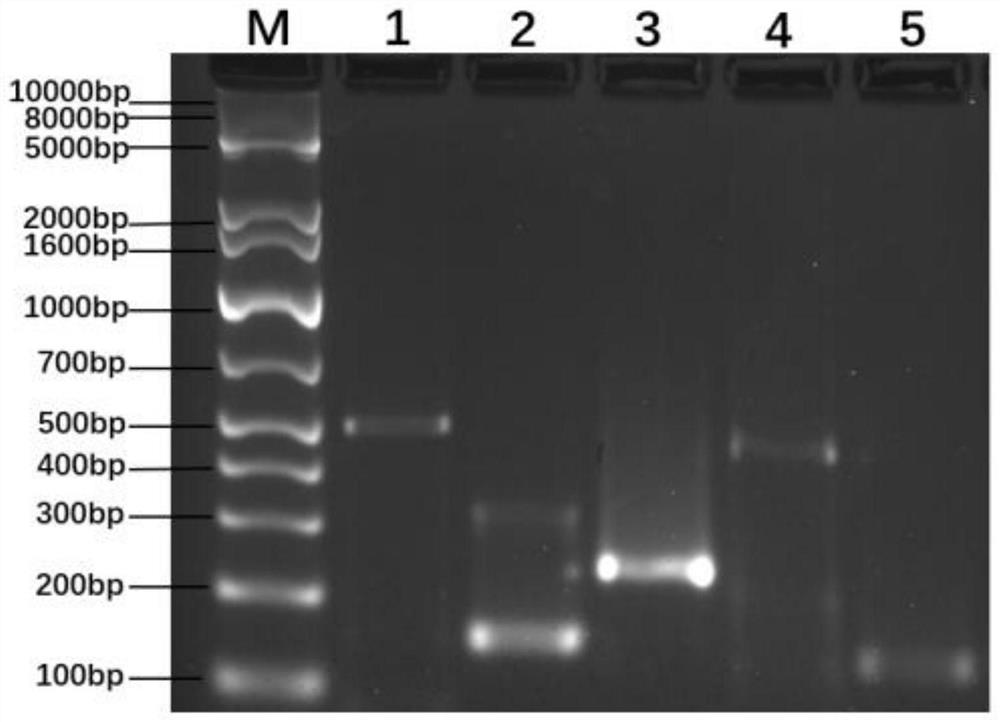
The invention discloses a method for rapidly detecting urogenital tract mycoplasmas based on RPA in the technical field of mycoplasma. The method for rapidly detecting urogenital tract mycoplasmas based on RPA comprises the following steps: S1, designing and screening primers and probes: according to a 16SrRNA gene and a gap gene of MH, strictly designing five pairs of primers by using an oligo 5software according to an amplification kit analysis and design manual and performing bLAST verification. The kit is used for screening the optimal RPA primer. According to the present invention, the same MH positive specimen is subjected to RPA amplification with the five pairs of primers respectively, and each 50 [mu]l of a reaction system contains 29.5 [mu]l of a primer-free rehydration buffer solution. The method can detect mycoplasmas rapidly, sensitively and immediately, and can effectively perform mycoplasma screening on women and males of the right age.
Owner:台州市中心医院
Urogenital tract infection pathogen multi-detection primer group and detection device comprising same
ActiveCN108531623ASimplify the experimental operation processHigh detection throughputBioreactor/fermenter combinationsBiological substance pretreatmentsSingle sampleMycoplasma hominis
Owner:无锡科智达科技有限公司
Primers, probe and kit for detecting mycoplasma genitalium and detection method
InactiveCN110894534AHigh sensitivityShort detection timeMicrobiological testing/measurementMicroorganism based processesNucleotideGlobin genes
The invention relates to the technical field of medical detection, in particular to primers for detecting mycoplasma genitalium, a probe for detecting mycoplasma genitalium, a kit for detecting mycoplasma genitalium and a detection method. The primers and the probe comprise a primer pair of nucleotide sequences shown in SEQ ID NO:1-2 as shown in the description and a probe of a nucleotide sequenceshown in SEQ ID NO:3 as shown in the description. The provided primers and probe can specifically bound to a mycoplasma genitalium outer membrane protein B gene and a human beta-globin gene, the detection sensitivity and specificity are significantly improved, the positive rate of low-value samples reaches 100%, and detection results are accurate and reliable so that the primers, probe and kit for detecting mycoplasma genitalium and the detection method can be applied to clinical detection of mycoplasma genitalium.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Culture medium for the isolation of clinical samples of species such as mycoplasma including those which are not culturable in traditional media
ActiveUS20180080005A1Effectively and quickly to cultivateSatisfies needBacteriaMicrobiological testing/measurementMicroorganismMycoplasma penetrans
The present invention relates to a culture medium specific for microorganisms of Mycoplasma genus and, in particular, for species of hardly cultivable Mycoplasma (Mycoplasma genitalium, Mycoplasma pneumoniae, Mycoplasma penetrans, Mycoplasma fermentans, Mycoplasma pirium and / or Mycoplasma hyorhinis). The present invention also provides an in vitro method for the growth and / or isolation and / or identification of species belonging to the Mycoplasma genus as well as growth supports using such culture medium.
