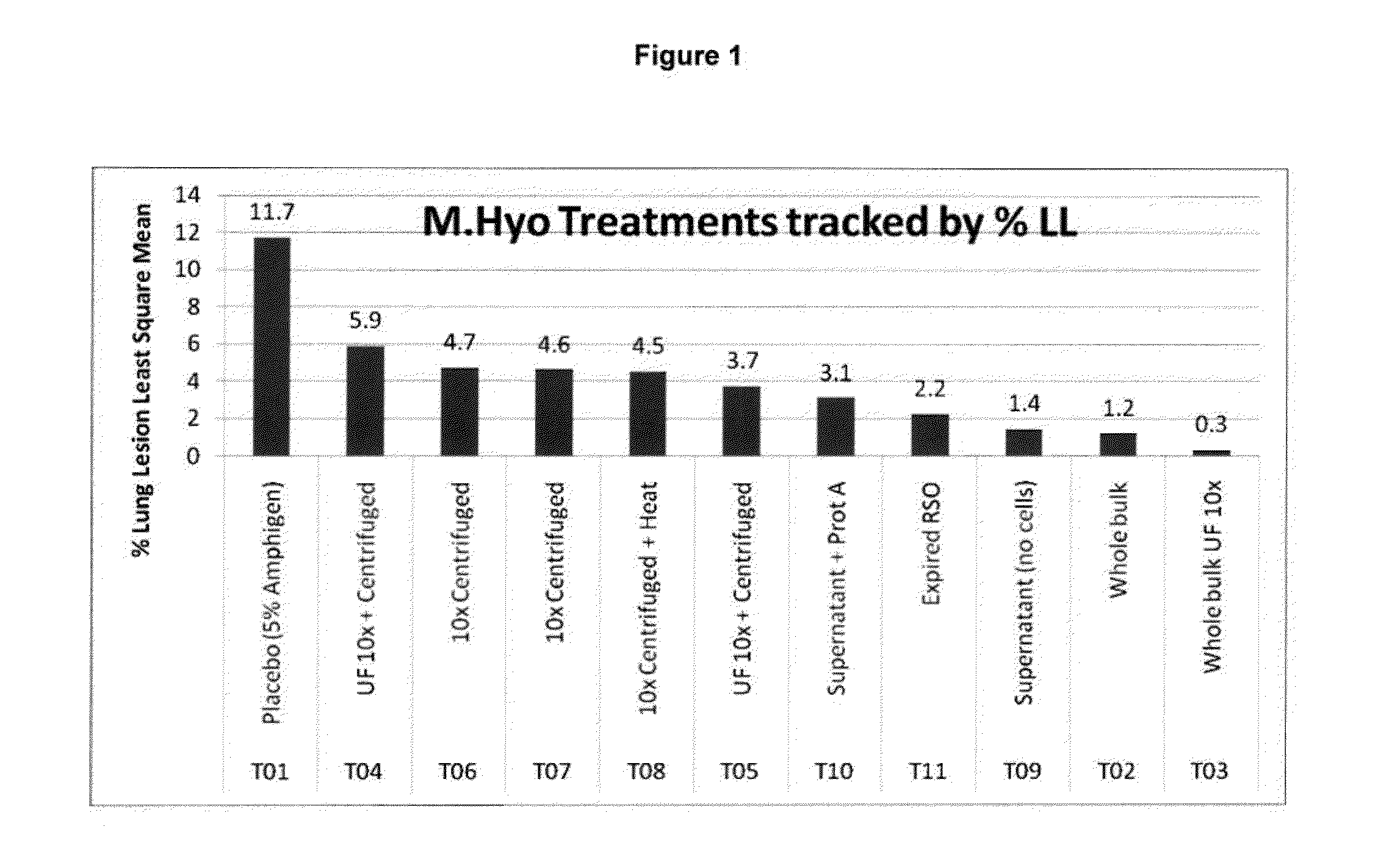
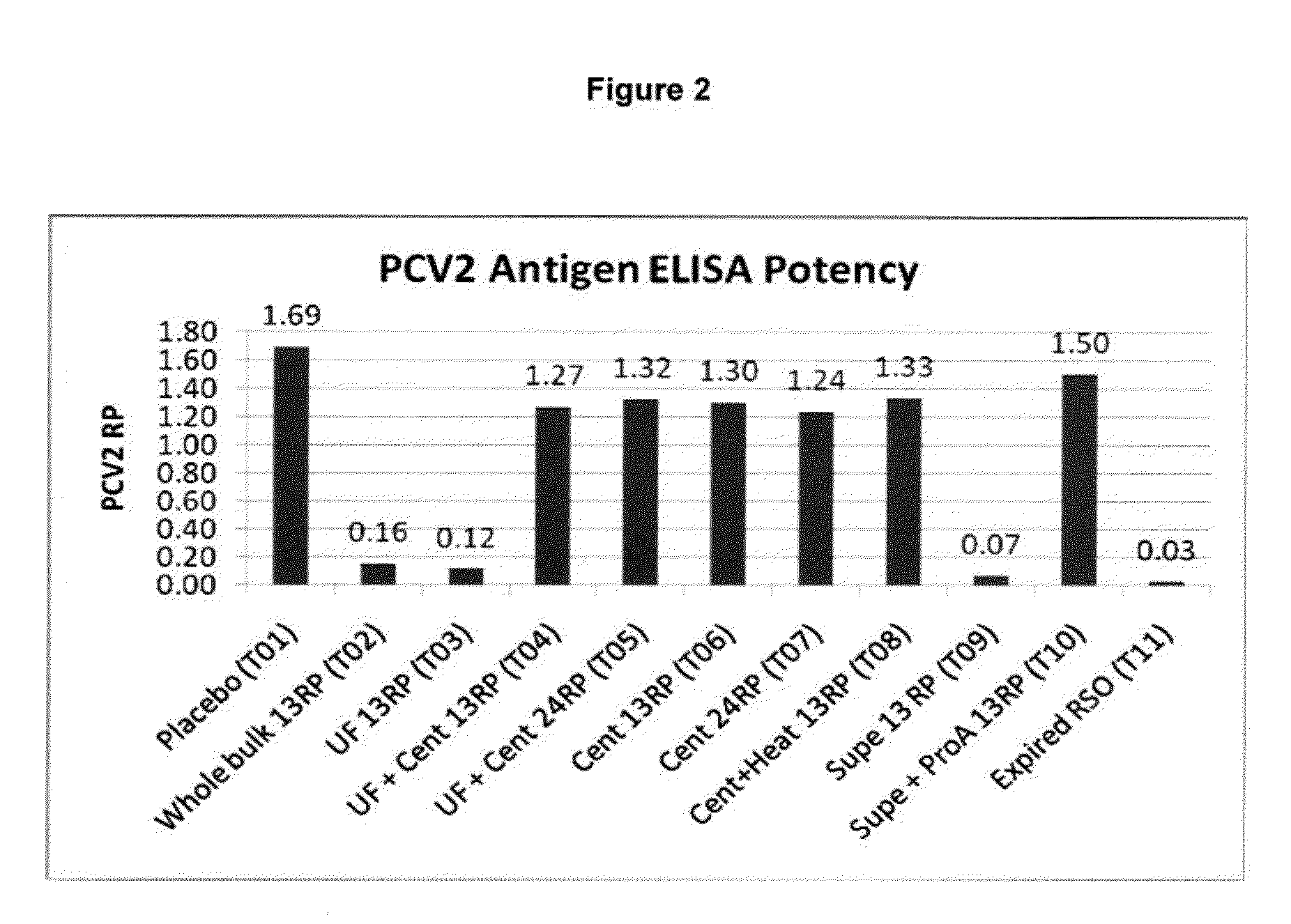
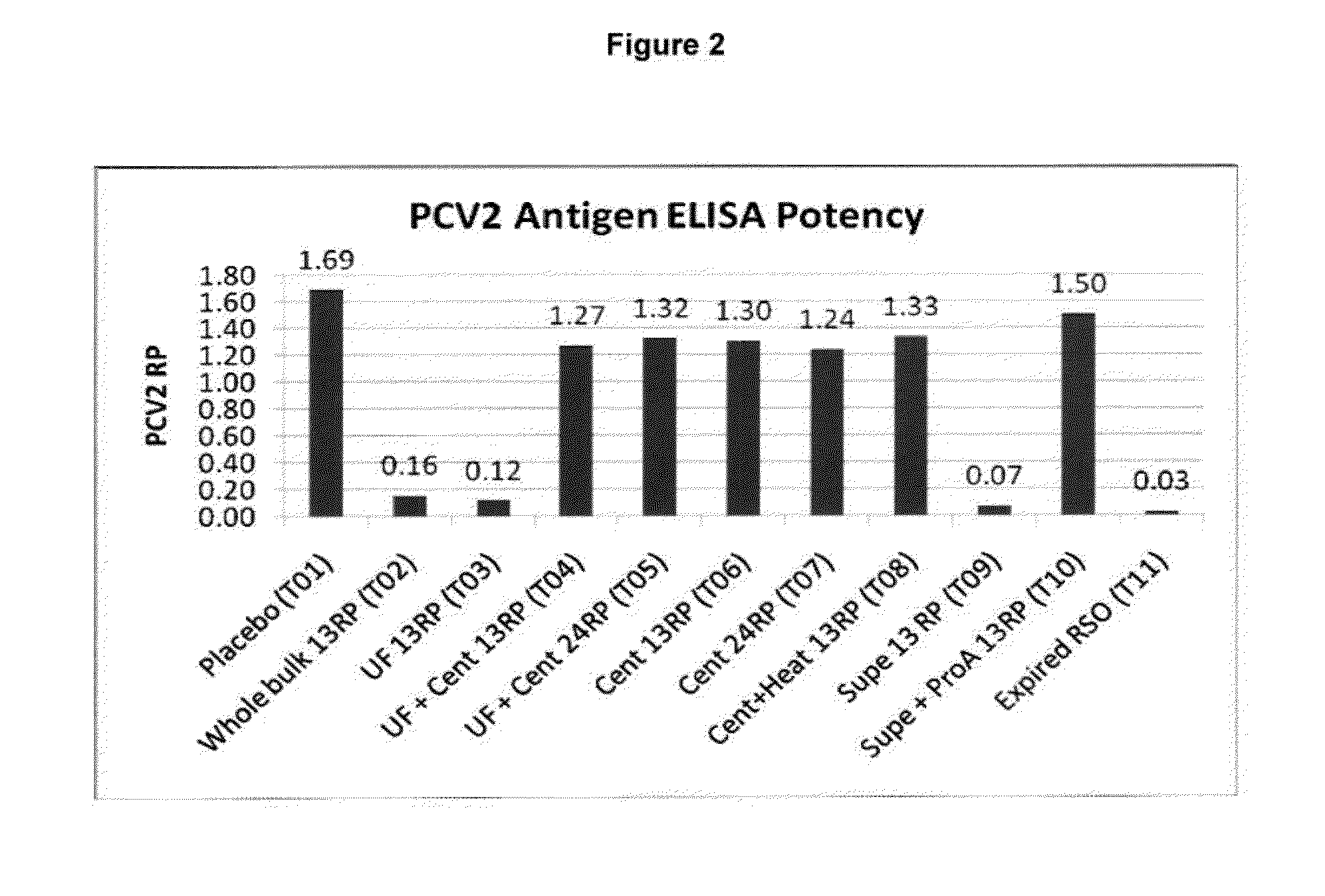
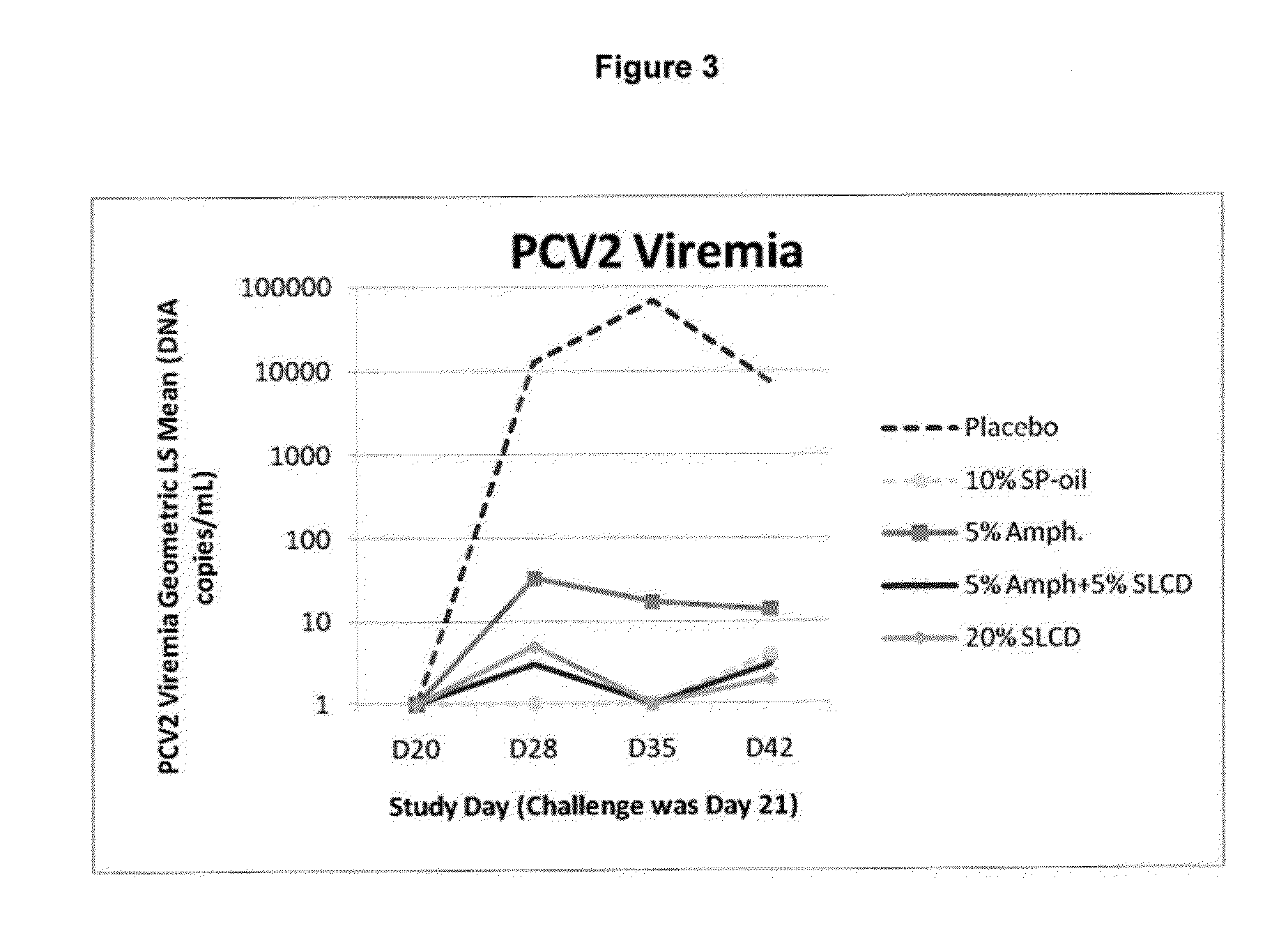
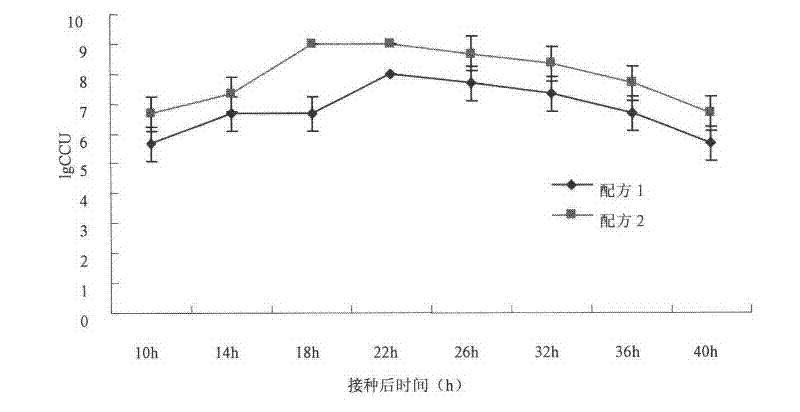
Patents
Literature
172 results about "Mycoplasma hyorhinis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mycoplasma hyorhinis is a member of the Mycoplasmatales family. This bacterium is often found as a commensal in the respiratory tract of pigs, and rarely in the skin of humans. It is thought to facilitate and exacerbate the development of diseases such as porcine enzootic pneumonia and porcine reproductive and respiratory syndrome (PRRS). Rarely, it may cause mycoplasma arthritis, mycoplasmal polyserositis or mycoplasma septicaemia in piglets without the involvement of other bacteria. This presents as polyarthritis or polyserositis.
Human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit and preparation method and application thereof
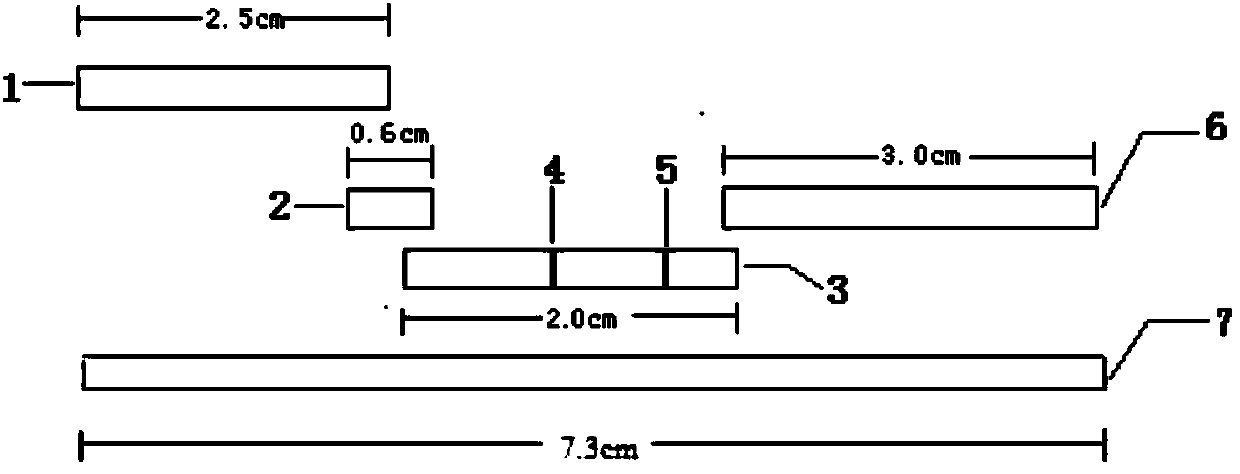
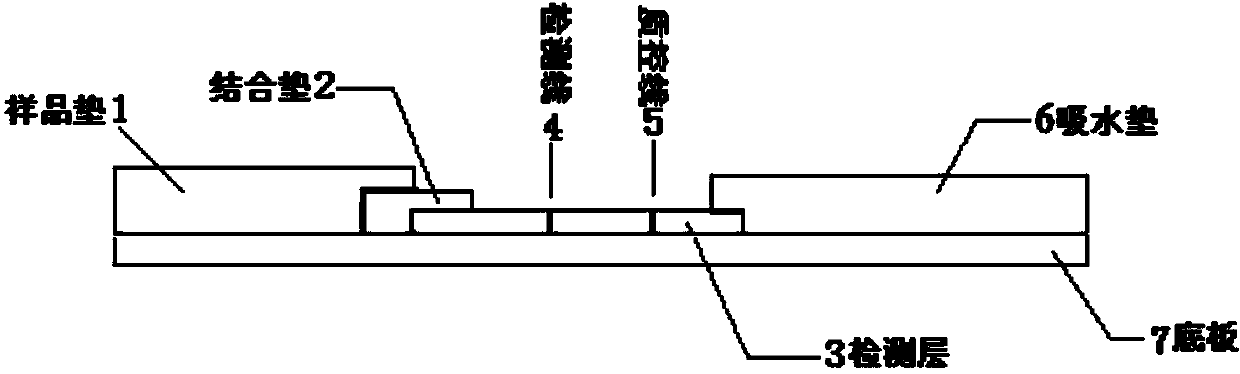
The invention provides a human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit and a preparation method and application thereof. The assay kit comprises a detection card and a silver-stained sensitivity-enhanced pad, wherein the detection card is composed of a bottom plate, a sample pad, an absorbent pad, a conjugate pad and a detection layer; the conjugate pad is coated with a colloidal gold-marked polyclonal antibody mixture of colloidal gold marked rabbit anti-human mycoplasma pneumoniae P1 protein and P30 protein; the detection layer is composed of a solid phase nitrocellulose membrane with a detection line and a quality control line; the detection layer is bonded on the bottom plate, the conjugate pad and the absorbent pad are partially overlapped with the detection layer respectively and are bonded with the detection layer and the bottom plate respectively; the sample pad and the conjugate pad are partially overlapped to be bonded with the conjugate pad and the bottom plate respectively; and the silver-stained sensitivity-enhanced pad consists of a AgNO3 pad and a restoring pad. The human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit can effectively improve the detection sensitivity of the human mycoplasma pneumoniae, has the strong specificity and has the high application value in the aspects of clinical diagnosis of human mycoplasma pneumoniae, etiology identification, epidemiological investigation and the like.
