Mycoplasma genitalium nucleic acid real-time fluorescence PCR detection primer, probe and kit
A technology of Mycoplasma genitalium and detection kit, which is applied in the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc., can solve the problems of false positive detection of MG, no practicality, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Development of real-time fluorescence quantitative PCR kit for mycoplasma genitalium nucleic acid
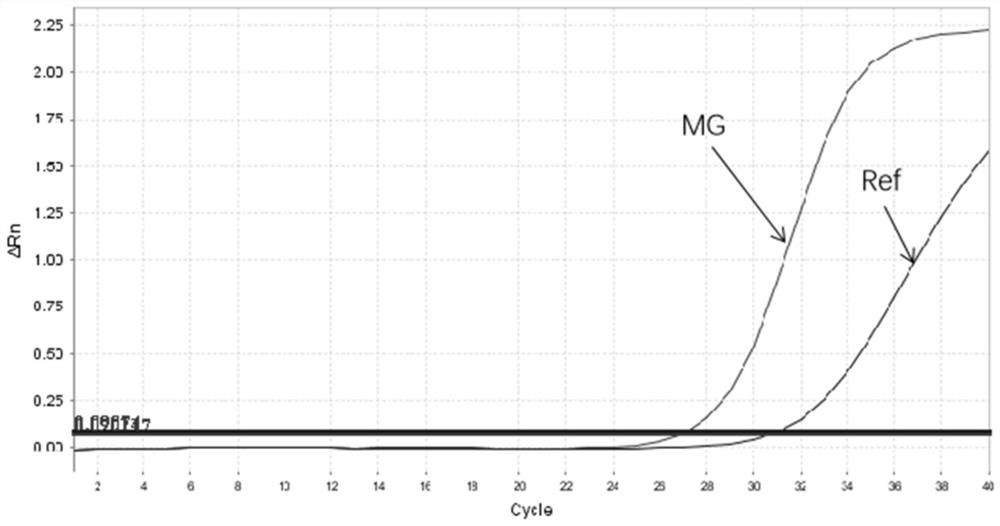
[0028] According to bibliographical reports, the present invention finally selects and designs primer probes for the MgPa gene region of Mycoplasma genitalium, selects 10 kinds of Mycoplasma genitalium MgPa operon full-lengths in NCBI database and carries out sequence comparison, and designs primer probes in its conserved regions, thereby ensuring A pair of primers and a probe can detect all strains of Mycoplasma genitalium, and they are compared by BLSAT to ensure that the primer probe can only be compared to Mycoplasma genitalium, but not to other pathogenic bacteria.
[0029] At the same time, in this embodiment, the human RNaseP gene was used as an internal reference gene to design specific primers and probes.
[0030] Primers and probes in the present invention are as follows:
[0031] a) Amplification primers and probes for Mycoplasma genitalium MG (conserved regio...
Embodiment 2
[0058] accuracy:
[0059] The kit described in Example 1 was used to detect 3 positive vaginal swabs from clinics diagnosed as Mycoplasma genitalium infection, as shown in Table 2, the results were all positive, and the positive coincidence rate was 100%.
[0060] Table 2 Test results of clinical samples
[0061]
[0062] specificity:
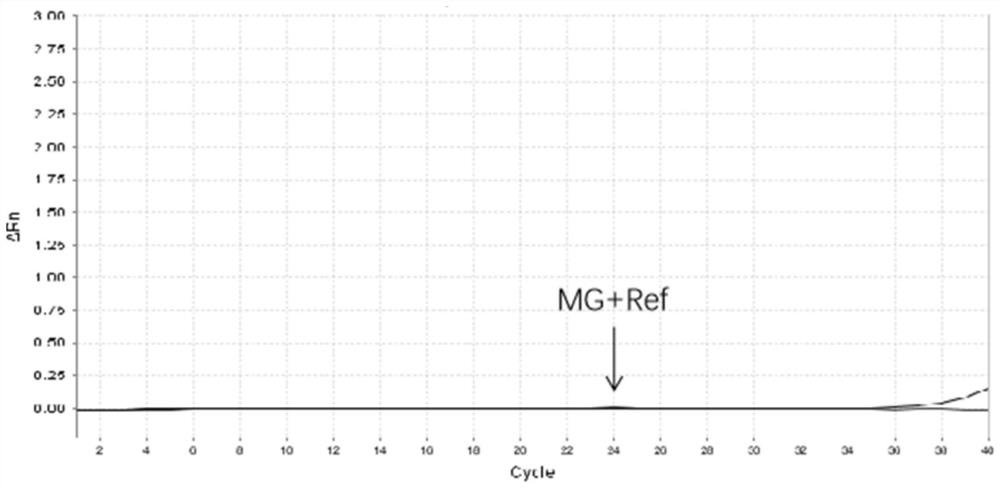
[0063] The kit described in Example 1 was used to detect 13 kinds of pathogenic bacteria such as Mycoplasma pneumoniae, Ureaplasma urealyticum, Mycoplasma hominis, and Chlamydia trachomatis. As shown in Table 3, the test results were all negative, and the coincidence rate was 100%.
[0064] Table 3 Test results of 9 kinds of pathogenic bacteria
[0065]
[0066]
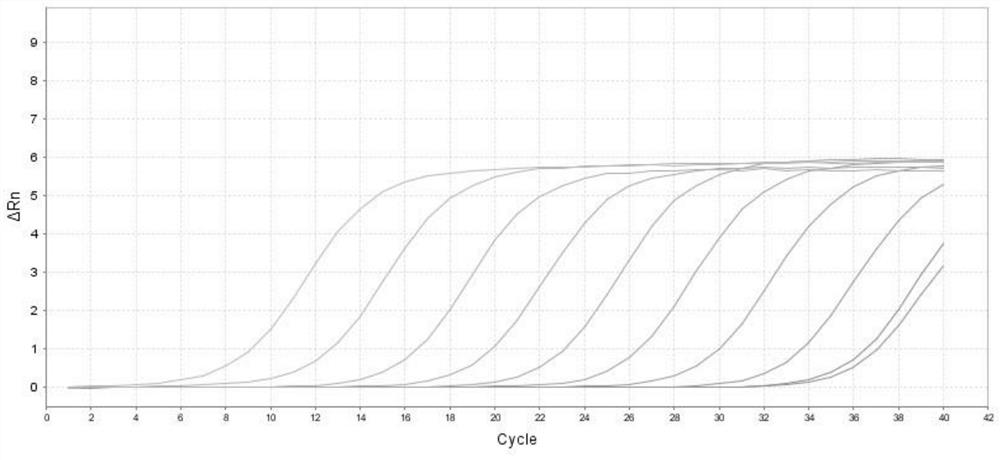
[0067] Repeatability:
[0068] The kit described in Example 1 was used to detect 2 self-built positive enterprise reference products, each sample was repeated 10 times, and the coefficient of variation (CV, %) of the test result Ct value was ≤ 5%.
[0069] The formula for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com