Owner:C P M DI CLAUDIO PIERMATTEI E C SAS
Method for detecting microorganisms belonging to Mycoplasma pneumoniae and/or Mycoplasma genitalium
ActiveUS8940496B2Rapidly and specifically diagnosedMicrobiological testing/measurementImmunoglobulins against bacteriaMycoplasmaMycoplasma pneumoniae
Owner:MITSUBISHI CHEM MEDIENCE
Method for detecting genital tract mycoplasmas based on loop-mediated isothermal amplification and micro-fluidic chip
PendingCN111304346AHigh sensitivityImprove featuresMicrobiological testing/measurementMicroorganism based processesFluorochrome DyeBiomedical engineering
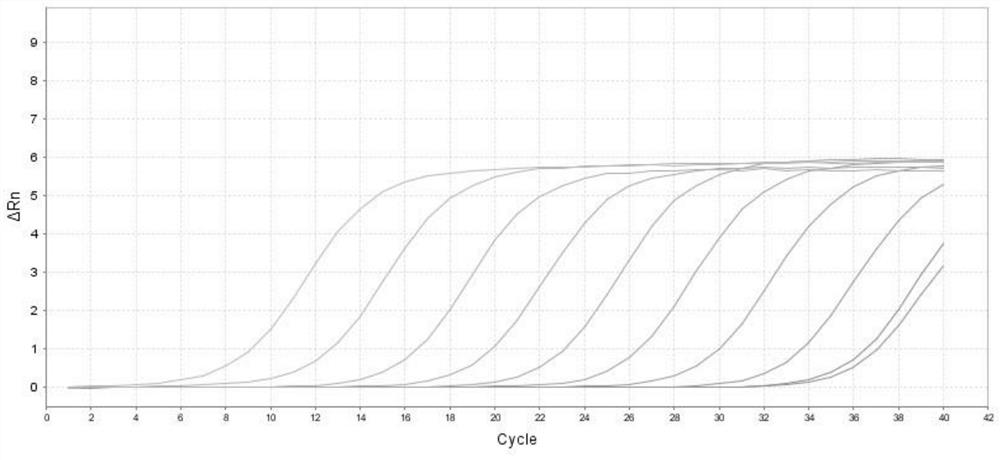
The invention discloses a method for detecting genital tract mycoplasmas based on loop-mediated isothermal amplification and a micro-fluidic chip and belongs to the technical field of pathogen detection and diagnosis. The method comprises the following steps: performing primer designing; manufacturing the micro-fluidic chip; preparing an isothermal amplification reaction system; supplying a sampleto the chip; and performing nucleic acid amplification, and judging and reading results. An isothermal amplification technique and a micro-fluidic chip technique are used in the method, polymerase with a chain replacement function is reacted under an isothermal condition of 63 DEG C, real-time fluorescence detection is implemented by using a fluorescence dye mixing method, and a sample with amplification positive can generate an ''S''-shaped amplification curve similar to real-time fluorescence. The micro-fluidic chip successfully prepared for four micro-fluidic chips has good sensitivity, specificity and accuracy, and the whole detection process can be completed within 1 hour. The minimum detection limits of Uu, Mg and Mh are 1000 copies / [mu] L, the minimum detection limit of Up is 5000copies / [mu] L, and the method has wide application prospects in clinical use.