Owner:HUBEI UNIV OF TECH +1
Mycoplasma vaccine, method of making, and application thereof
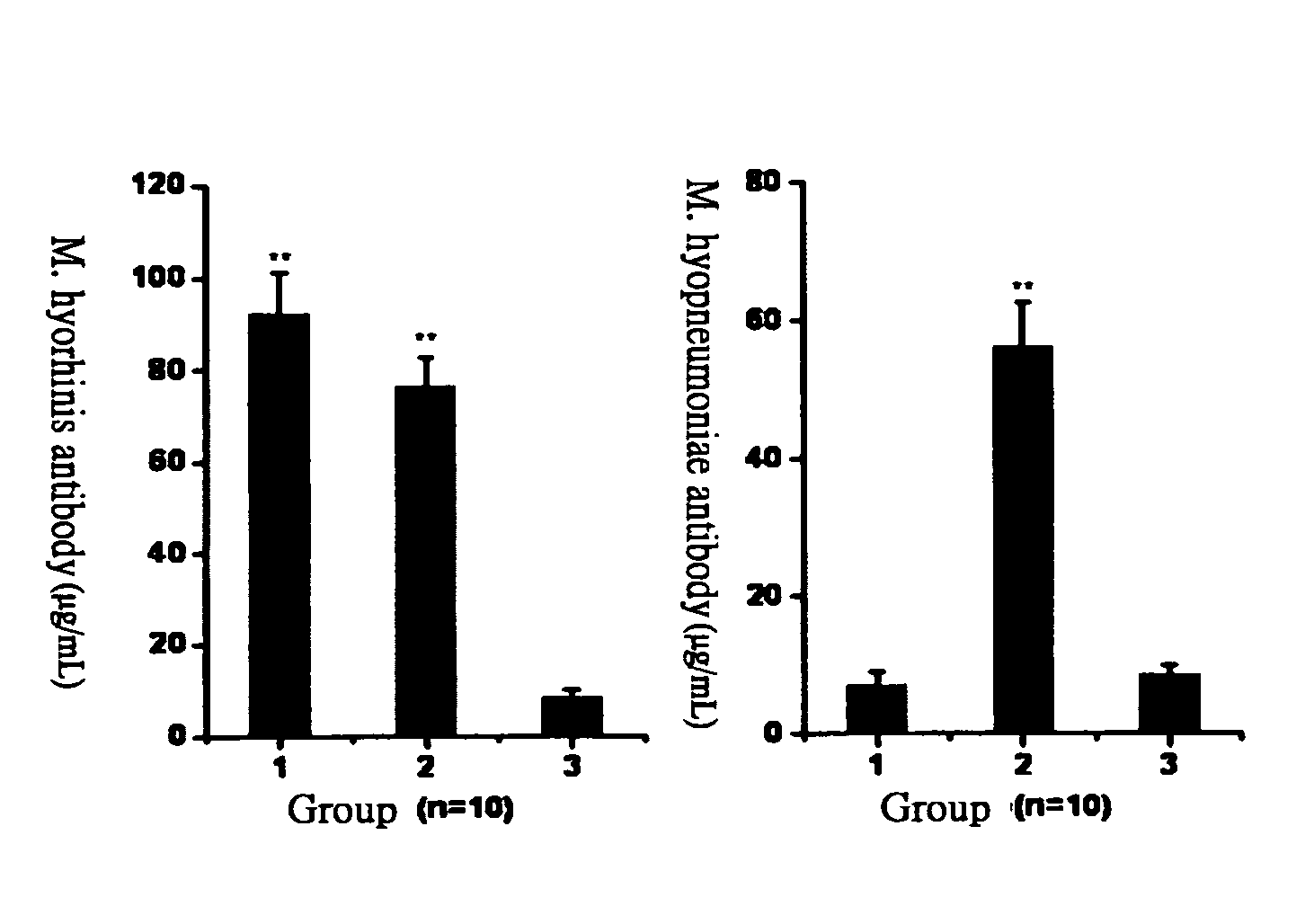
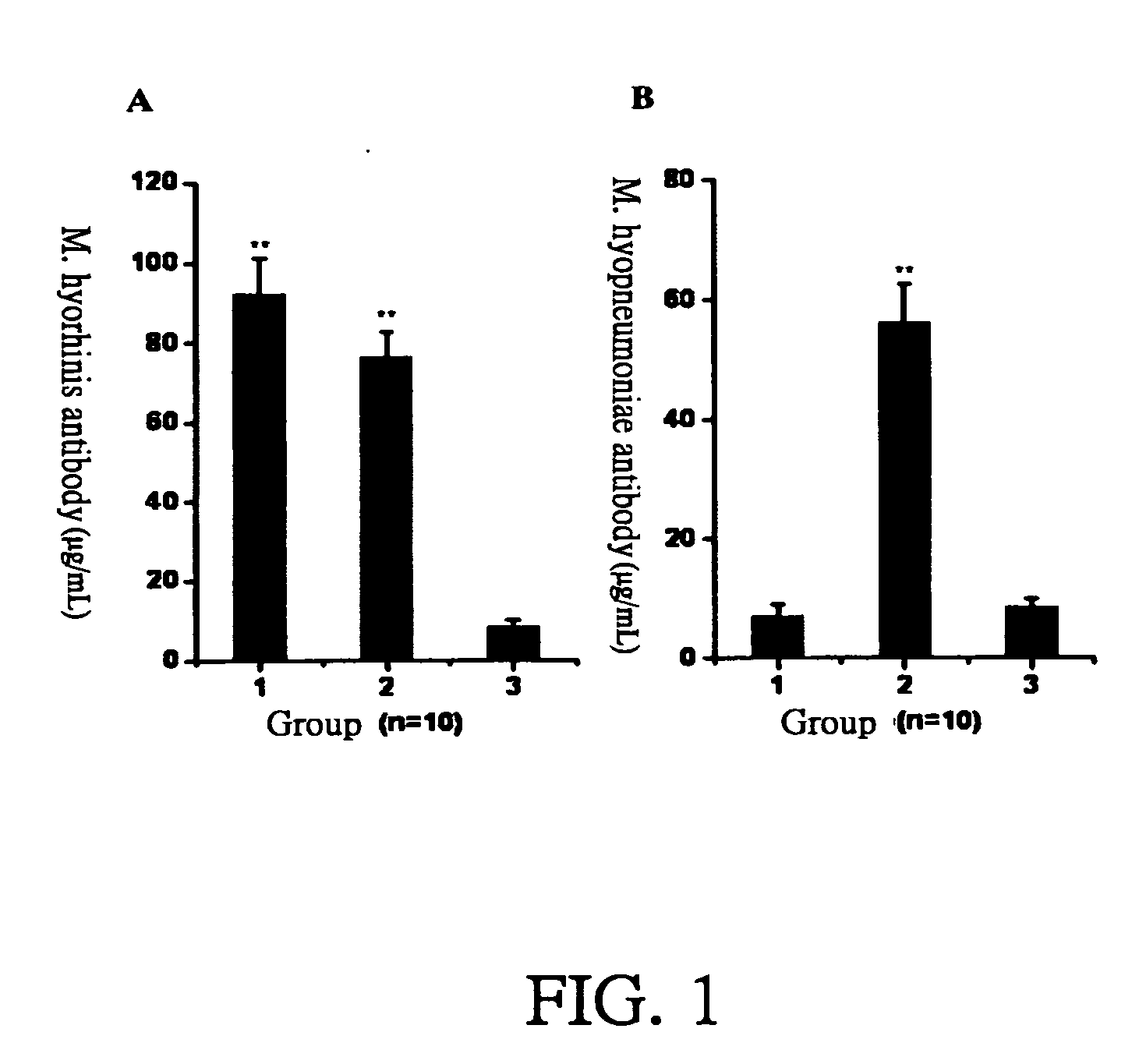
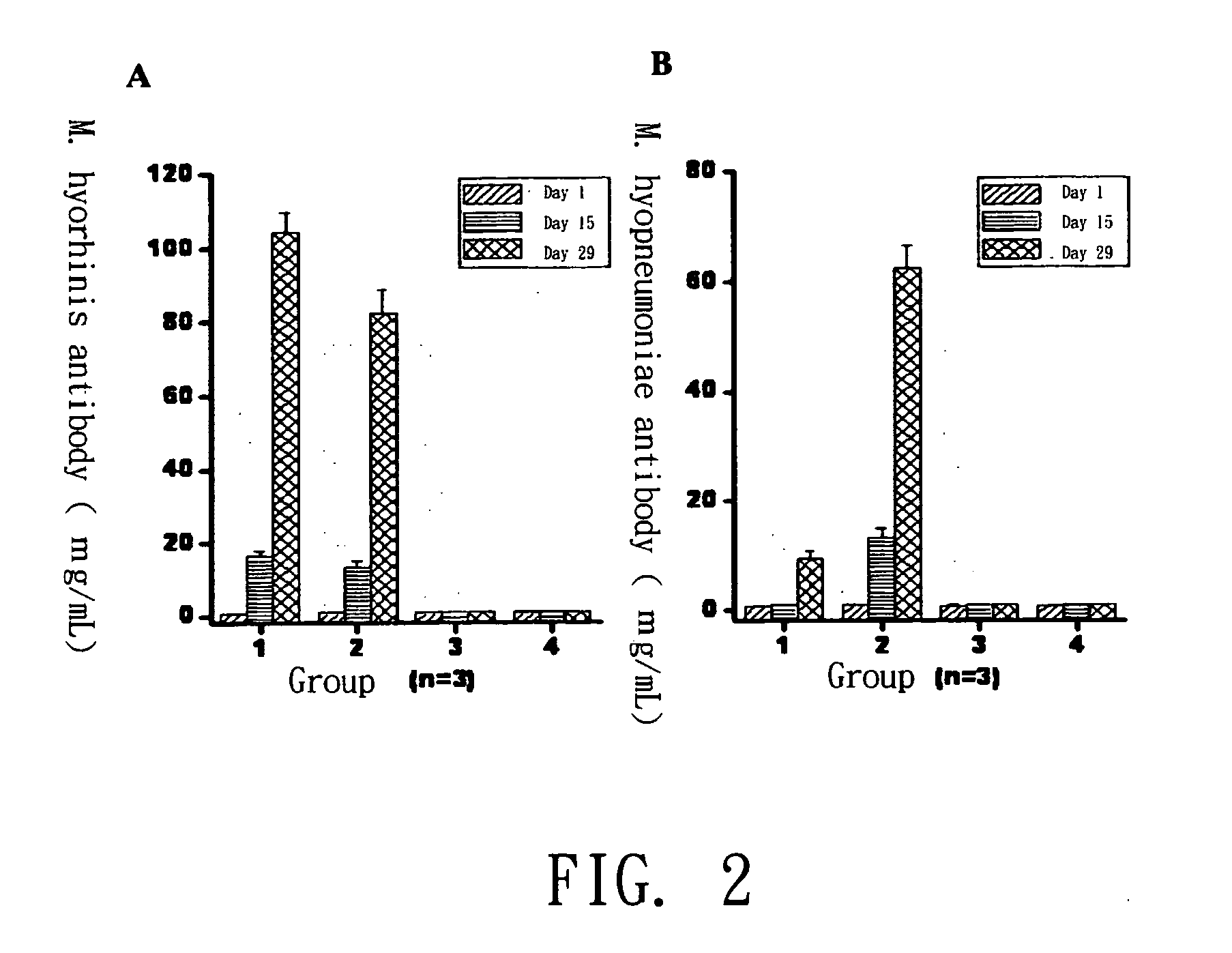
The present invention provides a mycoplasma vaccine, its preparation and application thereof. The foregoing mycoplasma vaccine comprises inactivated Mycoplasma hyorhinis ATIT-7 only or the mixture of inactivated Mycoplasma hyorhinis ATIT-7 and inactivated Mycoplasma hyopneumoniae, which effectively prevents the infection of swine enzootic pneumonia in pigs.
