Human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit and preparation method and application thereof
A technology for immunochromatographic detection and mycoplasma pneumoniae, which is applied in the field of medical detection, can solve the problems of inability to implement bedside detection, inconvenient clinical application, and insufficient convenient and fast time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
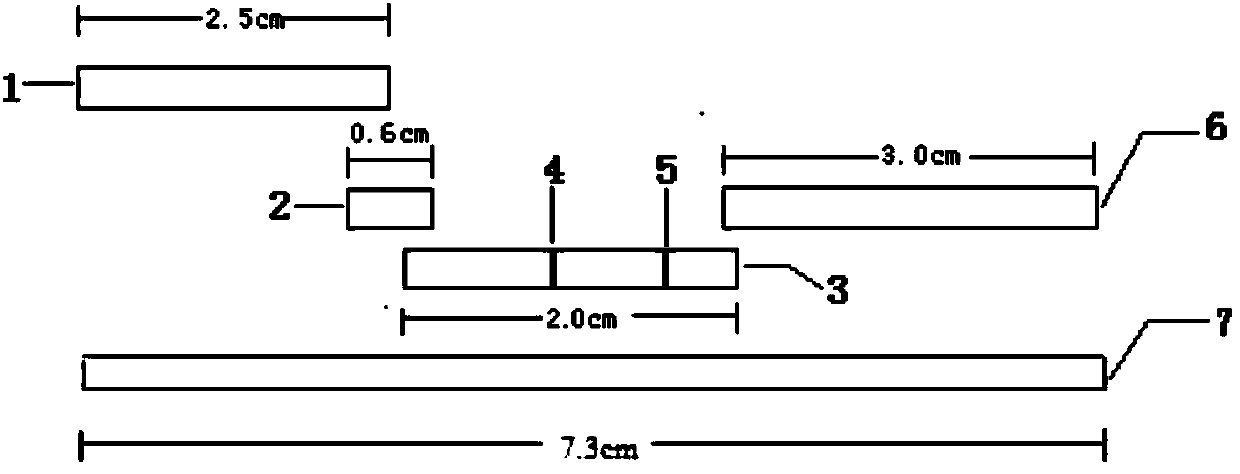
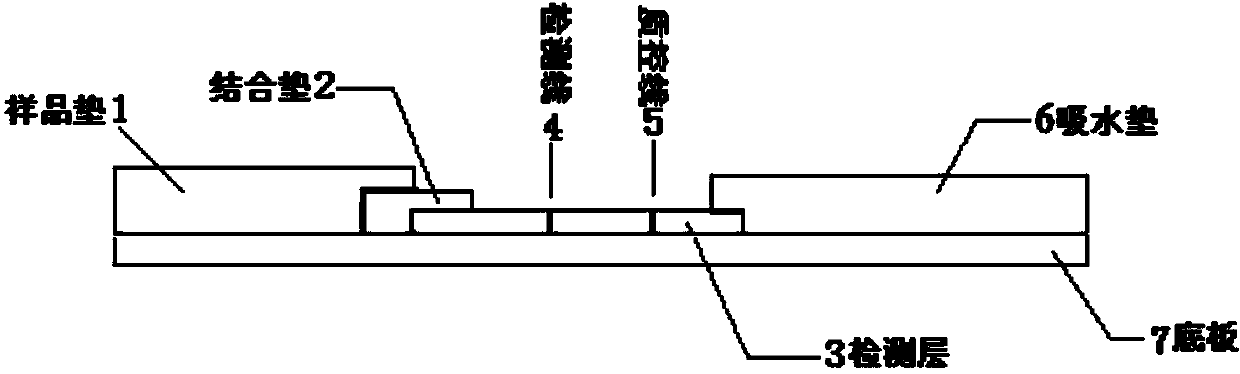
[0084] 1. Preparation of conjugated pads
[0085] (1) Preparation and purification of recombinant P1-His and P30-His fusion proteins:
[0086] Bioinformatics analysis was carried out on human Mycoplasma pneumoniae membrane proteins P1 and P30 to obtain the peptides with the most abundant antigenic epitopes in their extracellular domains; to find their corresponding gene coding sequences, according to the codon preference in Escherichia coli, After codon optimization, enzyme cleavage sites were introduced at the 5' and 3' ends respectively, and the entire gene sequence was chemically synthesized, and marked as p1 and p30 at the same time; see the sequence list for the sequence; the two gene sequences According to the method of molecular biology, respectively cloned into the expression vector pET-28a (+) and then transformed into Escherichia coli to express the recombinant P1-His, P30-His fusion protein; the recombinant P1-His fusion protein exists in the bacterium in a soluble ...
