Genital tract pathogen nucleic acid detection kit
A technology for detecting kits and pathogens, which is applied to the determination/inspection of microorganisms, microorganisms, and methods based on microorganisms. It can solve the problems of expensive, time-consuming, and false positives for supporting instruments, and achieve lower detection costs, less inconvenience for patients, and lighten the The effect of financial burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A nucleic acid detection kit for reproductive tract pathogens, comprising a first detection component, a second detection component, and a third detection component in a freeze-dried state that are simultaneously subjected to fluorescence quantitative PCR detection after adding a detection sample, and the first detection component is used to detect trachoma For Chlamydia and Neisseria gonorrhoeae, the second detection component is used to detect Ureaplasma urealyticum and Mycoplasma hominis, and the third detection component is used to detect Mycoplasma genitalium;
[0034] Among them, the first to third detection components are all made of trehalose, mannitol, PEG and detection reagent composition mixed and then freeze-dried, and the detection reagent composition of the first to third detection components are all made of 10×PCR buffer , dNTPs, detection primer pair, internal reference primer pair, detection probe, internal reference probe, MgCl 2 , BSA, r-Taq enzyme, U...
Embodiment 2
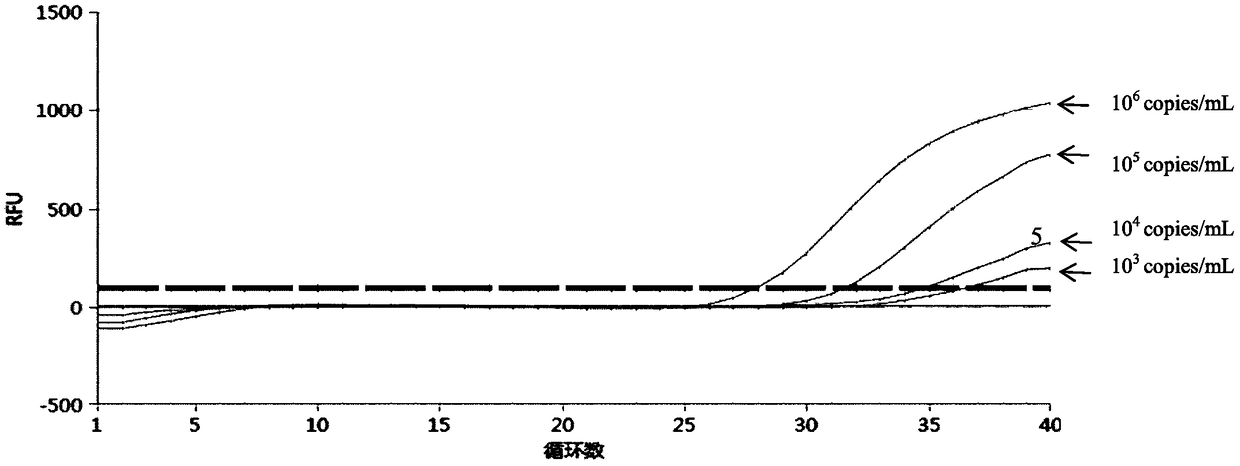
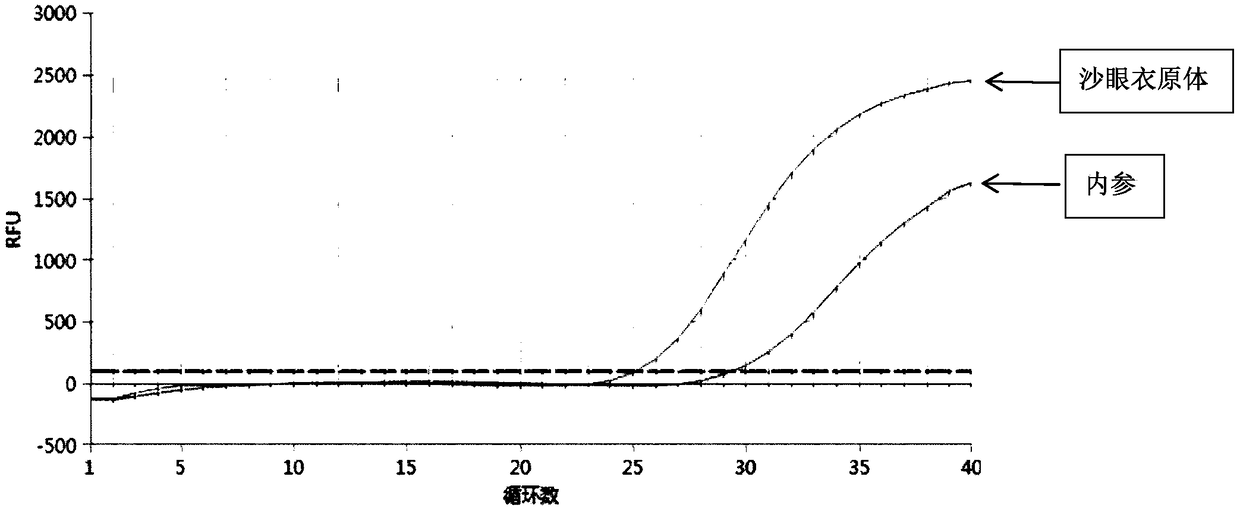
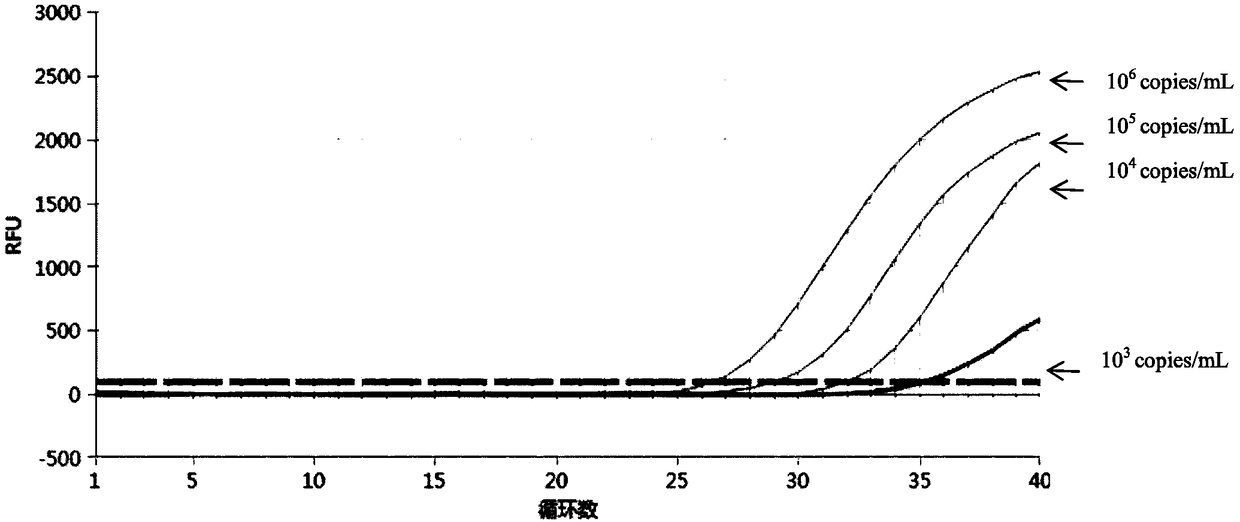