Owner:ANIMAL TECH INST TAIWAN
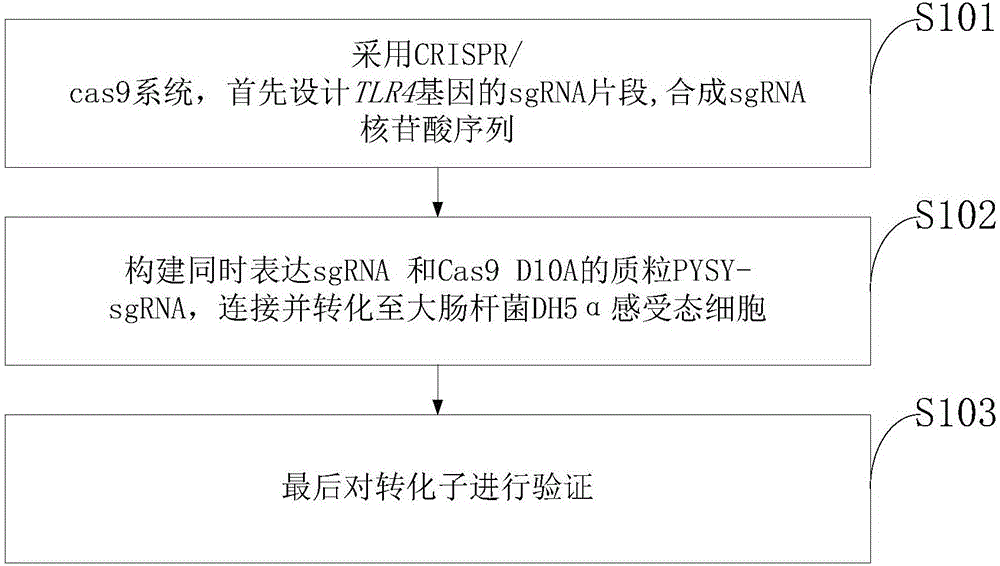
Goat TLR4 gene knock-out vector and construction method thereof
InactiveCN106755097AThe method is simple and fastHigh knockout efficiencyNucleic acid vectorVector-based foreign material introductionEscherichia coliCompetent cell
The invention discloses a goat TLR4 gene knock-out vector and a construction method thereof. The construction method comprises the following steps: firstly, designing an sgRNA fragment of the TLR4 gene by adopting a CRISPR / cas9 system, synthesizing an sgRNA nucleotide sequence, constructing and simultaneously expressing the sgRNA and plasmid PYSY-sgRNA of Cas9 D10A, connecting and transforming to an Escherichia coli DH5 alpha competent cell, and verifying the transformant; and judging and proving by enzyme digestion and sequencing that the TLR4 gene knock-out vector is constructed correctly. The invention adopts the CRISPR / cas9 for constructing the vector, and provides a theoretical basis for subsequently acquiring a goat TLR4 gene deletion type alveolar epithelial cell system, and studying the immune response molecular mechanism of mycoplasma pneumonia infection.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE ANHUI ACAD OF AGRI SCI
Mycoplasma hyopneumoniae culture medium and preparation method thereof
ActiveCN102154167ARich in nutrientsIncrease the titer of live bacteriaBacteriaMicroorganism based processesPenicillinMycoplasma culture
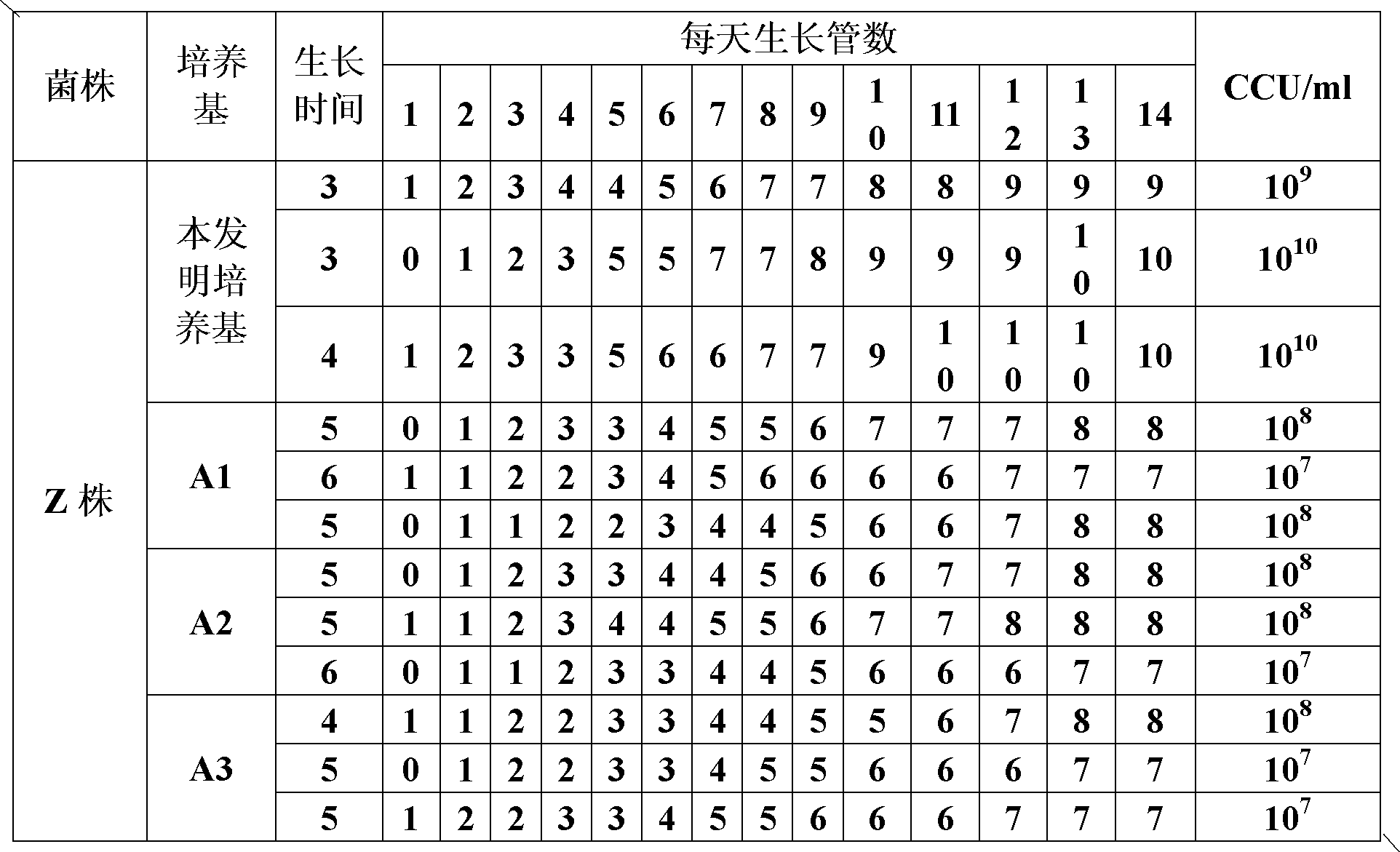
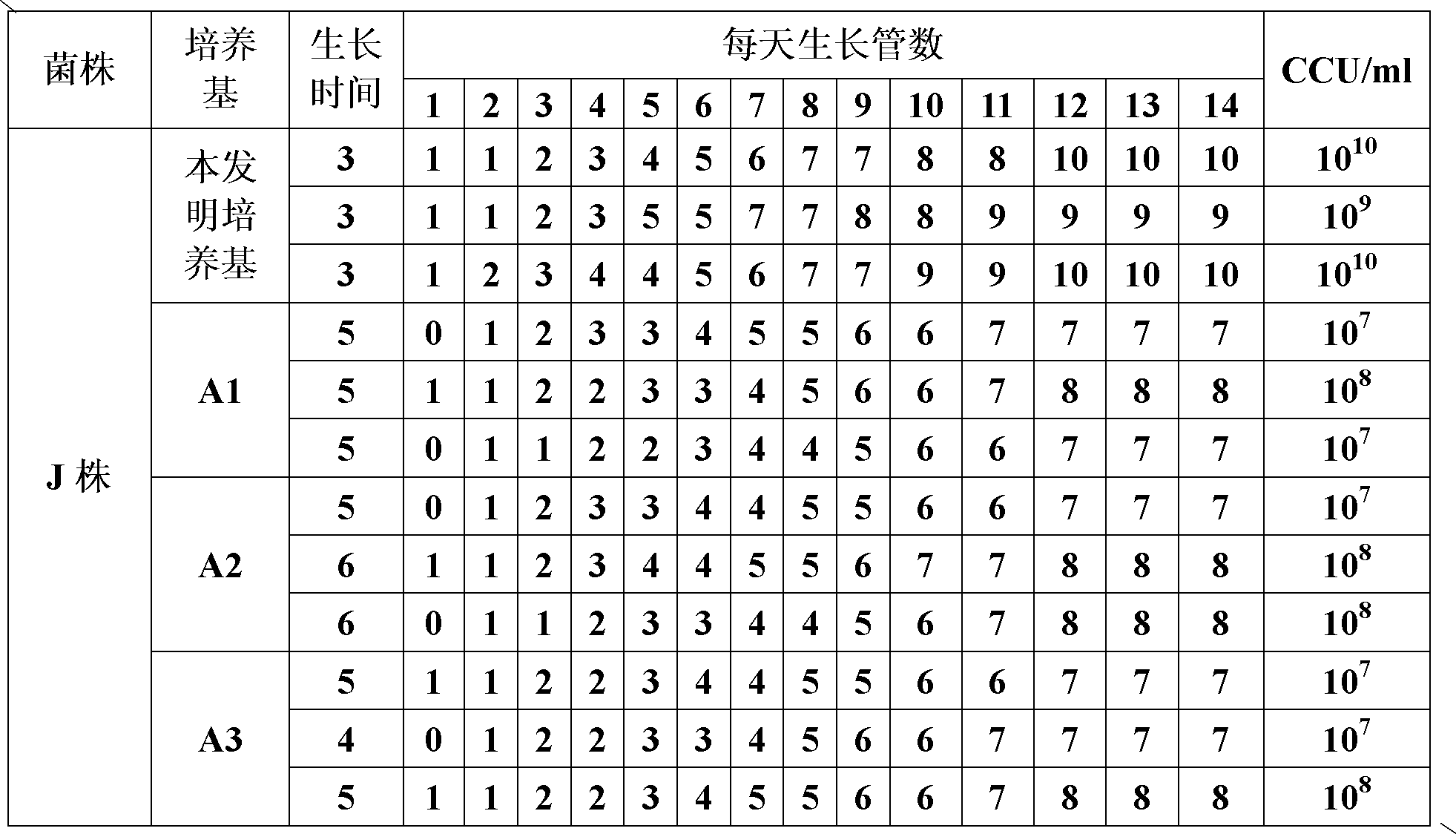
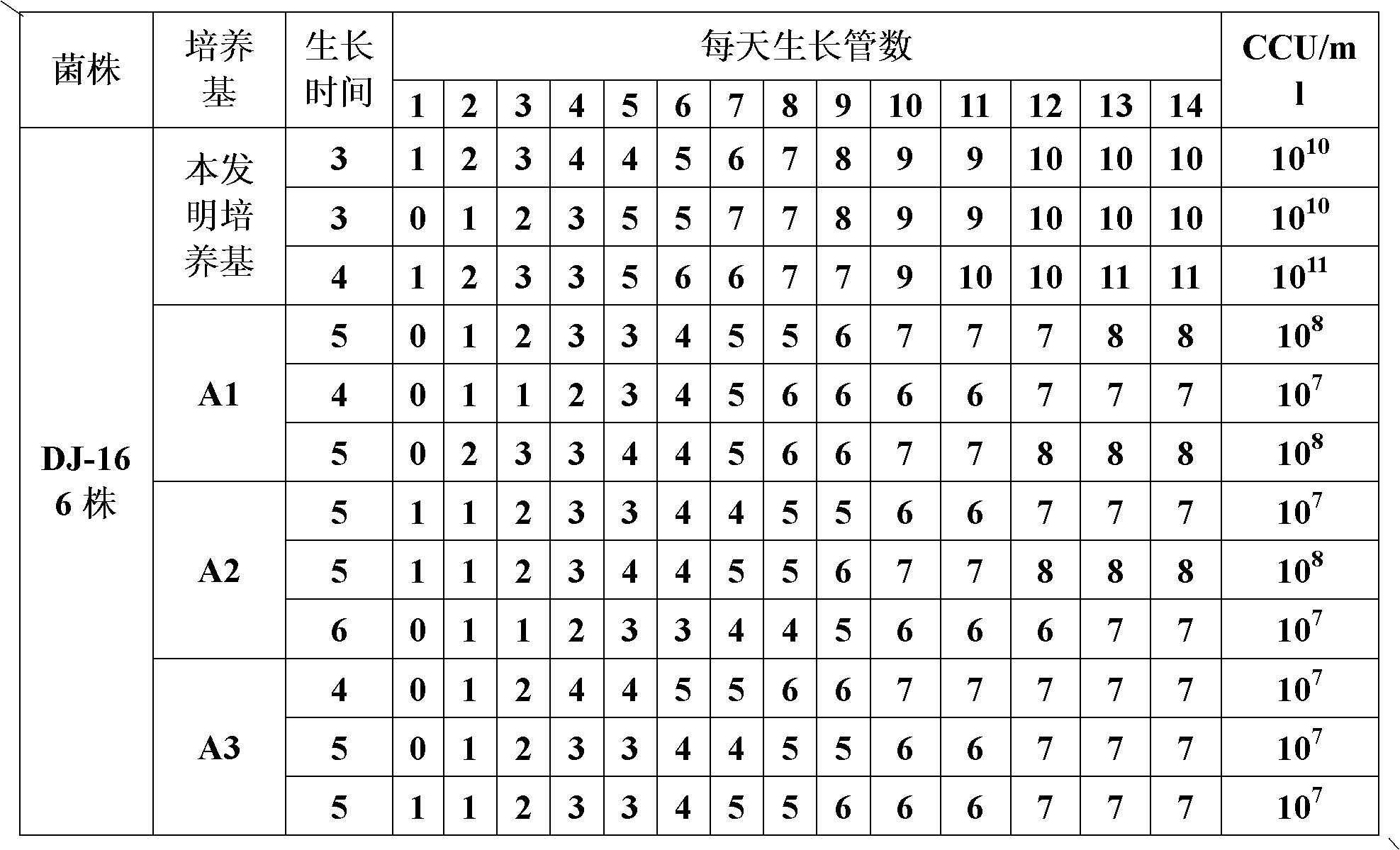
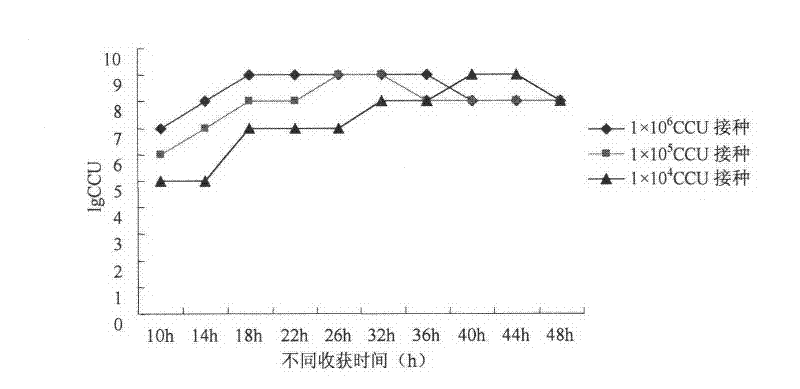
The invention provides a mycoplasma hyopneumoniae culture medium and a preparation method thereof, belonging to the technical field of veterinary biology. The mycoplasma hyopneumoniae liquid culture medium comprises the components as follows: brain heart infusion, lactalbumin hydrolysate, PPLO (pleuropneumonia-like organism) broth, yeast extract powder, proteose peptone, sodium thiosulfate, Hank's liquid, sodium pyruvate, 0.1% phenol red solution, penicillin and deionized water. The preparation method comprises the following steps of: adding health horse serum before using, and adding agar into the liquid culture medium to obtain a solid culture medium of mycoplasma hyopneumoniae. The viable bacteria titer of the mycoplasma hyopneumoniae culture medium can reach 1*109CCU / ml-1*1010CCU / ml; the viable bacteria titer and the separation sensibility are far higher than those of the existing culture medium, and the mycoplasma hyopneumoniae is fast in growth speed and high in the separation sensibility; and the preparation method of the culture medium is simple in technology, strong in operability, and suitable for industrial large-scale production.