Owner:重庆市第四人民医院
Quintuple fluorescent PCR (polymerase chain reaction) quick and hypersensitive detection kit and application thereof
ActiveCN102888464BGuaranteed accuracyGuaranteed reliabilityMicrobiological testing/measurementFluorescence/phosphorescenceMycoplasma hominisFluorescent pcr
The invention relates to a real-time PCR (polymerase chain reaction) method for quintuply detecting target nucleic acid in a nucleic acid extracting solution in a single PCR reaction vessel, which is used for detecting Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis and Mycoplasma genitalium in a sample.
Owner:苏州华益美生物科技有限公司
Genital tract pathogen nucleic acid detection kit
InactiveCN109457037AImprove stabilityReduce inconvenienceMicrobiological testing/measurementMicroorganism based processesFreeze-dryingMycoplasma hominis
The invention discloses a genital tract pathogen nucleic acid detection kit which comprises a first detection component, a second detection component and a third detection component which are freeze-dried and simultaneously perform fluorescent quantitative PCR (polymerase chain reaction) detection after detection samples are added. The first detection component is used for detecting Chlamydia trachomatis and Neisseria gonorrhoeae, the second detection component is used for detecting mycoplasma urealytium and mycoplasma hominis, and the third detection component is used for detecting mycoplasmagenitalium. The kit can simultaneously detect five nucleic acids such as the Chlamydia trachomatis, the Neisseria gonorrhoeae, the mycoplasma urealytium, the mycoplasma hominis and the mycoplasma genitalium in a genital tract, detection of five pathogenic microorganisms is integrated into a consumable, reagent stability is improved by the aid of a freeze-drying process, the kit is disposable whenbeing used by each user, only single sampling and one-step operation are needed, laboratory operators are greatly free, detection cost is reduced, inconvenience of a patient is decreased, and the financial burden of the patient is relieved.
Owner:MERLIN BIOMEDICAL (XIAMEN) CO LTD
Mycoplasma genitalium nucleic acid real-time fluorescence PCR detection primer, probe and kit
InactiveCN113186316ASensitive and accurate detectionGood repeatabilityMicrobiological testing/measurementDNA/RNA fragmentationNucleic acid detectionRNase P
The invention discloses a mycoplasma genitalium nucleic acid real-time fluorescence PCR detection primer, a probe and a kit. The kit comprises an amplification reaction solution, a mycoplasma genitalium nucleic acid detection solution, a positive reference substance and a negative reference substance, wherein the mycoplasma genitalium nucleic acid detection liquid comprises specific primers and probes aiming at mycoplasma genitalium and a human RNase P gene, the sequences of the primers aiming at the mycoplasma genitalium are as shown in SEQ ID NO.1 and SEQ ID NO.2, and the sequence of the probe aiming at the mycoplasma genitalium is as shown in SEQ ID NO.3. According to the primer, the probe and the kit for real-time fluorescence PCR detection of the mycoplasma genitalium, provided by the invention, the mycoplasma genitalium can be rapidly, accurately and sensitively detected, the repeatability of an experimental result is good, the precision is high, the detection time period is short, and the detection can be completed within 70 minutes as short as possible. Therefore, the detection time is greatly saved, and the clinical diagnosis efficiency can be accelerated.