[0053] Detect respectively Chlamydia trachomatis (CT), Mycoplasma genitalium (MG), Ureaplasma urealyticum (UU), Neisseria gonorrhoeae (NG) and Mycoplasma hominis (MH) pathogen (dry powder of pathogen) with embodiment 1 reproductive tract pathogen nucleic acid detection kit All were purchased from ATCC and reconstituted into 10 6 bacteria solution per mL), specifically, add 100-200 μL of bacteria solution to the first to third detection components, and place it in a fluorescent quantitative PCR instrument for amplification detection according to the following amplification procedure: pre-denaturation at 95°C for 3 minutes; Add 40 cycles, 95°C for 15s, 60°C for 30s (collect fluorescence)
[0054] The fluorescent PCR diagram for detecting Chlamydia trachomatis (CT) alone is as follows figure 1 and figure 2 Shown, the fluorescent PCR picture of detecting Mycoplasma genitalium (MG) alone image 3 and Figure 4 Shown, the fluorescent PCR picture of detecting Ureaplasma urealyti...
Embodiment 3
[0056] The nucleic acid detection kit for reproductive tract pathogens in Example 1 was used to detect the swab sample 1 and swab sample 2 of the patient's vaginal and cervical secretions from the Xiamen Maternal and Child Health Hospital, respectively, and soak the swab sample 1 and swab sample 2 with 1mL physiological saline , shake for 120s, and obtain sample stock solution 1 and sample stock solution 2.
[0057] Add 200-500 μL of sample stock solution 1 to the first to third detection components, cover the lid, put it into the matching automatic PCR analysis system, and run it for 40-60 minutes to obtain the following detection results, that is, Neisseria gonorrhoeae (NG) positive (Such as Figure 11 shown).
[0058] Add 200-500 μL sample stock solution 2 to the first to third detection components respectively, cover the lid, put it into the matching automatic PCR analysis system, and run it for 40-60 minutes to obtain the detection results as follows, that is, Mycoplasma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com