Owner:兆丰华生物科技(南京)有限公司 +1
One dose vaccination against mycoplasma infections of pigs
ActiveUS8444989B1Bacterial antigen ingredientsBacteriaVaccines AdministeredMycoplasma pneumoniae Infections
The present invention provides a one phase, aqueous vaccine composition for immunizing an animal against infection by Mycoplasma hyopneumoniae, comprising: an immunizing amount of a Mycoplasma hyopneumoniae bacterin, an acrylic acid polymer in the concentration range between 0.8 and 1.2 mg / ml, and a pharmaceutically acceptable carrier, and substantially no oil. It is especially useful for immunizing a pig against infection by Mycoplasma hyopneumoniae for at least 20 weeks after a single administration, which effective immunity is reached within 4 weeks after vaccination.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Kit for jointly detecting respiratory tract pathogen through multiple fluorescent PCR method
ActiveCN107058622ANo further action requiredShorten the course of the diseaseMicrobiological testing/measurementAgainst vector-borne diseasesPositive controlFluorescence
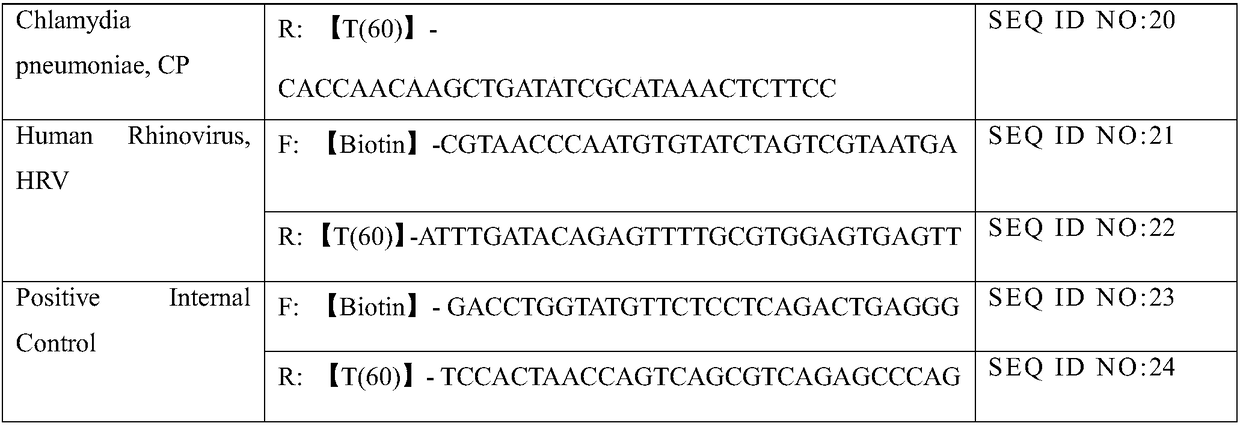
The invention provides a kit for jointly detecting respiratory tract pathogen through a multiple fluorescent PCR method. The kit comprises six components: reaction liquid A, reaction liquid B, reaction liquid C, enzyme mixed liquid, positive control and negative control, and comprises 11 common respiratory tract pathogen detections (general type of influenza virus A, influenza virus B, respiratory syncytial virus, 1 / 2 / 3 type of human parainfluenza virus, adenovirus, mycoplasma pneumoniae, chlamydia pneumonia, legionella pneumophila, streptococcus pneumonia, haemophilus influenza, A streptococcal); the amplification is performed through three reaction buffers, and each reaction buffer contains four fluorescent channels, 90% pathogen infection on the clinic can be checked.
Owner:DEBIQI BIOTECH XIAMEN
Mycoplasma bovis monoclonal antibody, and preparation method and application thereof
ActiveCN103509756AStrong variabilityEasy to operateImmunoglobulins against bacteriaMicroorganism based processesMycoplasma bovis antigenMycoplasma antigen
The invention discloses a mycoplasma bovis monoclonal antibody, and a preparation method and an application thereof. The preparation method of the mycoplasma bovis monoclonal antibody comprises the following steps: 1, preparing a mycoplasma bovis antigen; 2, immunizing mice by the antigen; 3, preparing a hybridomas cell secreting the mycoplasma bovis monoclonal antibody and monoclonal antibody ascites; and 4, purifying the above obtained monoclonal antibody. In the invention, mice are immunized by a mycoplasma bovis geographical strain HB0801 antigen, a hybridomas cell strain 1C11, CCTCC NO:C201218, which can efficiently secrete the mycoplasma bovis monoclonal antibody, is obtained through a hybridomas cell technology, the generated monoclonal antibody can be specifically combined with mycoplasma bovis and mycoplasma agalactiae, and the monoclonal antibody is utilized to establish sandwich ELISA for detecting the mycoplasma bovis antigen. The method has the advantages of simple operation, short required time and high sensitivity.
Owner:HUAZHONG AGRI UNIV
LAMP primer composite for detecting respiratory pathogens and kit of LAMP primer composite
InactiveCN107099619ADoes not affect amplificationMeet quality control requirementsMicrobiological testing/measurementMicroorganism based processesColor changesBiology
The invention relates to a primer composite for detecting respiratory pathogens. The primer composite comprises at least one group of a mycoplasma pneumoniae group, a chlamydia pneumoniae primer group, an influenza A / B virus primer group, a parainfluenza virus primer group, an adenovirus primer group and a respiratory syncytial virus primer group. The invention further relates to a kit comprising the primer composite. The kit further comprises a micro-fluidic chip wrapping primers, and a macroscopic indicator. The invention further relates to a detection method adopting the primer composite. The detection method comprises the steps of primer composite coating, to-be-detected sample nucleic acid extraction, LAMP reaction and visual result interpretation. The kit and the method are applied to the micro-fluidic chip for visual judgment, instant detection of the seven respiratory pathogens is rapidly and accurately realized, and a result is judged by a naked eye by color change, so that the kit and the method are simpler, more convenient and quicker in practical applications, are easy to operate, and are suitable for site operation.
Owner:SHANGHAI IGENETEC DIAGNOSTICS CO LTD
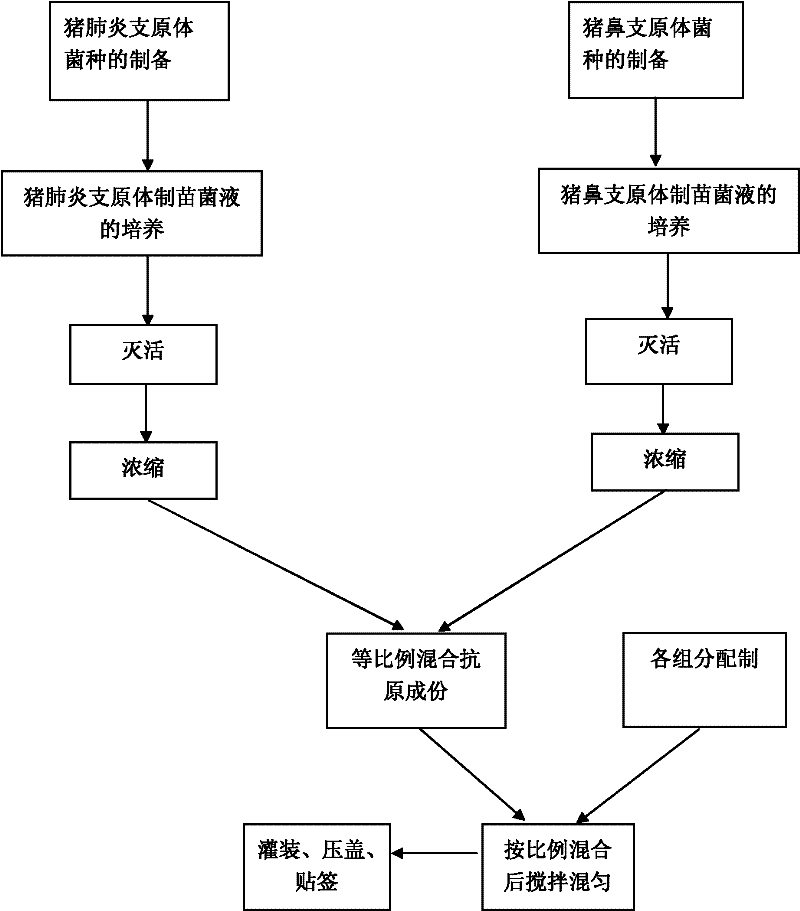
Mycoplasma hyopneumoniae and Mycoplasma hyorhinosus dual inactivated vaccine and preparation method thereof
ActiveCN102258776AStrong specificityGood immune effectAntibacterial agentsBacterial antigen ingredientsDiseaseImmune effects
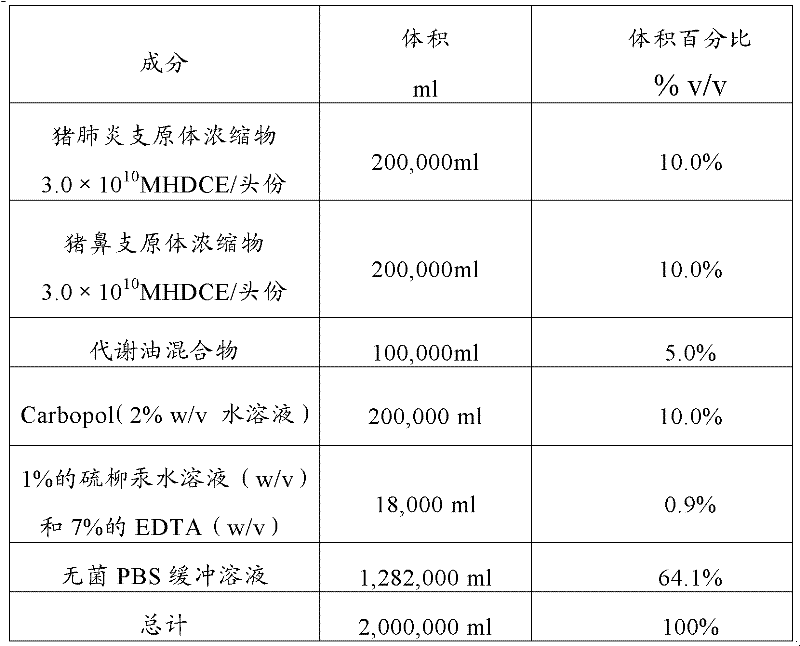
The invention provides a combined inactivated vaccine against mycoplasma hyopneumoniae (MHP) and mycoplasma hyorhinis. The combined inactivated vaccine contains the MHP and the mycoplasma hyorhinis with preferred content as well as a Carbomer adjuvant with concentration of 10% (V / V). The invention further provides a preparation method of the combined inactivated vaccine against the MHP and the mycoplasma hyorhinis. The preparation method comprises the following steps: preparing production strains; preparing bacterium solution for producing seedlings; and inactivating, concentrating and blending to obtain the vaccine. The combined inactivated vaccine has the advantages of strong specificity and good immunity, thus solving the problem of specific infection caused by the MHP in the current domestic breeding farm and obtaining the mycoplasma hyorhinis vaccine under a blank state at home and abroad at present. The combined inactivated vaccine has the beneficial effects that the step of vaccine inoculation is simplified, trouble caused by a plurality of inoculation and easily produced side effects are avoided, and vaccine cost is saved, thus being especially applicable to preventing andtreating mixed infection diseases in the breeding farms at home and abroad and the like; and compared with the existing single vaccine, application range is widened and immune effect is enhanced.
