Gene fragment, recombinant vector and use thereof for improving expression level of exogenous gene in mammalian cells
An exogenous gene and gene technology, applied in the field of genetic engineering, can solve the problems of reducing the half-life of mRNA, unfavorable RNA secondary structure, and lack of verification of expression, so as to overcome the position effect, have good application prospects, and reduce losses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
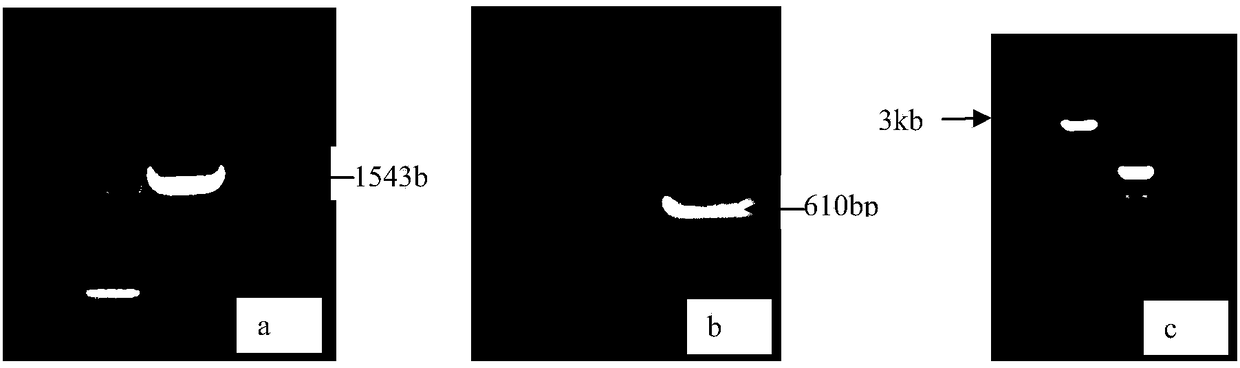
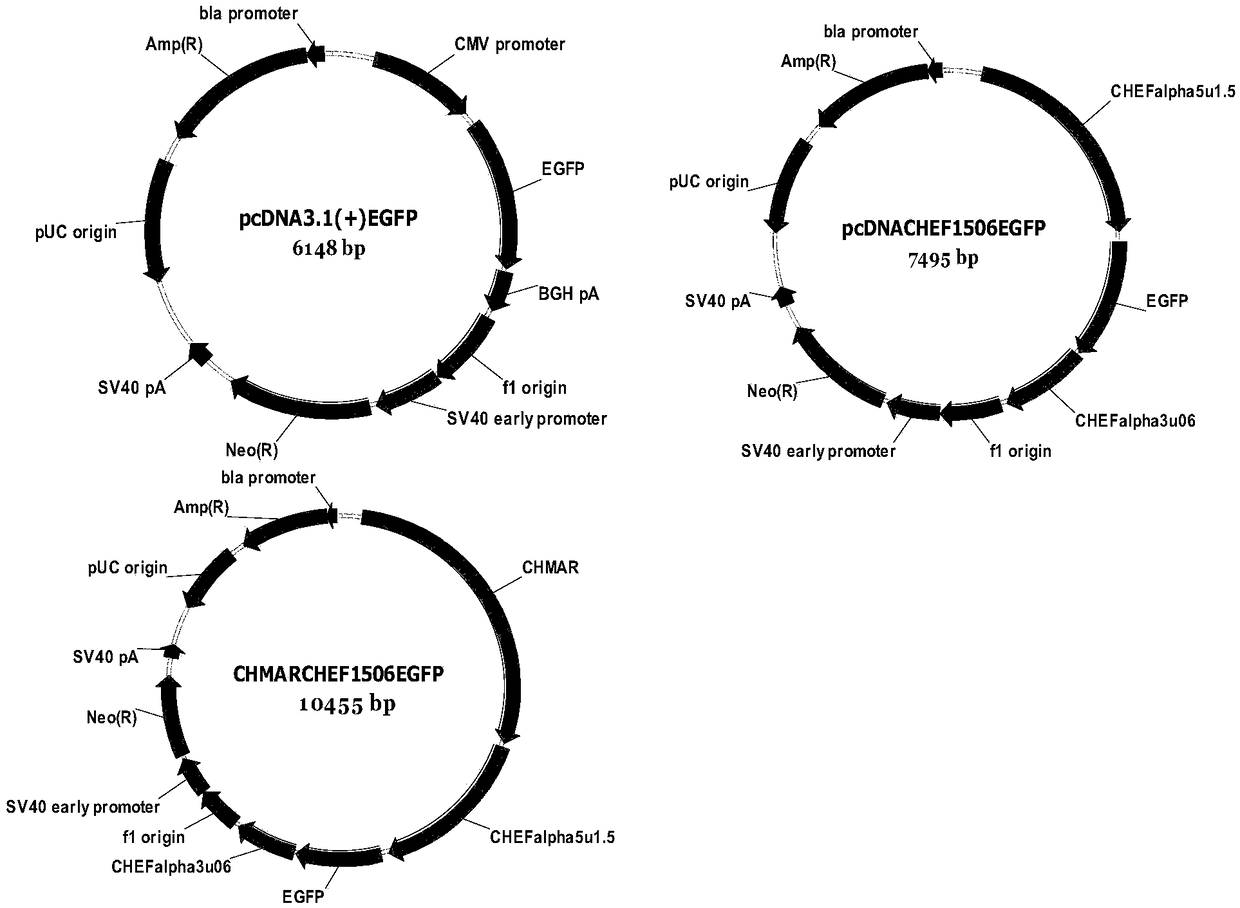
[0026] Embodiment 1 Construction of the recombinant vector of the present invention and its effect verification
[0027] 1. Construction method
[0028] (1) Cloning of the transcriptional regulatory sequence of CHO cell EF-alpha1 and the MAR sequence of chicken lysozyme.
[0029] 1. Extraction of CHO cell genomic DNA sequence
[0030] CHO cells were cultured with D / F medium 10% serum until the cell square flask was overgrown, and digested with trypsin. Genomic DNA of CHO cells was extracted according to the operating instructions of Transgen's EasyPure Genomic DNA kit.
[0031] 2. Cloning of the gene transcription regulatory sequence of CHO peptide elongation factor 1