Owner:北京华诺奥美基因生物科技有限公司
Nucleic acid group, kit and detection method for intrauterine microbiological detection of pregnant and lying-in women
PendingCN110904269AThe detection process is fastHigh sensitivityMicrobiological testing/measurementMicroorganism based processesPhysiologyUterus
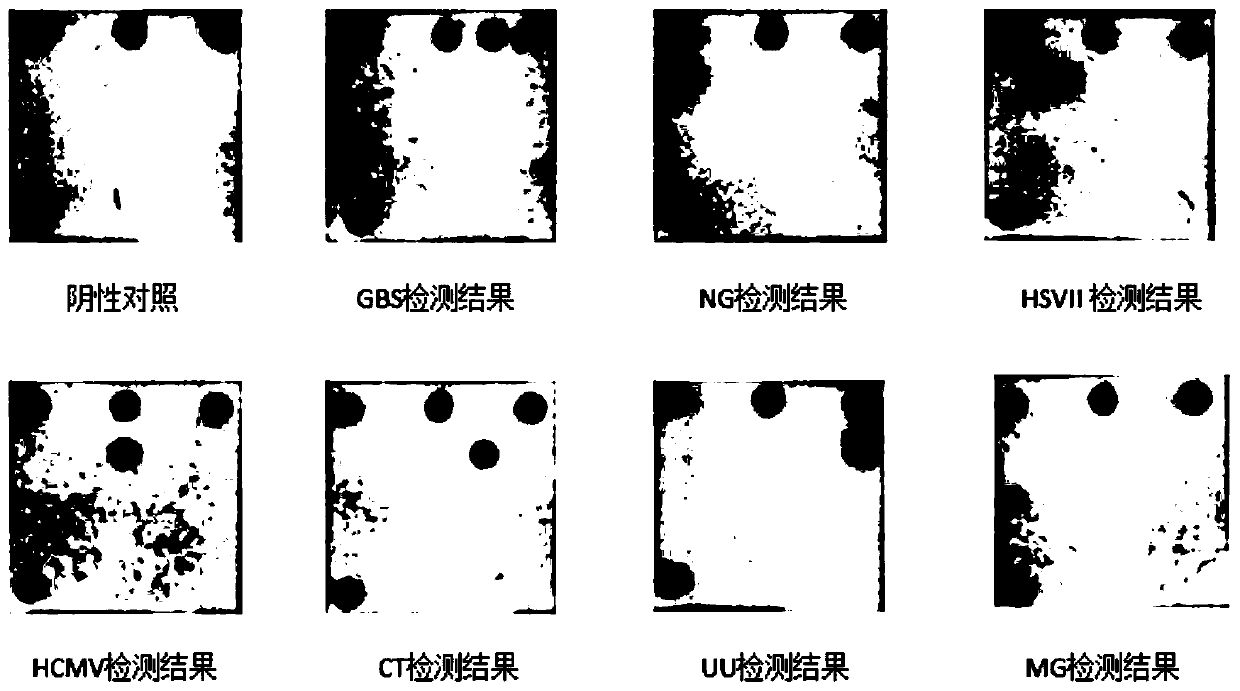
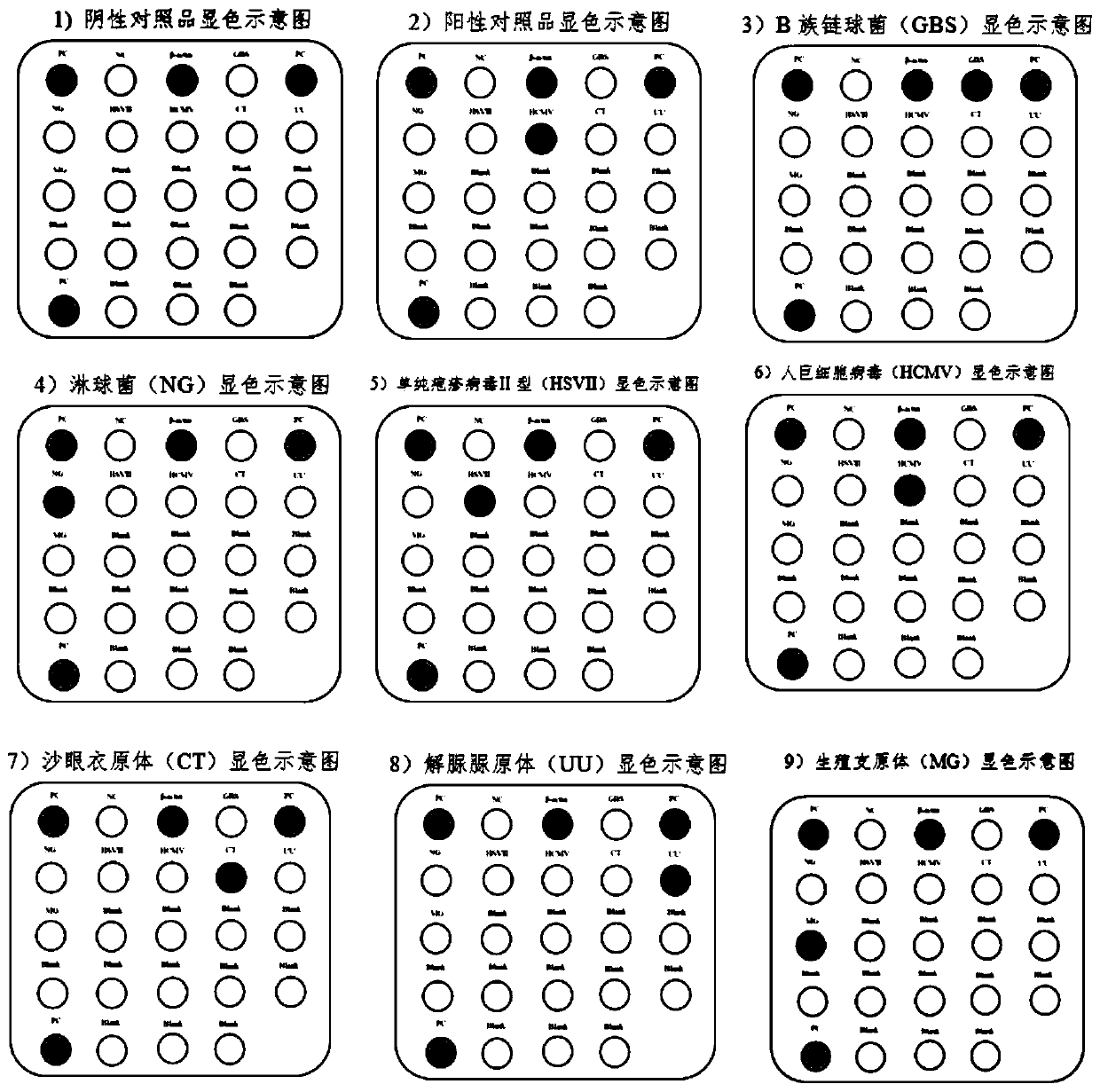
The invention discloses a nucleic acid group, a kit and a detection method for intrauterine microbiological detection of pregnant and lying-in women, and relates to the technical field of membrane chip detection. The nucleic acid group disclosed by the invention comprises at least one from a first primer pair, to a seventh primer pair; the sequences from the first primer pair to the seventh primerpair are shown as SEQ ID NO.3-16. The nucleic acid group can be used for simultaneously detecting pathogenic microorganisms such as group B streptococcus, gonococcus, herpes simplex virus II, human cytomegalovirus, chlamydia trachomatis, ureaplasma urealyticum, mycoplasma genitalium and the like infected in uterus of pregnant and lying-in women; when the nucleic acid group and the detection method provided by the invention are used for detection, the characteristics of high detection speed, high sensitivity, good specificity, high flux, convenience in operation and the like are achieved.