Owner:PU LIKE BIO ENG
Preparation method of mycoplasma gallisepticum and mycoplasma synoviae bivalent inactivated vaccine
ActiveCN103479995AImproving immunogenicityAvoid infectionAntibacterial agentsBacterial antigen ingredientsImmune effectsMycoplasma synoviae
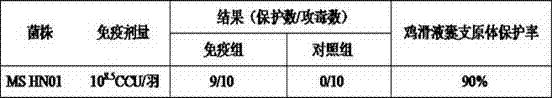
The invention relates to a preparation method of a mycoplasma gallisepticum and mycoplasma synoviae bivalent inactivated vaccine. The method comprises the steps as follows: a mycoplasma gallisepticum virulent CR strain and a mycoplasma synoviae HN01 strain which have good immunogenicity are inoculated on a proper culture medium for cultivation, so that a culture is acquired; and the culture is inactivated through a formaldehyde solution and then is mixed with an oil emulsion adjuvant and emulsified, so that the mycoplasma gallisepticum and mycoplasma synoviae bivalent inactivated vaccine is prepared. The mycoplasma gallisepticum and mycoplasma synoviae bivalent inactivated vaccine is used for preventing mycoplasma gallisepticum and mycoplasma synoviae diseases, and can realize immunization, prevent two pathogens at the same time and reduce the workload of immunization; and the prepared vaccine is stable in performance, good in immune effect, and more suitable for actual production of China.
Owner:兆丰华生物科技(南京)有限公司
Mycoplasma Hyopneumoniae Vaccine
ActiveUS20130266601A1Antibacterial agentsSsRNA viruses positive-senseImmunoglobulin IgEMycoplasma hyopneumoniae
This invention provides an immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M.hyo) whole cell preparation, wherein the soluble portion of the M.hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
Colloidal gold method detection test strip and reagent kit for IgG antibody of respiratory disease and preparation method of reagent kit
InactiveCN102928589AEasy to detectQuick checkMaterial analysisBovine respiratory diseasePrimary screening
The invention discloses a colloidal gold method detection test strip and a reagent kit for an IgG antibody of a respiratory disease and a preparation method of the reagent kit. The test strip determines the IgG antibody by using a principle of an immunocapture method; respiratory herpes viruses, adenoviruses, influenzaviruses and an IgG antibody of mycoplasma pneumoniae can be detected jointly by one operation; the operation process is simplified; the test strip is simple, convenient, rapid and accurate in detection, suitable for mass detection and applicable to primary screening and epidemiological survey; and a result is distinct and easy to distinguish.
Owner:JIANGSU KEYGEN BIOTECH CORP LTD
A temperature sensitive vaccine strain of mycoplasma hyopneumoniae and uses thereof
ActiveCN102458462AAntibacterial agentsBacterial antigen ingredientsGene listMycoplasma hyopneumoniae
The present invention relates to a Mycoplasma hyopneumoniae vaccine strain comprising a mutation in at least one of the genes listed or as deposited with the National Measurements Institute (Australia) under accession number NM04 / 41259, which strain is temperature sensitive and attenuated, a vaccine comprising such strains and methods and uses thereof.
Owner:BIOPROPERTIES +1
Vaccine composition, preparation method and application thereof
ActiveCN104248760APlay a role in immune enhancementMaintain stabilityAntiviralsAntibody medical ingredientsLevamisolePolymer
The invention provides a vaccine composition, which comprises an immune amount of a porcine circovirus antigen, an immune amount of a mycoplasma hyopneumoniae antigen, and levamisole or its derivative, and a polymer. The vaccine composition not only can have an immunological enhancement effect on two antigens at the same time, but also has stability, and can be used for dilution of porcine reproductive and respiratory syndrome live vaccines.
Owner:PU LIKE BIO ENG
Primer set for respiratory tract infection pathogen detection, rapid diagnostic kit and detection method
InactiveCN108300803AImmediate rapid diagnosisMultiplexingMicrobiological testing/measurementAgainst vector-borne diseasesPolymerase LRecombinase
Belonging to the molecular biology field, the invention relates to a primer set for respiratory tract infection pathogen detection, a rapid diagnostic kit and a detection method. The primer set can achieve one-time detection of the following 9 respiratory tract infection pathogens: influenza A virus, influenza B virus, respiratory syncytial virus, parainfluenza virus type I, parainfluenza virus type II and parainfluenza virus type III, adenovirus, mycoplasma pneumonia, legionella pneumonia, chlamydia pneumonia and human rhinovirus. The invention adopts solid phase recombinase-polymerase constant temperature gene amplification method to detect respiratory multiple infection pathogens. The primer set adopted by the invention for respiratory tract infection pathogen detection has strong specificity and high amplification efficiency, can effectively and rapidly detect the 9 pathogens simultaneously, and realizes multiplex detection.
