A temperature sensitive vaccine strain of mycoplasma hyopneumoniae and uses thereof
A technology of mycoplasma hyopneumoniae and vaccine strains, applied in the application field of protecting pigs from mycoplasma pneumonia, can solve the problems of weakening the severity and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1: the preparation of vaccine strain
[0076] The Australian M. hyopneumoniae isolate LKR is a slaughtered sample of pig lung showing typical enzootic disease (Lloyd and Etheridge (1981), J. Comp. Path. 91:77-83). The isolate was cultured and maintained at the Mycoplasma Reference Culture Center at the University of Adelaide, South Australia. Cultures of this isolate were subsequently obtained from the Mycoplasma Group at the Victoria University of Melbourne.
[0077] After the culture was passed three times in vitro, it was treated with 200 mg / ml of N-methyl-N'-nitro-N-nitrosoguanidine (N-Methyl-N'-nitro-N-nitrosoguanidine (NTG)), using Mutations were performed as previously mentioned (Nonamura and Imada (1982) Avian Diseases 26: 763-775). The brief description is as follows: the culture of Mycoplasma hyopneumoniae strain LKR was grown in culture medium to the late logarithmic phase, and the pellet was obtained by centrifugation. Cells were washed with ph...
Embodiment 2
[0081] Embodiment 2: vaccine preparation
[0082] To prepare the vaccine, cultures of the Ts19 strain were grown in modified Friis medium containing phenol red (pH indicator) at 33°C until an acidic color change was observed, and the vaccine was titrated in a modified 96-well microtiter plate. in Friis medium. Serial ten-fold dilutions were performed in the first 10 of 12 columns of the microtiter plate. The last two columns were left uninoculated as media only (negative) controls. Use up to six microtiter plates per titration.
[0083] After incubation for up to three weeks, plates were scored for color change and the most likely number (MPN) was used to determine the average titer. The final average titer was expressed as color change units (CUU) per milliliter.
[0084] The Ts19 vaccine culture was kept as a "wet frozen" format for long-term storage at -70°C to -80°C. Alternatively, the Ts19 vaccine strain culture can be freeze-dried and stored at -20°C for a long term...
Embodiment 3
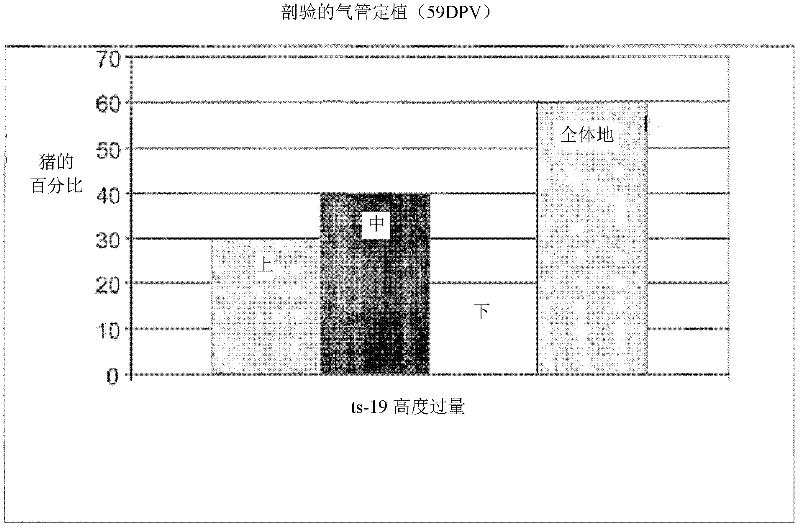
[0085] Example 3: Vaccine Safety and Colonization Study
[0086] In order to evaluate the safety of the ts19 vaccine strain, the study design had to use six-week-old pigs from a mycoplasma-uninfected herd. The study was conducted under the PC2 biosafety level standard set by the CSIRO (Commonwealth Serum Industry Research Organization) in Werribee, Victoria, Australia. All 20 pigs were randomly divided into two groups, 10 in each group (see Table 2).
[0087] Table 2: Safety studies of ts19 vaccine strains
[0088]
[0089] The vaccine was delivered by the intranasal route, so the safety of the vaccine was only tested on mucosal membranes. Group 1 (unvaccinated controls) as well as Group 2 (highly overvaccinated pigs) were clinically observed for 59 days (Table 2).
[0090] In this safety study, all pigs were monitored twice daily, including rectal body temperature, from the day before vaccination until four days after vaccination, and all pigs were monitored for respira...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com