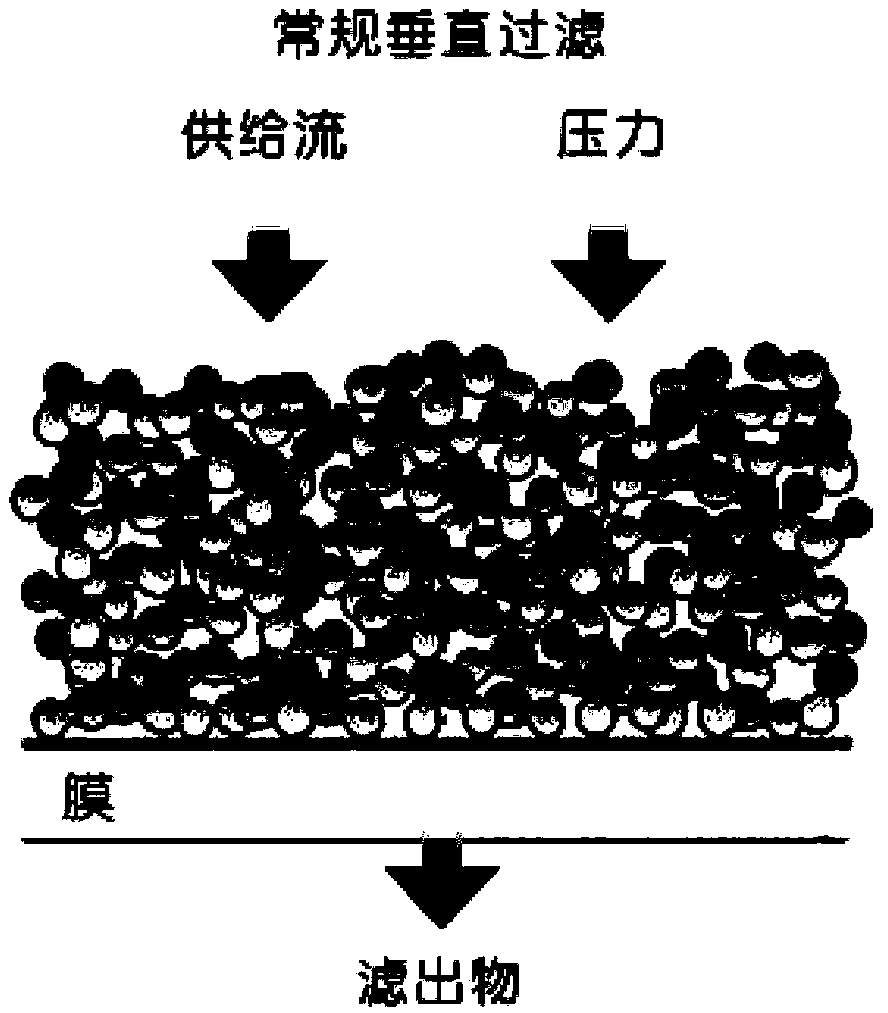
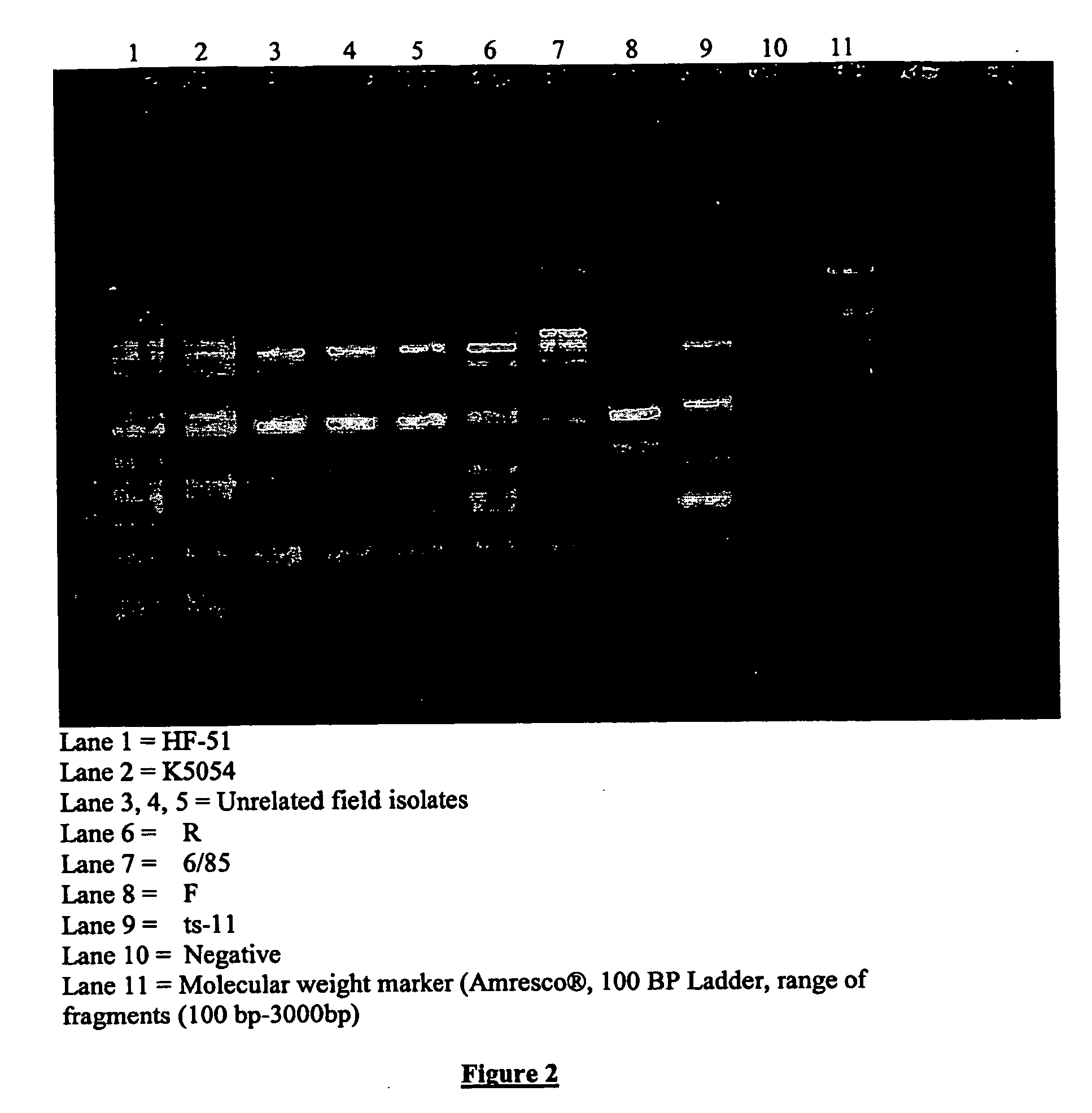
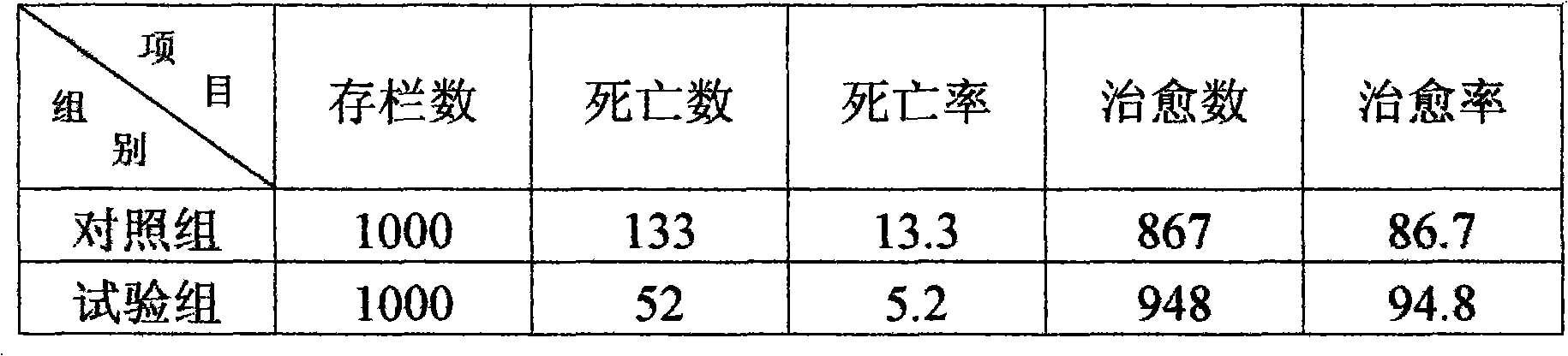
Patents
Literature
69 results about "Mycoplasma gallisepticum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mycoplasma gallisepticum (MG) is a bacterium belonging to the class Mollicutes and the family Mycoplasmataceae. It is the causative agent of chronic respiratory disease (CRD) in chickens and infectious sinusitis in turkeys, chickens, game birds, pigeons, and passerine birds of all ages.