Owner:博迪泰(厦门)生物科技有限公司
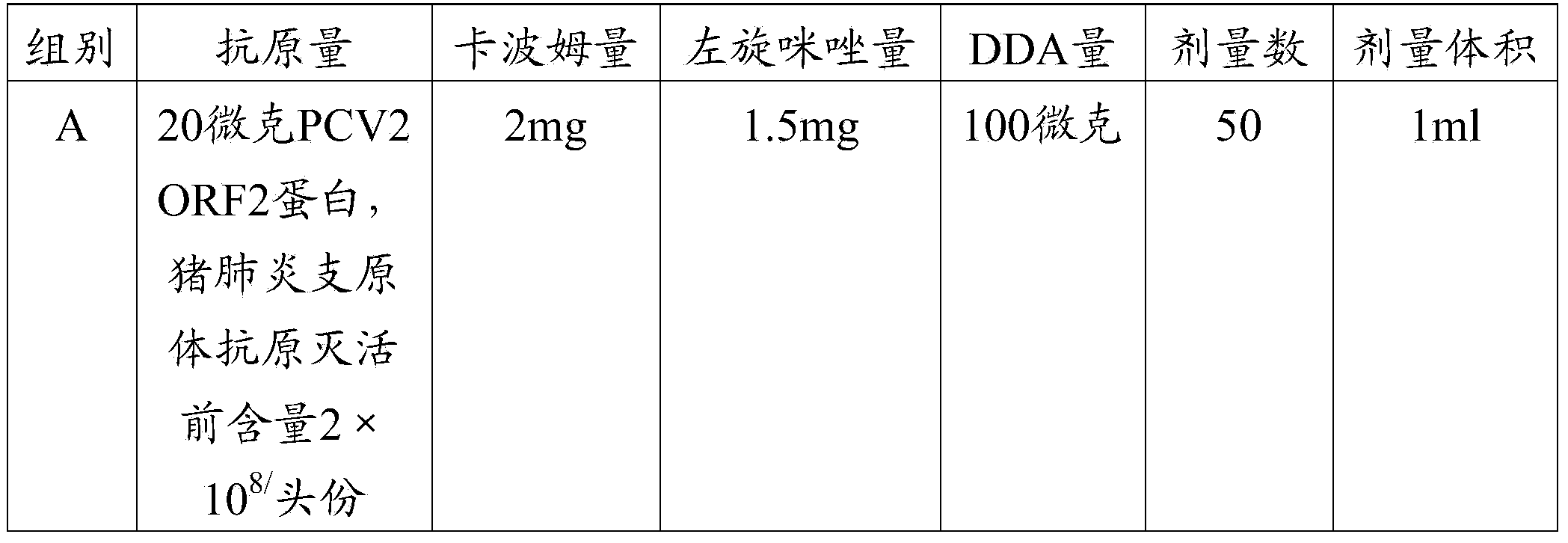
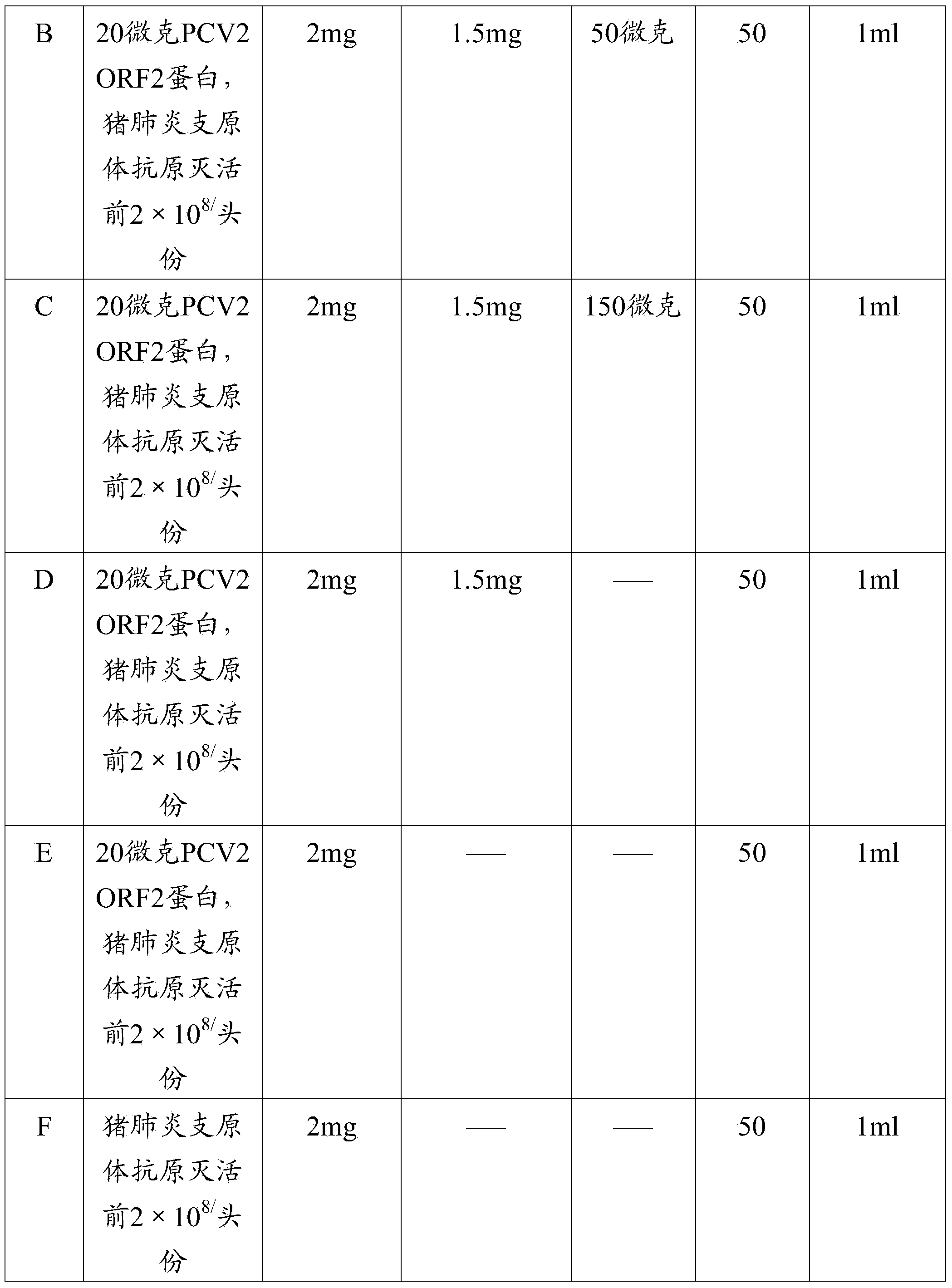
Pcv2 mycoplasma hyopneumoniae immunogenic compositions and methods of producing such compositions
ActiveUS20150174233A1Reduce incidenceReduce severityBacterial antigen ingredientsViral antigen ingredientsDiseaseMultivalent Vaccine
Multivalent combination vaccines are provided which include an immunological agent effective for reducing the incidence of or lessening the severity of M. hyo infection, preferably M. hyo bacterin, or an immunogenic composition comprising M. hyo bacterin, and at least one immunogenic active component of another disease-causing organism in swine, preferably PCV2 wherein the preferred PCV2 antigen for such a multivalent vaccine is PCV2 ORF 2 protein.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Mycoplasma bovis mutant strain with growth defect under cell coculture and application
ActiveCN109652357AMarked growth defectSignificantly small colony phenotypeBacteriaHydrolasesPhosphodiesteraseBovine embryo
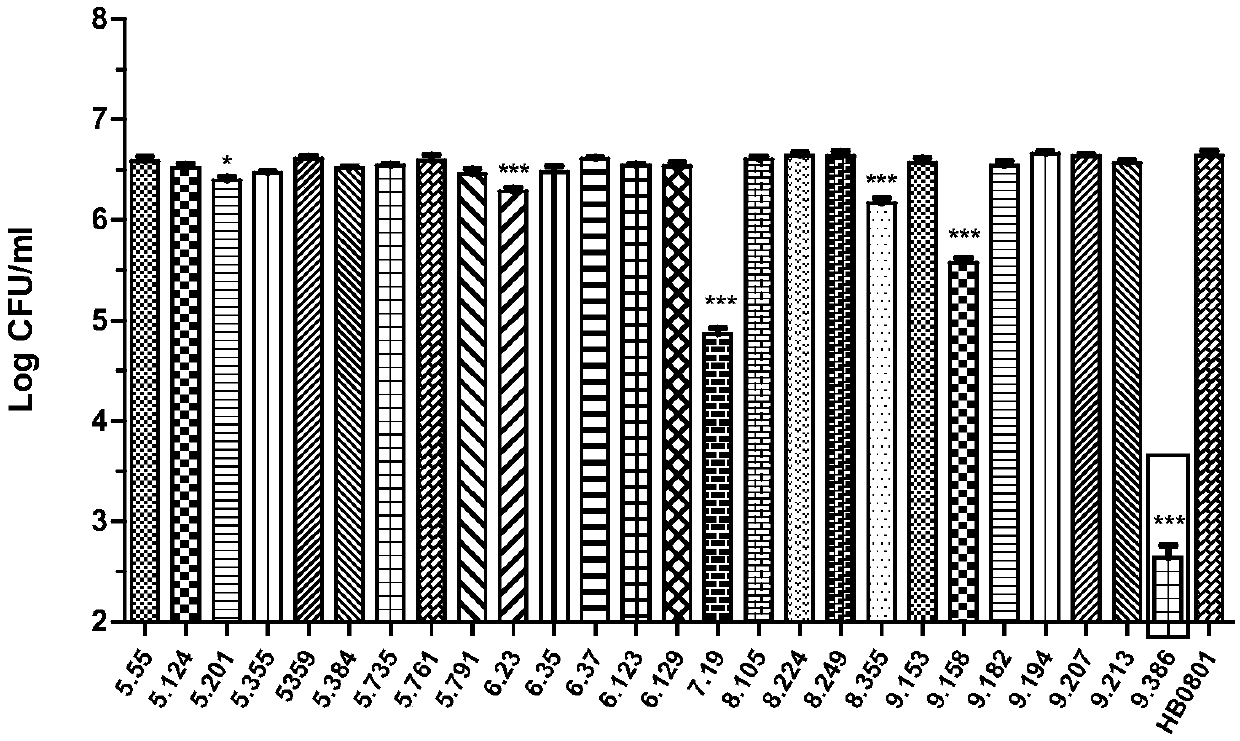
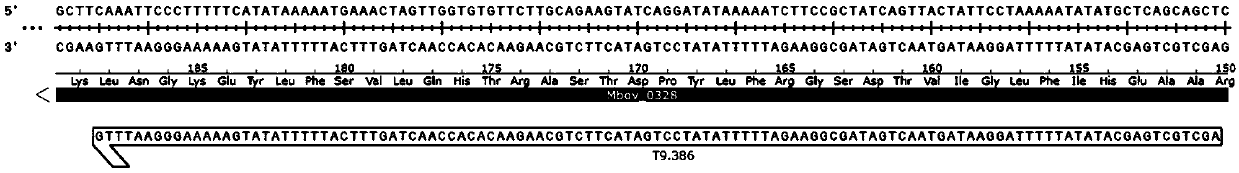
The invention belongs to the technical field of zoonosis prevention and treatment, and particularly relates to a mycoplasma bovis mutant strain with a growth defect under cell coculture and application. The mycoplasma bovis Mbov-0328 gene deletion mutant strain T9.386 is sifted out from a mycoplasma bovis gene mutant library, and the gene is coded to form cyclic dinucleotide phosphodiesterase. When the mutant strain and a bovine embryo pneumonocyte are co-cultured, the remarkable growth defect phenotype is shown; the mutant strain shows small colonial morphology on a PPLO solid culture medium.Proteomics between the mutant strain and a wild strain shows a remarkable difference expression spectrum. The mutant strain has 38 differential expression proteins, wherein 30 proteins are in up-regulated expression, and 8 proteins are in down-regulated expression. The mutant strain can be applied to the field of mycoplasma bovis metabolic physiology, pathopoiesia and immune prevention.
Owner:HUAZHONG AGRI UNIV
Detection method for specific antibodies IgM of mycoplasma pneumonia and influenza viruses based on micro-fluidic chip
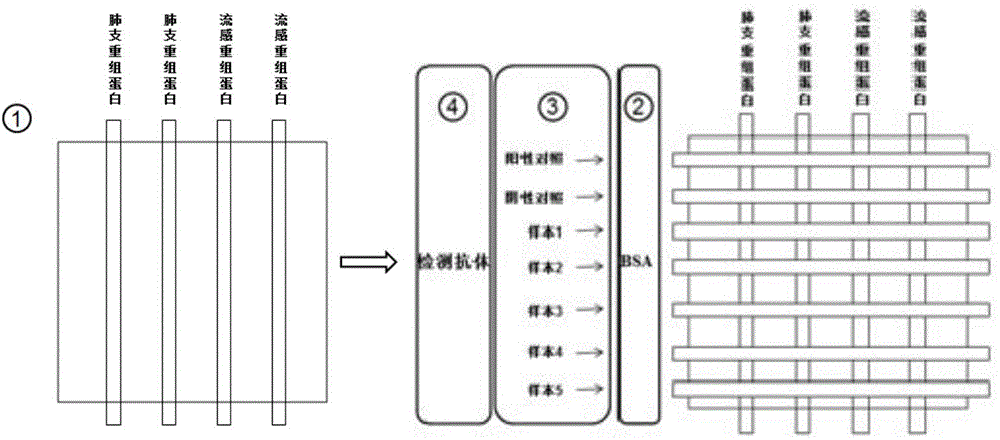
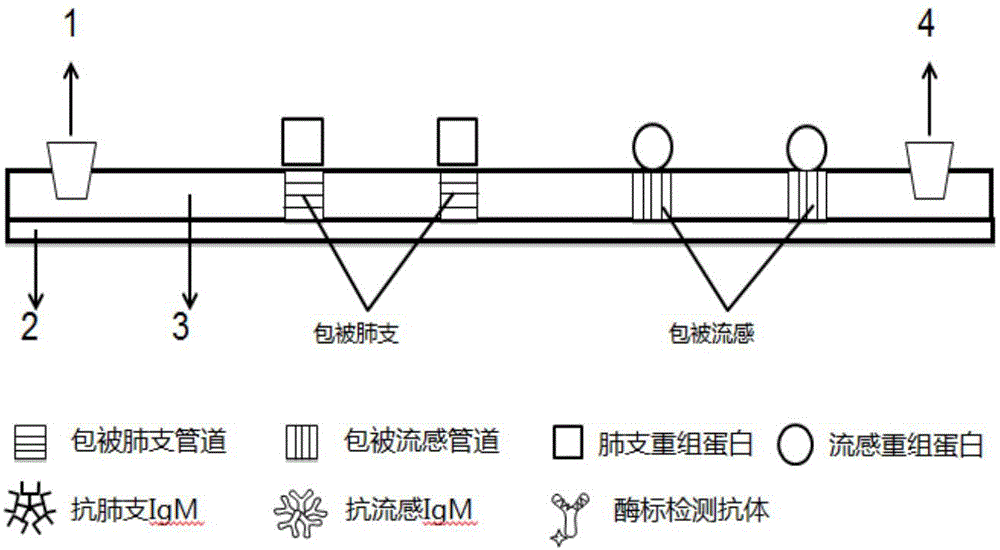
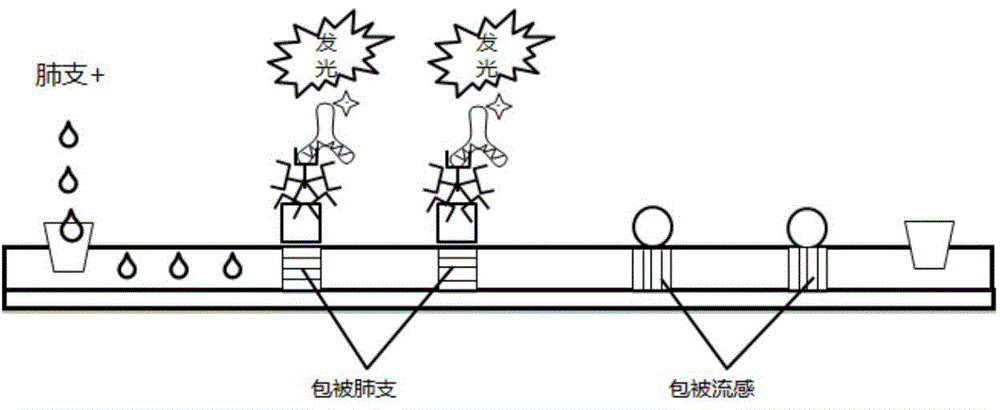
ActiveCN104360060AAvoid non-specific reactionsMeet testing needsChemiluminescene/bioluminescenceBiological material analysisSmall sampleSpecific antibody
The invention relates to a detection method for specific antibodies IgM of mycoplasma pneumonia and influenza viruses based on a micro-fluidic chip and application of the detection method. The method is characterized by taking the micro-fluidic chip with a plurality of pipelines as a reaction platform and detecting by using a chemical luminescent signal so as to independently or simultaneously detect the serum specific antibodies IgM of mycoplasma pneumonia and influenza viruses. As the micro-fluidic chip is combined with chemiluminiscence, the detection method integrates the advantages of rapidness, high efficiency and small sample amount of the micro-fluidic chip and high specificity of chemiluminiscence, also has the advantages of simplicity in operation, low cost, rapidness and the like, is a multi-target, real-time and high-sensitivity detection method and can be applied to rapid diagnosis of pneumonia and influenza.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) diagnostic kit of mycoplasma gallisepticum
InactiveCN102221616AIndirect ELISA method optimizationAntigenic stabilityBiological testingSorbentElution
The invention discloses an indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) diagnostic kit of mycoplasma gallisepticum, which comprises an ELISA plate coated by species specificity proteins PvpA, an elution buffer solution, an antibody diluting solution, an ELISA secondary antibody, a substrate color developing solution and a stop solution. The kit disclosed by the invention selects the species specificity proteins PvpA, covers the high-frequency variation regions: DR-1 and DR-2 regions of the PvpA proteins, has high specificity and immunogenicity, improves the specificity and sensitivity of the detection result, lowers the production cost and is suitable for popularization and use in grassroots veterinarian mechanisms.
