Mycoplasma hyopneumoniae and Mycoplasma hyorhinosus dual inactivated vaccine and preparation method thereof
A technology of Mycoplasma hyopneumoniae and double inactivated vaccine, which is applied in the directions of bacterial antigen components and antibacterial drugs, can solve the problems of weak specificity and easy mutation of Mycoplasma hyopneumoniae, achieve good immunity and prevent multiple plasma Occurrence of meningitis or arthritis, effect of vaccine cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
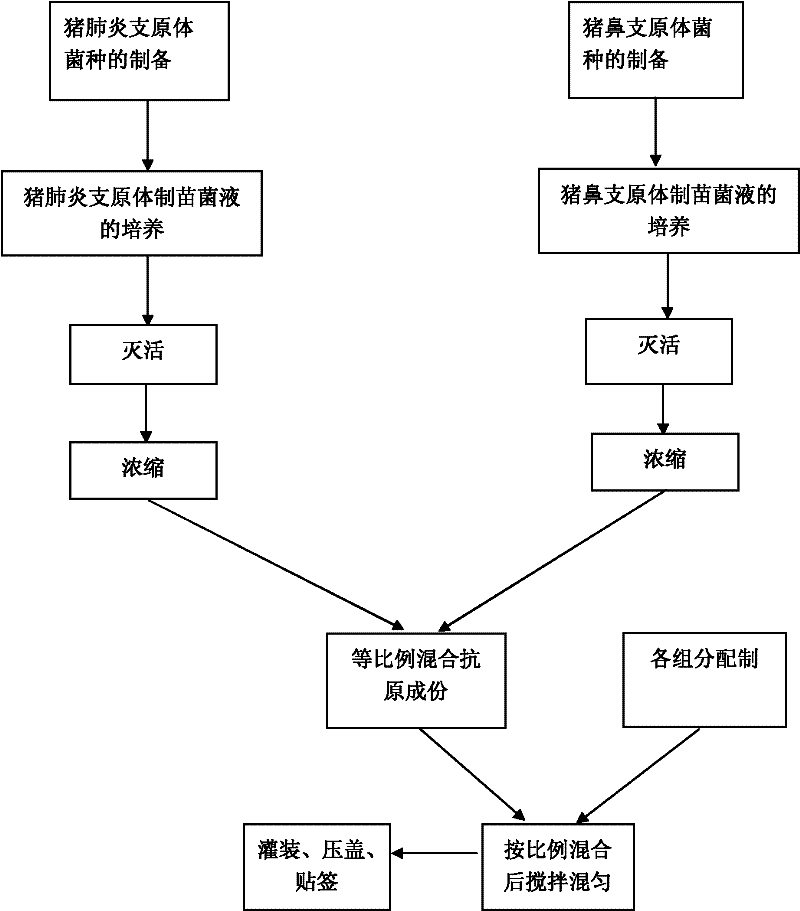
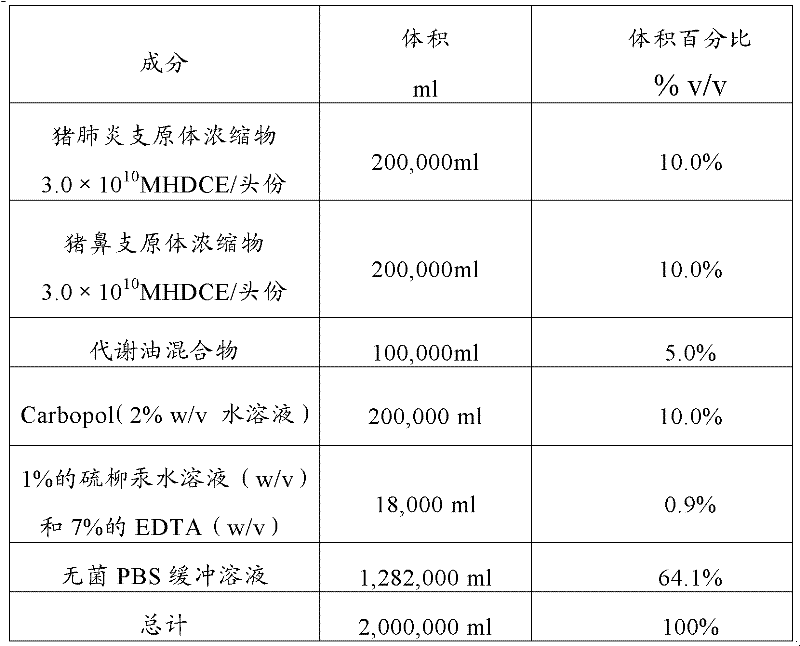
[0025] The preparation method of the dual inactivated vaccine of embodiment 1, mycoplasma swine pneumonia, mycoplasma hyorhinosum
[0026] The strain Mycoplasma suis pneumonia was selected as MR48 strain, which was preserved in the General Microbiology Center of China Microbiological Culture Collection Management Committee on September 19, 2006. The English name is Mycoplasma hyopneumoniae, and the address of the preservation unit is: Institute of Microbiology, Chinese Academy of Sciences, No. 1 Beichen West Road, Chaoyang District, Beijing. Mycoplasma hyorhinosum CVCC361 strain was purchased from China Veterinary Drug Control Institute.
[0027] The steps to prepare the dual vaccine are as follows:
[0028] 1). Bacteria culture
[0029] 1.1 Culture of swine mycoplasma pneumoniae
[0030] The selected strain of Mycoplasma suis pneumonia was MR48 strain, which was preserved in the General Microorganism Center of China Committee for the Collection of Microbial Cultures on Sep...
Embodiment 2
[0097] Embodiment 2 Mycoplasma hyopneumoniae, Mycoplasma hyopneumoniae dual inactivated vaccine and the immune effect comparison of using Mycoplasma hyopneumoniae inactivated vaccine alone
[0098] 1. Materials The trial product of Mycoplasma hyopneumoniae and Mycoplasma hyorhina dual inactivated vaccine in Example 1; American Schering-Plough inactivated vaccine against Mycoplasma suis pneumonia (J strain) (batch number 100806).
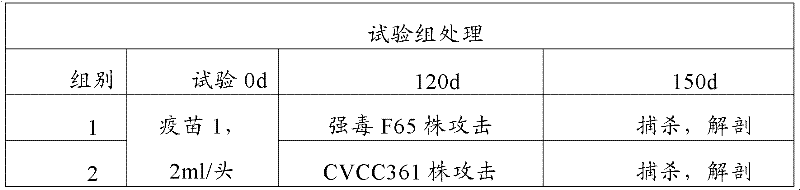
[0099] 2 Animal experiment design
[0100] Select 60 weaned piglets aged 21 to 25 days, and divide them into 6 groups, 10 pigs in each group (see the table below); each pig in the 1st and 2nd groups was injected intramuscularly with the double inactivated vaccine of Mycoplasma hyopneumoniae and Mycoplasma hyorhina respectively in the neck 2ml; each pig in the third group was intramuscularly injected with 2ml of the commercialized vaccine of Mycoplasma hyopneumoniae in the neck; the fourth and fifth groups were used as the challenge control, without v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com