Owner:SICHUAN HUAHAN TRIO BIOTECH CO LTD
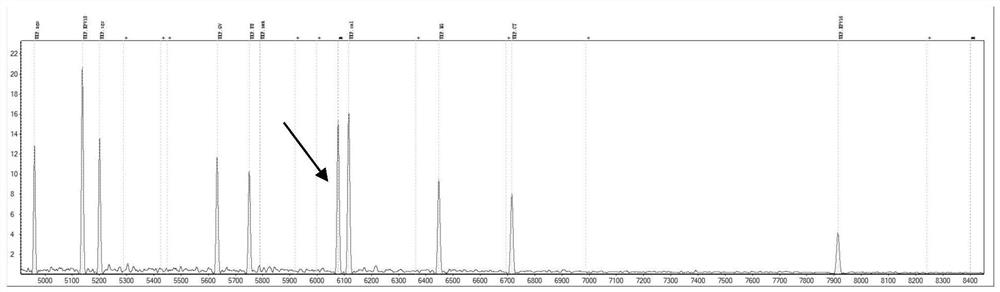
Primer composition for detecting genital tract pathogens through MALDI-TOF MS and application
ActiveCN113215322AHigh biological valueHigh detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationStaphyloccocus aureusChlamydiae
The invention provides a primer group for detecting specific typing sites of eight genital tract pathogenic bacteria and two human papilloma viruses by a time of flight mass spectrometry method, a product and a non-diagnostic detection method. The genital tract pathogenic bacteria comprise staphylococcus aureus, group B streptococcus, neisseria gonorrhoeae, gardnerella vaginalis, candida albicans, mycoplasma genitalium, mycoplasma urealyticum, chlamydia trachomatis, and HPV16 and HPV18 virus types. The invention provides a novel method for detecting 10 kinds of genital tract pathogens by combining multiple PCR with clinical mass spectrometry for the first time, integrates technologies of multiple PCR, single base extension, mass spectrometry detection and the like, can amplify a detection template through the PCR technology, can detect a trace sample through the mass spectrometry technology, integrates the advantages of the two technologies, is much superior to the method for detecting specific fragments of pathogens by singly using PCR, and has higher detection sensitivity.