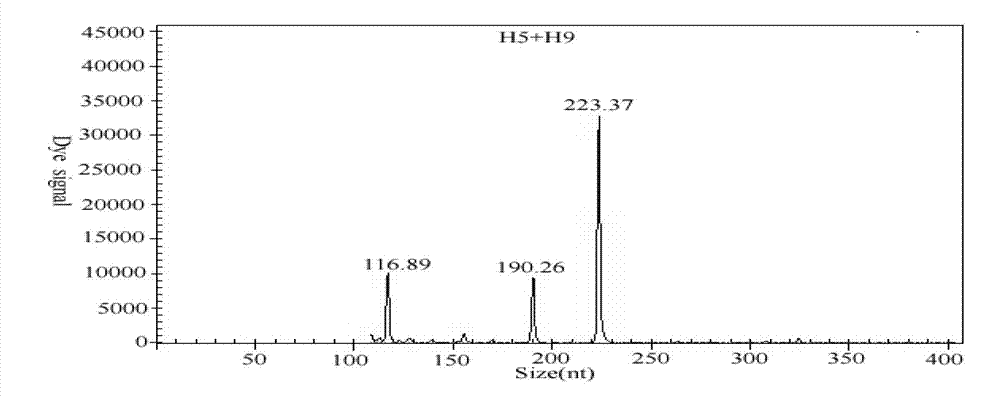
GeXP rapid detection kit capable of simultaneously identifying nine pathogens of chicken respiratory tract diseases
ActiveCN102899424AStrong specificityImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationInfectious laryngotracheitisRespiratory tract disease
The invention discloses a GeXP rapid detection kit capable of simultaneously identifying nine pathogens of chicken respiratory tract diseases. The kit is used based on a GeXP system and comprises ten polymerase chain reaction (PCR) primer pairs; the kit is used for identifying and detecting avian influenza virus, H5, H7 and H9 subtype avian influenza virus, newcastle disease virus, infectious bronchitis, infectious laryngotracheitis, mycoplasma gallisepticum, bursa synovialis mycoplasma and haemophilus paragallinarum; and the kit is good in specificity, high in sensitivity and can detect 100 copy / mu l. Compared with an identifying result of the conventional experiment method of a pathogen separation and hemagglutination inhibition experiment or a serology experiment and the like, the GeXP rapid detection kit has the advantage that the coincidence rate reaches 100 percent. The kit is generally used for detecting the main chicken respiratory tract diseases and the pathogens thereof, so that a simple and high-flux detection kit and a detection system are provided, an actual requirement is met, and the application prospect is wide.
Owner:GUANGXI VETERINARY RES INST
Use of a live attenuated Mycoplasma gallisepticum strain as a vaccine and vector for the protection of chickens and turkeys from respiratory disease
The present invention relates to a live cytadherence-deficient M. gallisepticum strain that does not express at least two of three proteins, the Gap-A molecule, crnA protein, and the 45 kDa protein, expressed by wildtype M. gallisepticum Strain R and its use as a vaccine for preventing and protecting birds, especially chickens and turkeys against the respiratory diseases attendant with wildtype Mycoplasma gallispeticum infection. The invention also relates to the use of the vaccine as a vector for the delivery of genes encoding protective antigens from other bacterial and viral avian pathogens, such as avian influenza virus. There is also disclosed a method for identifying the attenuated cytadherence-deficient M. gallisepticum Rhigh or a strain thereof.