Embodiment 1
[0134] Embodiment 1 (preparation embodiment)
[0135] Conjugate pad preparation
[0136] (1) Preparation and purification of recombinant P1-His and P30-His fusion proteins
[0137] (1) Cloning of related genes
[0138]Bioinformatic analysis was performed on human Mycoplasma pneumoniae membrane proteins P1 and P30 (the accession numbers in the NCBI protein database are AAK92040 and ABR09215), respectively, to obtain the peptides with the most abundant antigenic epitopes in their extracellular conserved domains, and to find their The corresponding DNA coding sequence was codon-optimized according to the codon preference of Escherichia coli, and the restriction site NdeI was introduced at the 5' end, and the termination signal TAA and the restriction site XhoI were introduced at the 3' end, respectively. The whole gene sequence was synthesized (the whole sequence synthesis was completed by GenScript Biotechnology Co., Ltd., and the artificially synthesized gene fragments were a...
Embodiment 2
[0176] Embodiment 2 (preparation embodiment)
[0177] Preparation of sample pads
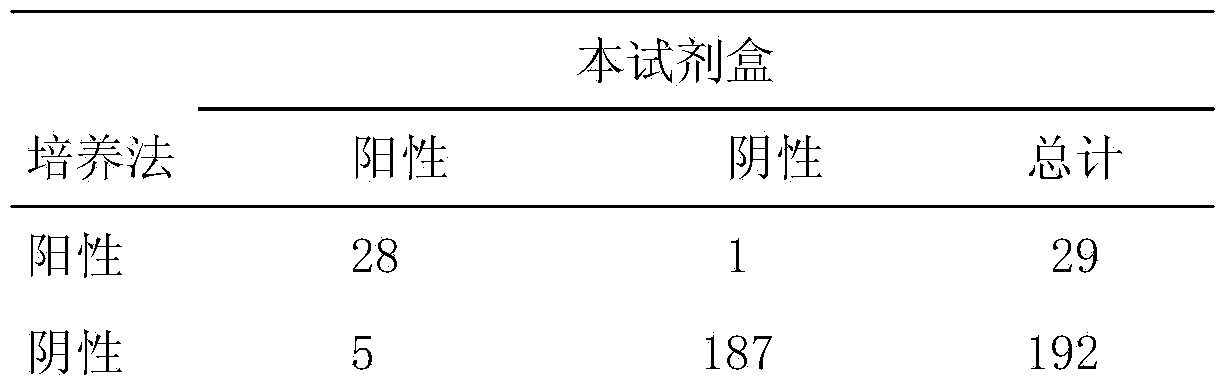
[0178] Prepare sample pad treatment solutions with different formulations, observe the release effect of colloidal gold-labeled antibodies, and optimize through multiple orthogonal experiments to obtain the optimal sample pad treatment solution formulation (that is, described in the present invention). Take a piece of glass cellulose membrane, soak it in the sample pad treatment solution for at least 3 hours, then place it in a biological safety cabinet at 37°C, ventilate and dry it, and cut it into a size of 4cm*2.5cm / strip to prepare the sample pad , sealed and dry at room temperature. Tests have proved that the use of the sample pad greatly improves the release rate of the colloidal gold-labeled antibody on the binding pad and achieves a better application effect.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com