[0032] According to the sequence design of Genbank's report following primers:
[0033] CHEF1:5-ata acgcgt GCAGATCCGT CGAGCTCTCG GCCACCGAGC-3
[0034] CHEF2:5-ata gctagc ACACCTTAAA AAAAAAGTTC GAAGAATACC-3
[0035] CHEF3:5-ata tctaga AATATTACCC CTAACACCTG CCACCCCAGT C-3
[0036] CHEF4:5-ata tccgga AGCAAAGCC...
Embodiment 2
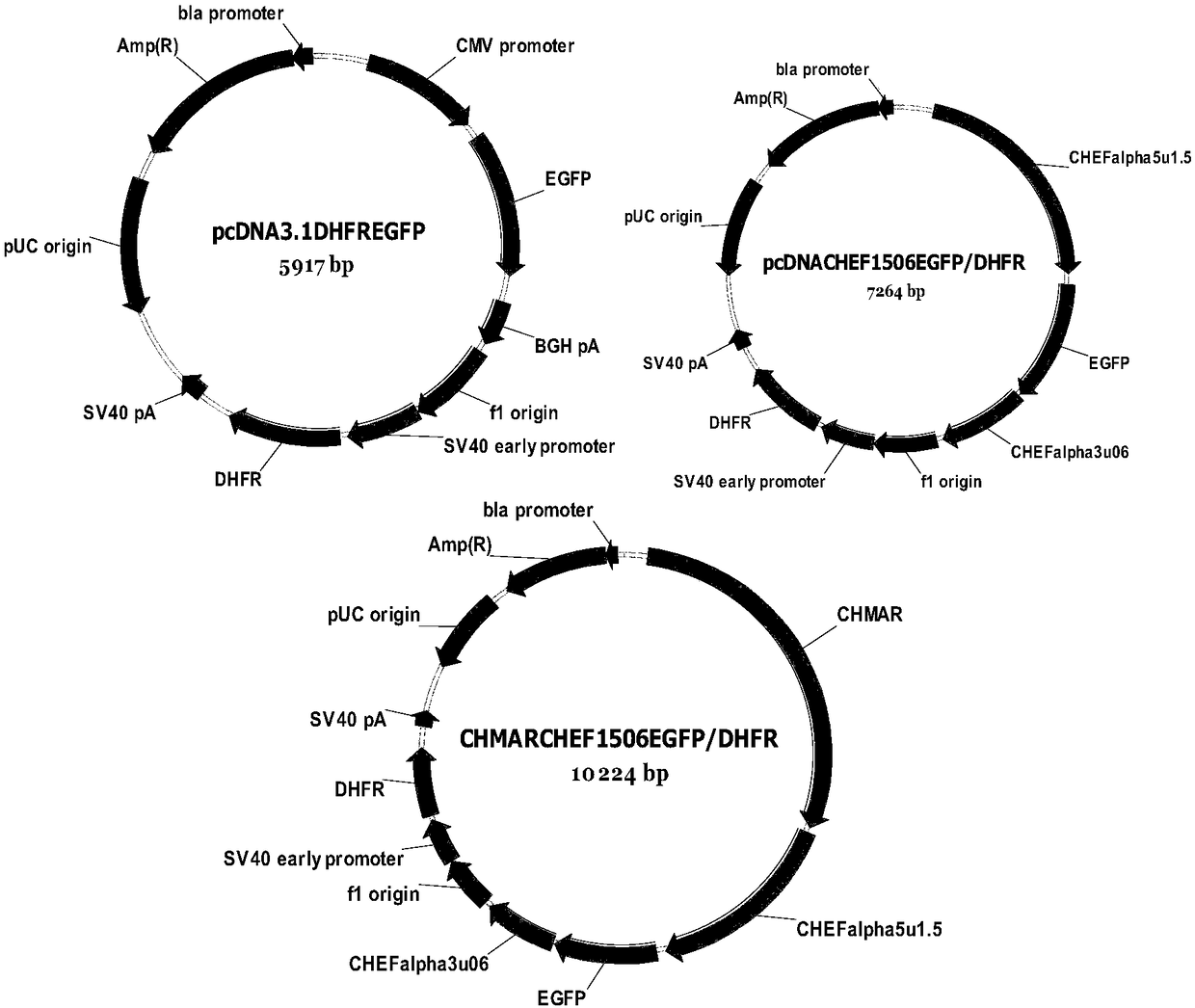
[0061] Example 2 Expression of exogenous protein using the combination carrier CHMARCHEF1506 / DHFR of the present invention
[0062] Example 1 shows that the carrier CHMARCHEF1506DHFR expresses good fluorescence intensity of EGFP, indicating that the gene 5' and 3' end regulatory sequences of CHO endogenous peptide elongation factor 1 and the chicken lysozyme MAR sequence are used to express foreign genes in CHO cells The effect is better, and the exogenous gene aldalimumab is further used to verify the effect of this vector.
[0063] On CHMARCHEF53 / DHFR through the NheI and XhoI restriction sites, the aldalimumab heavy chain gene (the sequence of the aldalimumab heavy chain gene is shown in SEQ ID NO.14) was constructed into the vector CHMARCHEFaldalimumabHC / DHFR ( Figure 5); the aldalimumab light chain gene (the sequence of the aldalimumab light chain gene is shown in SEQ ID NO.15) is also connected into the vector CHMARCHEF1506 / EGFP through the same digestion site through t...
Embodiment 3
[0067] Example 3 Study on the stability of exogenous protein expressed by the combined vector CHMARCHEF1506 / DHFR of the present invention
[0068] The five strains of cells that were highly expressed in the shake flask of Example 2 were obtained by 3x10 5 / ml cell density inoculation, 3-4 days the cells grow to 2 million / ml, and then press 3x10 5 / ml density and have been subcultured in this way for 50-60 days in shake flasks. The results showed that the expression of aldalimumab in the obtained high-expression cell lines did not change significantly after subculture for about 60 days, and the final subculture cells were cultured in serum-free medium supplemented with glucose for 14 days, and the five cell lines expressed The amounts are about 1-1.3 g / L, indicating that the high-expression vector constructed in this way can achieve stable and high-yield expression of foreign proteins in CHO cells.
[0069] According to literature reports, if the culture conditions are optimi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com