Owner:BIOYONG TECH +1
Detection product for detecting genital tract pathogens by MALDI-TOF MS and application
ActiveCN113215321AHigh biological valueHigh detection sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesType specificReproductive tract
The invention provides a primer group, a product and a non-diagnostic detection method for detecting typing specific sites of 8 genital tract pathogenic bacteria and 2 human papilloma viruses by a time-of-flight mass spectrometry method. The genital tract pathogenic bacteria comprise staphylococcus aureus, group B streptococcus, neisseria gonorrhoeae, gardnerella vaginalis, candida albicans, mycoplasma genitalium, mycoplasma urealyticum, chlamydia trachomatis, HPV16 virus types and HPV18 virus types. A novel method for detecting 10 genital tract pathogens by clinical mass spectrometry combined with multiple PCR is firstly provided, the multiple PCR, single base extension, mass spectrometry detection and other technologies are integrated into a whole, a detection template is amplified through the PCR technology, a trace sample is detected through the mass spectrometry, and the advantages of the two technologies are synthesized. The method is far superior to that for detecting pathogen specific fragments by singly using PCR, and has higher detection sensitivity.
Owner:BIOYONG TECH +1
Quick detection test paper for ureaplasma urealyticum (uu) neutralization
InactiveCN101566620AFull of nutritionEasy to prepareMicrobiological testing/measurementBiological testingNutritionFiltration
The invention relates to a quick detection test paper for ureaplasma urealyticum (uu) neutralization, in particular to a quick detection test paper used for uu neutralization after a large amount of clinical antibiotics is used and a preparation method thereof. The quick detection test paper for neutralization can detect out the positive result of genital tract mycoplasma after the large amount of clinical antibiotics is used, thereby having the function of the clinical diagnosis guidance and the clinical treatmen and the significant meaning of preventing the abuse of antibacterial drugs is. The quick detection test paper for uu neutralization is rich in nutrition and easy to prepare, has satisfied clinical application effect, solves the difficulty of bedside inoculation, is less polluted, has simple detection method, short time, strong selectivity, sensitive color change, high detection rate and good stability, can obtain a liquid for filtration enrichment and achieve the effects of permanently observing the growth condition of a colony after a sample is inoculated for 24 hours and can practically and reliably neutralize the corresponding clinical antibiotics; the products of reaction of the quick detection test paper for uu neutralization and the antibiotics do not sterilize experimental uu but inhibit the growth of the experimental uu; and the quick detection test paper for uu neutralization can rapidly and accurately detect the uu after a large amount of clinical antibiotics is used.
Owner:曲奕
Preparation method of nucleic acid fingerprint database for detecting genital tract pathogens by MALDI TOF-MS (matrix-assisted laser desorption/ionization time-of-flight mass spectrometry)
ActiveCN113355456AHigh biological valueMicrobiological testing/measurementDNA/RNA fragmentationNeisseria gonorrhoeaeChlamydiae
The invention provides a preparation method of a nucleic acid fingerprint database for detecting specific sites of eight genital tract pathogenic bacteria and two human papilloma virus types by a time-of-flight mass spectrometry. The genital tract pathogenic bacteria comprise staphylococcus aureus, group B streptococcus, neisseria gonorrhoeae, gardnerella vaginalis, candida albicans, mycoplasma genitalium, mycoplasma urealyticum, chlamydia trachomatis and virus types of HPV16 and HPV18 . The invention provides a novel method for detecting 10 genital tract pathogens by combining multiple PCR with clinical mass spectrometry for the first time, integrates multiple PCR, single base extension, mass spectrometry detection and other technologies, and establishes the preparation method of the nucleic acid fingerprint database, so that the model can amplify a detection template through the PCR technology, also can be used for detecting trace samples through the mass spectrometry technology, integrates the advantages of the two technologies, is far superior to a method for detecting a specific fragment of the pathogens by singly using PCR (Polymerase Chain Reaction), and has relatively high detection sensitivity.
