LAMP primer composite for detecting respiratory pathogens and kit of LAMP primer composite
A primer composition and primer set technology, applied in the field of nucleic acid amplification, can solve the problems of difficult on-site detection, time-consuming and laborious, cumbersome operation process, etc., and achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] This example describes the microfluidic chip used in the present invention.
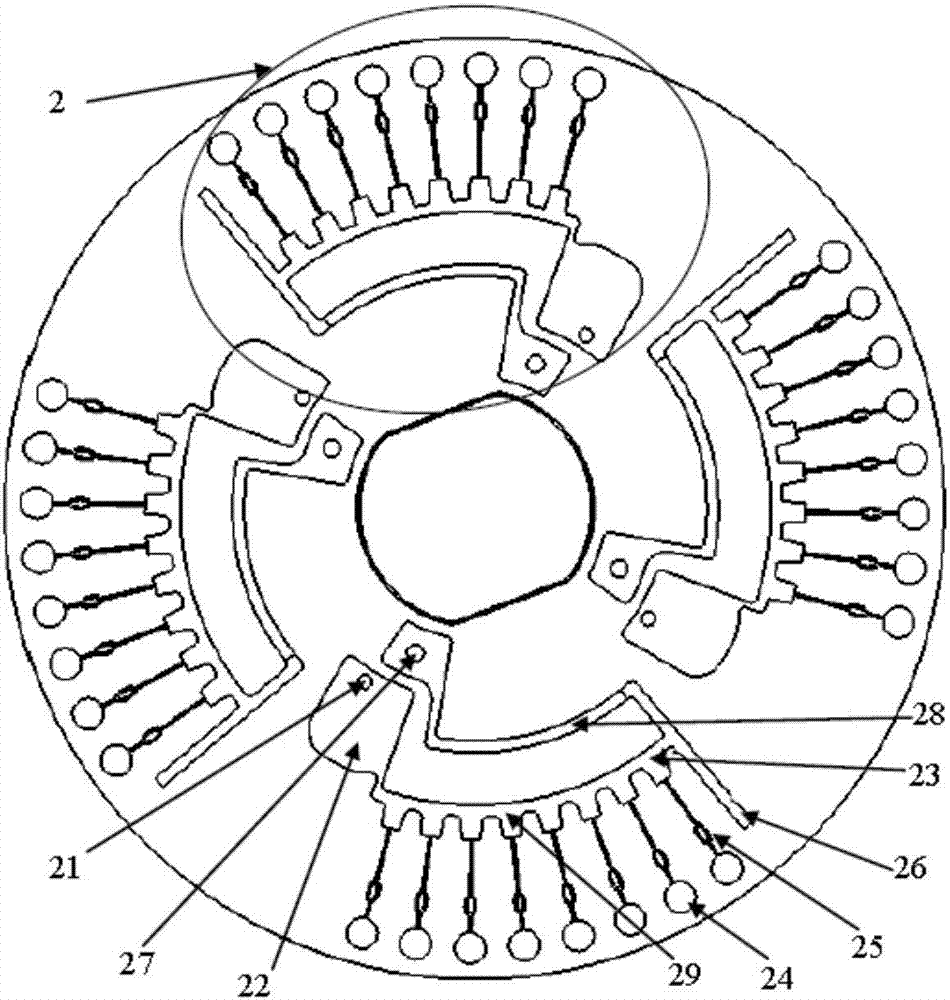
[0073] Such as figure 1 As shown, the microfluidic chip used in the present invention is a disc-shaped microfluidic chip, which includes four reaction detection parts 2, and each reaction detection part 2 includes a sample injection hole 21, a liquid storage area 22, and a reserved area 23 , reaction hole 24, ball valve 25, waste liquid tank 26, exhaust hole 27, first arc channel 28 and second arc channel 29, above-mentioned first arc channel 28 and second arc channel 29 are located on the film 1 , the first arc channel 28 connects the reserved area 23 and the exhaust hole 27, the second arc channel 29 connects the sample hole 21, the liquid storage area 22 and the reserved area 23, and the above-mentioned ball valve 25 is located in the reserved area 23 and the reaction Between wells 2, each reaction detection section has 8 reaction wells.