Owner:UNIV OF CONNECTICUT
Method for improving proliferation capability of mycoplasma gallisepticum in vaccine production
ActiveCN102168073AImprove proliferative abilityHigh titerAntibacterial agentsBacterial antigen ingredientsMycoplasma cultureVaccine Production
The invention relates to a method for improving proliferation capability of mycoplasma gallisepticum in vaccine production, vaccines are prepared through the process steps of preparing seeds, preparing a culture medium of the mycoplasma gallisepticum, preparing vaccine-making bacterial liquid and preparing the inactivated vaccines, after improvement, the content of PPLO (pleuropneumonia-like organism) broth, porcine serum and glucose is increased, MEM (minimum essential medium) ingredients are removed, and the mixture ratio of the ingredients in the culture medium are more scientific and the nutritional value is higher after adjustment. The bacterial liquid concentration of a semi-finished product prepared by the method is as high as 1.0*1013CCU / ml-1.0*1014CCU / ml, and the bacterial liquiddoes not need to be concentrated and can be directly or indirectly used for preparing the inactivated vaccines after dilution, thereby not only simplifying the production process, but also reducing the production cost.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Multiply-fluorescence PCR (polymerase chain reaction) detection kit for identifying strong and weak strains of mycoplasma gallisepticum
ActiveCN102605072AGood fast sensitivityImprove scalabilityMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceInverse polymerase chain reaction
The invention discloses a multiply-fluorescence PCR (polymerase chain reaction) detection kit for identifying strong and weak strains of mycoplasma gallisepticum. Primers used consists of a first primer, a second primer, a third primer and a fourth primer, the sequences of the first primer, the second primer, the third primer and the fourth primer in the sequence table are respectively a sequence1, a sequence 2, a sequence 4 and a sequence 5. The experiment indicates that on one hand, high amplification and excellent specificity of the PCR technology and quick sensitivity of the fluorescencedetection technology are sufficiently used in the agent and detection method; on the other hand, primer pairs universal to the strong and weak strains of the mycoplasma gallispticum and primer pairs special for weak strains and probes are assembled into a fluorescent PCR system for multiply-fluorescence PCR detection, and mutual interference is avoided, and detection sensitivity and specificity are improved further.
Owner:GUANGXI VETERINARY RES INST
Mycoplasma Gallisepticum immune body immune colloidal gold fast detecting reagent kit and its application
The invention belongs to the immunity application field, which relates to the related fields of animal molecular biochemistry and the immunology, etc. The invention discloses a reagent kit respectively used for fast testing antibody of Mycoplasma Gallisepticum Strain, which comprises a case body, and the test paper card and sample diluent equipped inside the case body, wherein, the test paper card is formed by the absorption pad, nitrocellulose membrane, gold-label pad and sample pad affixed in turn on the non-absorbent supporting flake by taking the sample pad, absorption pad and the pMGA1.2 recombinant proteins coated with the Mycoplasma Gallisepticum Strain as the detection line and the pMGA1.2 recombinant proteins coated with anti-mouse IgG as nitrocellulose membrane of the quality control line. The reagent kit used for testing the corresponding antibody has the obvious advantages of strong specificity, high sensitivity, easy operation and fast diagnosis.
Owner:HUAZHONG AGRI UNIV
Indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) diagnostic kit of mycoplasma gallisepticum
InactiveCN102221616AIndirect ELISA method optimizationAntigenic stabilityBiological testingSorbentElution
The invention discloses an indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) diagnostic kit of mycoplasma gallisepticum, which comprises an ELISA plate coated by species specificity proteins PvpA, an elution buffer solution, an antibody diluting solution, an ELISA secondary antibody, a substrate color developing solution and a stop solution. The kit disclosed by the invention selects the species specificity proteins PvpA, covers the high-frequency variation regions: DR-1 and DR-2 regions of the PvpA proteins, has high specificity and immunogenicity, improves the specificity and sensitivity of the detection result, lowers the production cost and is suitable for popularization and use in grassroots veterinarian mechanisms.