Owner:SOUTH CHINA AGRI UNIV
Mycoplasma bovis immunity-related protein P22, nucleotide sequence for coding same and application thereof
The invention discloses a mycoplasma bovis immunity-related protein P22, a nucleotide sequence for coding the same and an application thereof. A mycoplasma bovis Hubei strain is taken as a sample, and an efficient two-dimensional electrophoresis system of a mycoplasma bovis whole protein is established, so that a two-dimensional electrophoretogram with high resolution and high repeatability is obtained; an immunity-related protein is screened in combination with Western blot, and is named P22 protein; and as proved by a prokaryotic expression result of the protein, a Western blot rest result of a recombinant protein and a mycoplasma bovis positive serum is negative, and a Dot blot test result is positive. The protein is proved to be possibly a conformation-dependent immunity-related protein of mycoplasma bovis, and the mycoplasma bovis immunity-related protein P22 plays a guidance role in diagnosing, preventing and treating a mycoplasma bovis disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
LAMP (loop-mediated isothermal amplification) kit for detection of mycoplasma pneumoniae and special LAMP primer for detection of mycoplasma pneumoniae
InactiveCN105238860AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesLoop-mediated isothermal amplificationPneumonitis
The invention discloses a LAMP (loop-mediated isothermal amplification) kit for detection of mycoplasma pneumoniae and a special LAMP primer for detection of mycoplasma pneumoniae. The special LAMP primer for detection of mycoplasma pneumoniae is designed according to a specificity conservative target sequence of a mycoplasma pneumoniae P1 gene (GenBank number: CP002077.1). The LAMP primer is formed by six primers including outer primers MP-16F3 and MP-16B3, inner primers MP-16FIP and MP-16BIP and loop primers MP-16LF and MP-16LB. By the aid of the LAMP kit and the special LAMP primer for detection of mycoplasma pneumoniae, quickness, convenience, high efficiency, high specificity and high sensitivity in qualitative detection of the mycoplasma pneumoniae in samples of pure bacteria, sputum, bronchoalveolar lavage fluid, throat swabs and the like can be realized without complicated instruments, and a new technical platform is provided for detection of the mycoplasma pneumoniae.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION +1
Mycoplasma pneumonia mosaic antigen, antigen detection reagent, and preparation method of both
InactiveCN107573417AStrong specificityEasy to culture and purifyBiological testingHybrid peptidesAntigenMycoplasma pneumonia
The invention provides a mycoplasma pneumonia mosaic antigen amino acid sequence containing an amino acid sequence as shown in SEQ ID NO:1 as well as a full-gene synthesized mycoplasma pneumonia mosaic antigen full-gene sequence containing an amino acid sequence as shown in SEQ ID NO:2. The invention also provides a method for constructing the two gene sequences. The invention also provides a preparation method of the mycoplasma pneumonia mosaic antigen containing full-gene synthesis, a mycoplasma pneumonia detection kit and a preparation method thereof. Mp recombinant mosaic antigen is selected as a mark material and is applied to a gold immunochromatography system, and the detection system is directly marked and captured, so the sensitivity is greatly improved, the specificity of the antigen is high, the antigen is easy in cultivation and purification, and cost is reduced; and a new method for detecting mycoplasma pneumoniae IgG rapidly and accurately is provided for clinical use, and a good market prospect is achieved.
Owner:HANGZHOU CLONGENE BIOTECH
Primer for detecting sheep mycoplasma pneumoniae
PendingCN107502676AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationFluorescencePcr method
The invention provides a primer for detecting sheep mycoplasma pneumonia. The sequence of the primer comprises an upstream primer: 5'-GGGACTTCGGGACTTATTGGA-3', and a downstream primer: 5'-CACGAGATGCAAACTGATTTACTTG-3'. According to a p80 gene sequence of KM435069.1 logged in GenBank, a specific primer is designed, a real-time fluorescence quantification PCR (polymerase chain reaction) method is established for a p80 gene of sheep mycoplasma pneumonia for a first time, and the method is good in specificity, high in sensitivity and good in repeatability and can be applied to diagnosis and epidemiological investigation on sheep mycoplasma pneumonia.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Chinese medicinal composition for treating ovine mycoplasma pneumoniae
InactiveCN102228611AHeat-clearing and detoxifyingWith cough and phlegmAntibacterial agentsRespiratory disorderMedicinal herbsSyndrome differentiation
The invention relates to a Chinese medicinal composition for treating ovine mycoplasma pneumoniae, which comprises the following components in part by weight: 15 to 25 parts of Vietnamese sophora root, 15 to 25 parts of indigowoad root, 15 to 25 parts of indigowoad leaf, 5 to 15 parts of baical skullcap root, 15 to 20 parts of barbed skullcap herb, 5 to 15 parts of caladium, 15 to 20 parts of Indian buead, 20 to 30 parts of astragalus, 15 to 20 parts of szechuan tangshen root, 10 to 20 parts of Szechuan lovage rhizome and 5 to 15 parts of liquoric root. In the Chinese medicinal composition, a pure Chinese medicinal herb preparation is developed by combining the research achievements of the pharmacology of modern Chinese veterinary medicines, formulating prescriptions elaborately and screening strictly according to the treatment based on syndrome differentiation and holism concept in Chinese veterinary science. The Chinese medicinal composition consists of the following 11 Chinese medicinal herbs of the Vietnamese sophora root, the indigowoad root, the baical skullcap root, the barbed skullcap herb, the caladium, the Indian buead, the astragalus, the Chinese angelica, the szechuan tangshen root, the Szechuan lovage rhizome, the liquoric root and the like, and has the effects of clearing heat, detoxicating, relieving cough, reducing sputum, tonifying qi and promoting blood circulation.
Owner:SRICK TIANJIN BIO TECH
Swine testicular cell strain ST-S suitable for suspension culture as well as acquisition method and application of swine testicular cell strain ST-S
Owner:JINYUBAOLING BIO PHARMA CO LTD +1
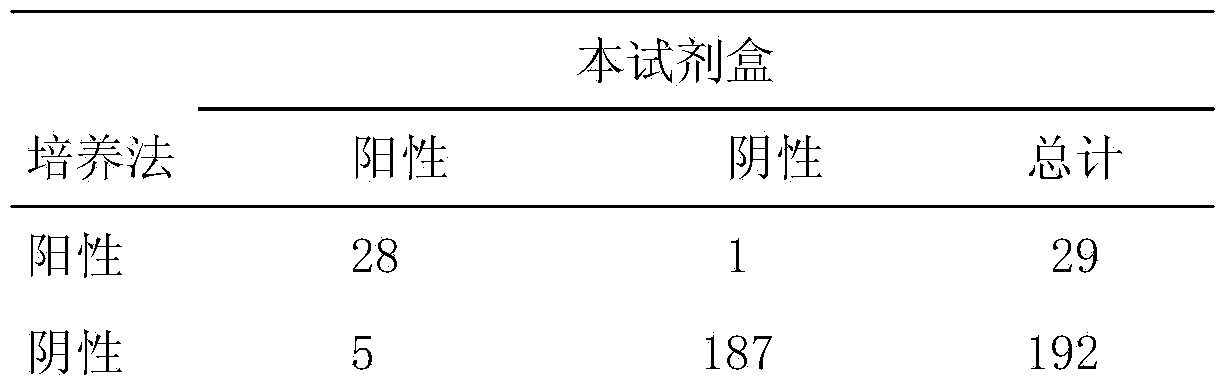
Kit for detecting mycoplasma hominis nucleic acid through PCR-fluorescence probe method and detecting method of kit
InactiveCN104988242AShort detection timeShort windowMicrobiological testing/measurementHuman DNA sequencingPositive control
The invention relates to a kit for detecting mycoplasma hominis nucleic acid through a PCR-fluorescence probe method and a detecting method of the kit. The kit comprises a nucleic acid extracting set and a nucleic acid amplification detecting set. The nucleic acid amplification detecting set comprises PCR reaction liquid, negative control and positive control. The PCR reaction liquid comprises DNA polymerase, UNG enzymes, reaction buffer liquid, a primer, a probe and Mg2+. The positive control comprises positive plasmids and human genomes. The negative control comprises human genomes. The probe is a Taqman fluorescence probe. The special primer probe is designed according to an MH polymerase conservative target sequence by means of the kit and the method, the MH is rapidly detected through the PCR-fluorescence probe method, and the result is more reliable and accurate. Compared with an existing culture method, operation is easy and convenient and the speed is fast when mycoplasma hominis is identified; the whole process is completed within 3 hours; specificity and flexibility are high, and the result is visual.
Owner:SHANGHAI REPODX BIOTECH CO LTD
PCV/Mycoplasma hyopneumoniae combination vaccine
ActiveUS9125885B2Antibacterial agentsBacterial antigen ingredientsImmunglobulin eMycoplasma hyopneumoniae
This invention provides a multivalent immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M. hyo) whole cell preparation; and a porcine circovirus type 2 (PCV2) antigen, wherein the soluble portion of the M. hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
Chinese isolate of Leachiii mycoplasma, isolation medium and purpose thereof
InactiveCN102344892AImproving immunogenicityTrigger immune responseAntibacterial agentsBacterial antigen ingredientsMicroorganismWhole body
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Lyophilized reagent capable of simultaneously distinguishing three pathogen nucleic acids
InactiveCN108411037AImprove stabilityAmplified detectionMicrobiological testing/measurementMicroorganism based processesLiquid nitrogenReaction system
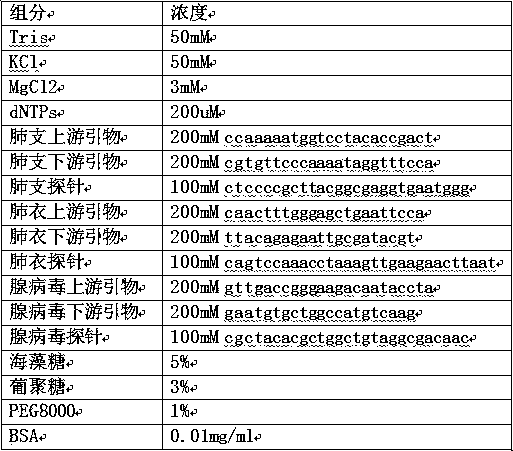
The invention discloses a lyophilized reagent for nucleic acid diagnosis. The lyophilized reagent comprises a lyophilized protecting agent which contains 2%-10% of trehalose, 2%-5% of glucan, 0.5%-2%of PEG8000 and 0.01mg-0.02mg / ml of BSA protein, and the lyophilized protecting agent contains mycoplasma pneumoniae, chlamydia pneumoniae and adenovirus primers and probes. The lyophilized reagent hasadvantages that since the lyophilized reagent contains three pathogen nucleic acids of mycoplasma pneumoniae, chlamydia pneumoniae and adenovirus, amplification detection of various pathogen nucleicacids can be realized, namely three targets can be simultaneously detected by one reaction system, and accordingly reagent and detection consumables are greatly saved, high detection throughput is realized, and a clinical application range is expanded. In application of the lyophilized reagent for lyophilization treatment, liquid nitrogen is adopted for quick pre-freezing of a liquid reagent, so that a subsequent lyophilization process is shortened, and stability of the lyophilized reagent is improved.
Owner:AUTOBIO DIAGNOSTICS CO LTD
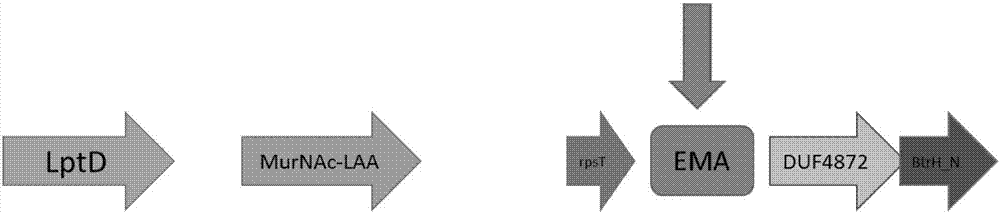
Primer combination for detecting PCR of Elizabethkingia meningoseptica
ActiveCN107083443AImprove featuresIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseStaphylococcus aureus
The invention discloses a primer sequence combination and a method for applying the primer sequence combination in PCR amplification. The amplification target in the method is specific fragments EMA inside the genome of Elizabethkingia meningoseptica, and the method shows excellent specificity and sensibility; and moreover, by using the method, the neisseria meningitidis, haemophilus influenzae, staphylococcus aureus, streptococcus pneumoniae, escherichia coli, listeria monocytogenes, mycoplasma pneumoniae, bordetella pertussis, klebsiella pneumoniae and mycobacterium tuberculosis and the other pathogenic bacteria which are likely to cause meningitis and diseases of the upper respiratory tract, and easy to be confused with the Elizabethkingia meningoseptica in an identification of bacteria, can be distinguished at one time. Additionally, the lower detection limit of the method can reach 10-4 ng per Mul.
Owner:ICDC CHINA CDC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com