[0074] The above-mentioned microfluidic chip perf...
Embodiment 2
[0077] This example is the LAMP primer composition and kit used in the present invention for detecting respiratory pathogens.
[0078] The LAMMP primer composition of the present invention includes at least one of the six pathogen primer sets. The respiratory pathogens include at least one of the following six pathogens: Mycoplasma pneumoniae, Chlamydia pneumoniae, influenza A / B virus, parainfluenza virus, adenovirus, respiratory syncytial virus. The six primer sets corresponding to the above pathogens are Mycoplasma pneumoniae primer set, Chlamydia pneumoniae primer set, Influenza A / B virus primer set, Parainfluenza virus primer set, Adenovirus primer set, Respiratory syncytial virus primer set, the primer set Quality control primer sets (negative quality control, positive quality control) are also included. See Table 1 for details of the primer sequences of the above primer sets.
[0079]The present invention relates to a kit containing the above-mentioned LAMP primer comp...
Embodiment 3
[0081] This embodiment is a non-diagnostic method for detecting respiratory pathogens using the primer composition and kit described in Embodiment 2, which includes the following steps:
[0082] 1. Cleaning steps of the microfluidic chip;
[0083] The microfluidic chip is cleaned by plasma, and the surface of the microfluidic chip is washed with a plasma cleaner, and air-dried with an inert gas.
[0084] 2. Coating of primer composition;
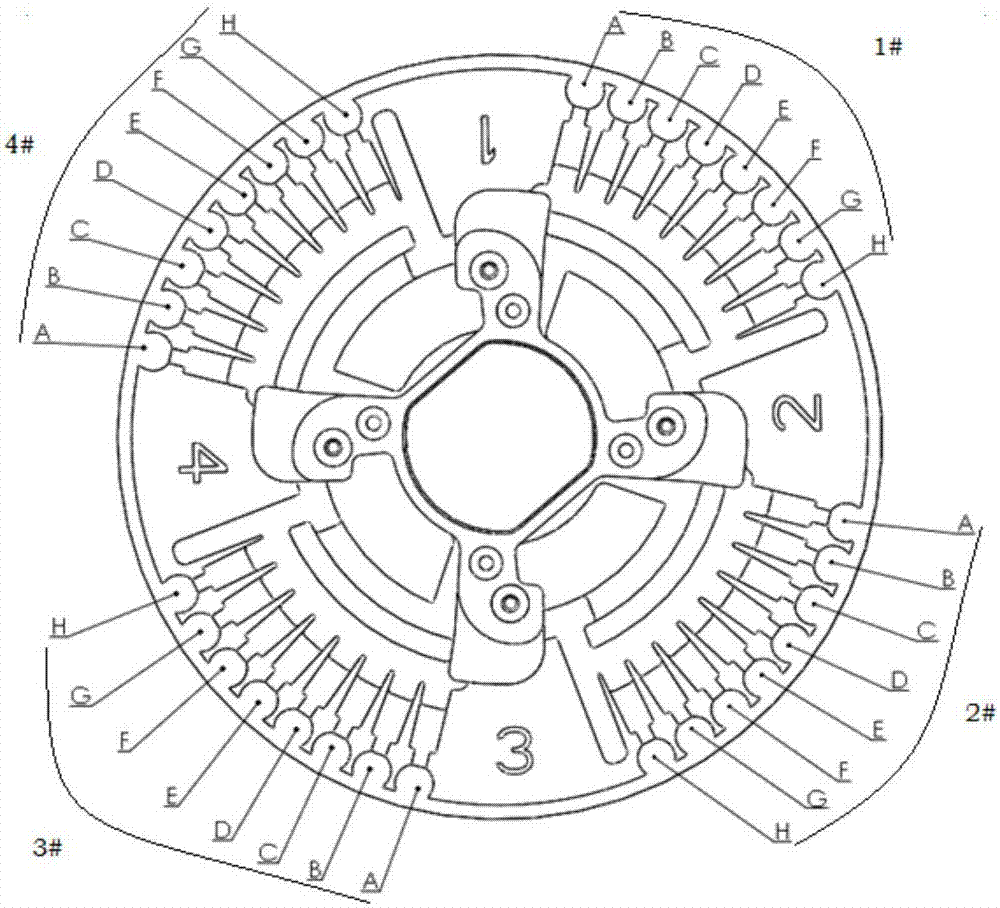
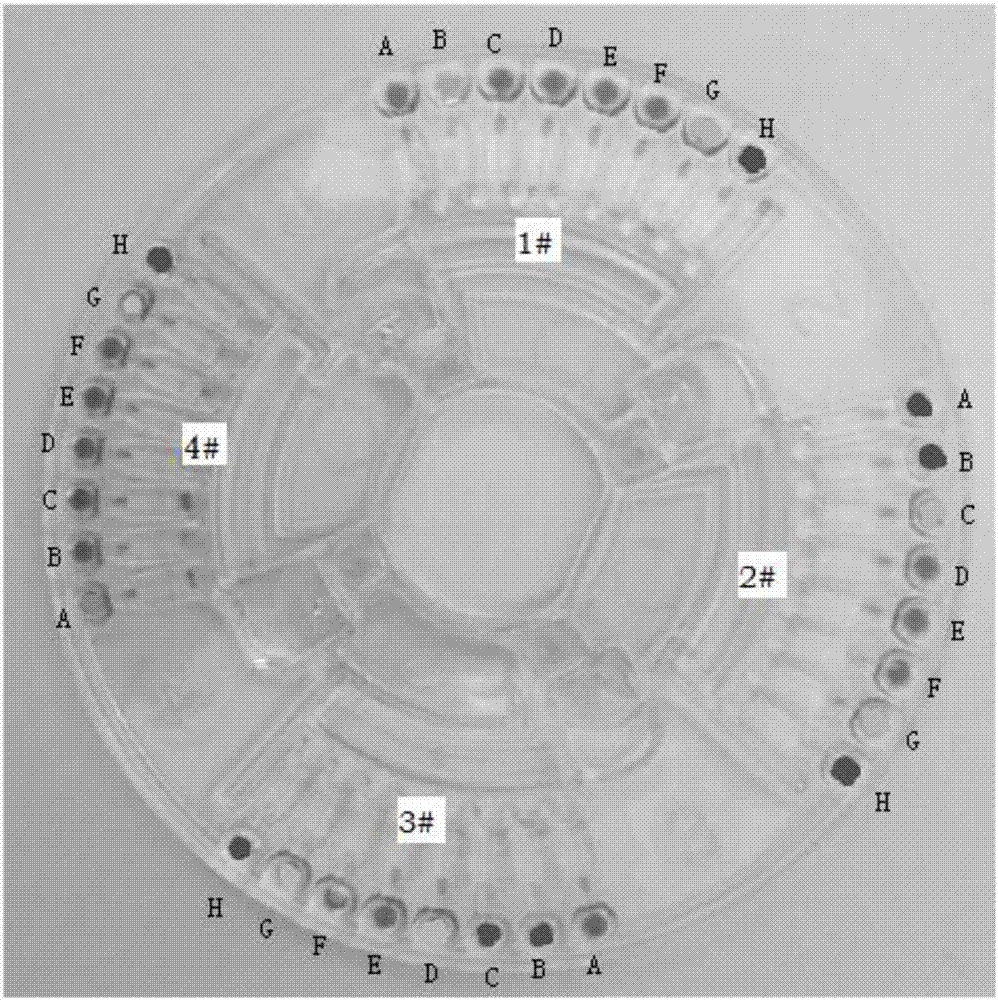
[0085] Such as figure 2 and image 3 As shown, the Mycoplasma pneumoniae primer set, Chlamydia pneumoniae primer set, Influenza A / B virus primer set, Parainfluenza virus primer set, Adenovirus primer set, Respiratory syncytial virus primer set, quality control primer set were mixed with agarose , prepared into a corresponding mixed solution, in the above mixed solution, containing 4-8 μM inner primer 1, 4-8 μM inner primer 2, 1 μM outer primer 1, 1 μM outer primer 2, the final concentration of agarose in the mixed solution is 0.1% (mass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com