Owner:BIOYONG TECH +1
Household intelligent detection reagent kit
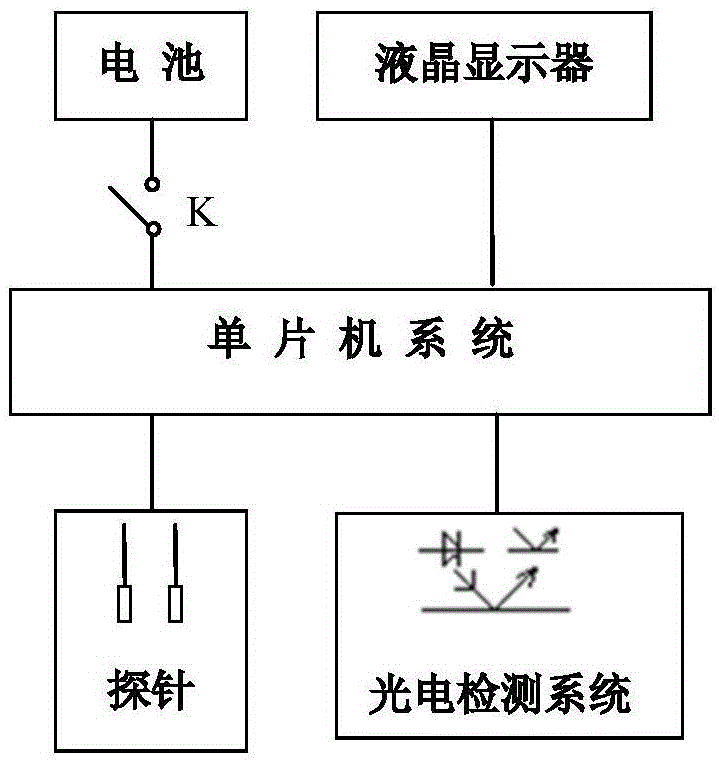
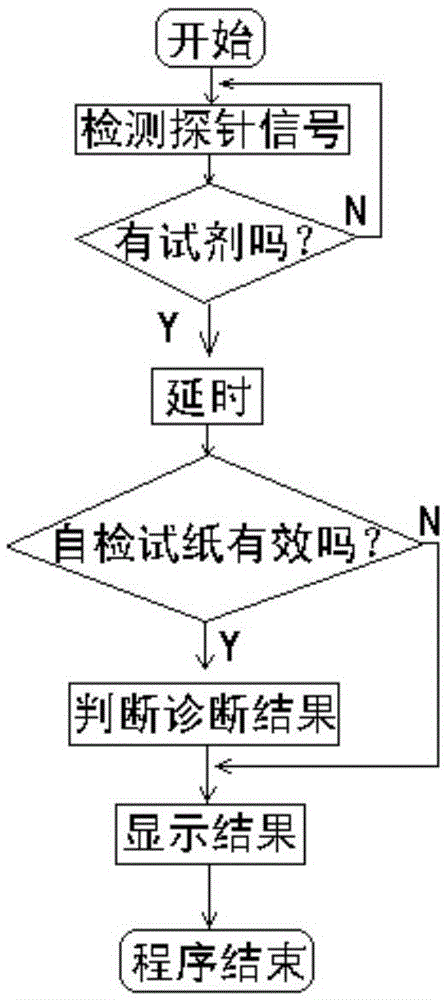
ActiveCN105675865AStrong specificityNo bloodMaterial analysisLiquid-crystal displayMicrocomputer system
The invention discloses a household intelligent detection reagent kit.The household intelligent detection reagent kit is composed of an electronic reading and analyzing part and a monoclonal antibody detection reagent.The electronic reading and analyzing part comprises a single-chip microcomputer system, a photoelectrical detection system, a battery, a switch K, a liquid crystal displayer, a combined gold-labeled detection probe and test paper.The combined gold-labeled detection probe, the battery, the switch K and the single-chip microcomputer system are sealed in a case, and the liquid crystal displayer is installed at a case window.The monoclonal antibody detection reagent comprises a monoclonal antibody for specific recognition of mycoplasma genitalium, a neisseria gonorrhoeae monoclonal antibody, a candida albicans monoclonal antibody, a goat-anti-mouse IgG antibody and the combined gold-labeled detection probe.The household intelligent detection reagent kit is used for directly detecting infective pathogens based on the monoclonal antibody technology, has the advantages of being high in specificity, free of blood sampling, capable of making an early diagnosis, high in speed and the like, has the automatic detection function, avoids interference of human factors, displays a detection result visually, and is easy and convenient to operate and low in cost.
Owner:西安昱子生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com