Owner:SOUTH CHINA AGRI UNIV
Recombinant Newcastle disease LaSota attenuated vaccine strain expressing mycoplasma gallisepticum TM1protein
InactiveCN102961743AAntibacterial agentsBacterial antigen ingredientsDiseaseMycoplasma gallisepticum
The invention relates to a recombinant Newcastle disease LaSota attenuated vaccine strain expressing a mycoplasma gallisepticum TM1protein, more particularly, the recombinant Newcastle disease LaSota attenuated vaccine is rLa-tPA-TM1. The invention also discloses a method for preparing the recombinant Newcastle disease LaSota attenuated vaccine and application of the recombinant Newcastle disease LaSota attenuated vaccine in the preparation of bivalent vaccines for controlling diseases caused by mycoplasma gallisepticum and Newcastle disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
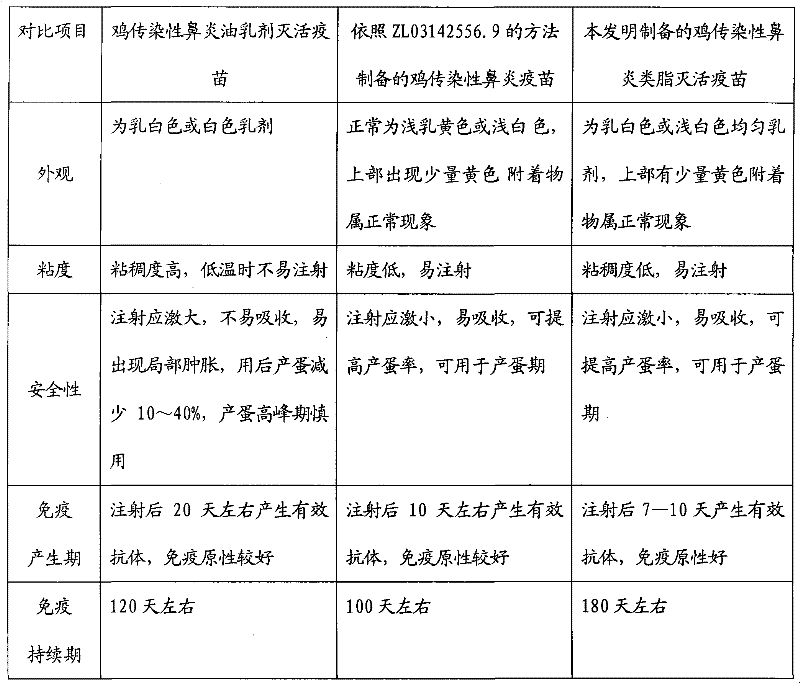
Preparation method of chicken infectious rhinitis lipid inactivated vaccine
InactiveCN102406934AReduce dosageReduce manufacturing costAntibacterial agentsSenses disorderAntigenImmune effects
The invention provides a preparation method of a chicken infectious rhinitis lipid inactivated vaccine, belonging to the technical field of biological products for animals. The preparation method is implemented by the following steps of: preparing a type A haemophilus paragallinarum inactivated antigen bacterial liquid and a type C haemophilus paragallinarum inactivated antigen bacterial liquid; uniformly mixing the type A haemophilus paragallinarum inactivated antigen bacterial liquid with the type C haemophilus paragallinarum inactivated antigen bacterial liquid in the volume ratio of 1:1, and pouring into an emulsifying tank; stirring with a mixer; slowly adding poplar bark lipid at a low speed till the final volume percentage concentration of the poplar bark lipid in the mixture is 2.0-3.8 percent; and continually stirring and emulsifying for 30-60 minutes to obtain the vaccine, wherein the final bacterium content of a type A strain and the final bacterium content of a type C strain in the vaccine are 1,000,000,000 cfu / ml. The vaccine has the advantages of easiness for absorbing, no toxic or side effect, short immunogenic time, long immune duration, good immune effect and capability of effectively preventing chicken infectious rhinitis and mycoplasma gallisepticum.
Owner:SHANDONG BINZHOU BOLAIWEI BIOTECH
Fused protein, gene therefor, recombinant vector, recombinant virus, and its use
InactiveUS7348422B2Enhanced infection prevention activityEasy to identifyAntibody mimetics/scaffoldsVirus peptidesA-DNAMycoplasma gallisepticum
A DNA coding for a fusion protein comprising a polypeptide having the antigenicity of Mycoplasma gallisepticum and a polypeptide derived from Herpesvirus outer membrane protein, in which the polypeptide derived from the outer membrane protein has been ligated with the polypeptide having the antigenicity of Mycoplasma gallisepticum at the N terminus thereof, is prepared. The DNA is inserted into a region non-essential to growth of Avipox virus and the resulting recombinant Avipox virus is provided as a more potent recombinant virus as an anti-Mycoplasma vaccine.
Owner:ZEON CORP
LAMP (Loop-Mediated Isothermal Amplification) assay kit for identifying virulent and avirulent strains of mycoplasma gallisepticum
ActiveCN102605071AAvoiding the False Positive ProblemImprove bindingMicrobiological testing/measurementMicroorganism based processesWater bathsNucleotide
The invention discloses an LAMP assay kit for identifying virulent and avirulent strains of mycoplasma gallisepticum. A primer group A provided by the LAMP assay kit consists of a primer group a and a primer group b; the primer group a consists of a primer 1 and a primer 2; the primer group b consists of a primer 3 and a primer 4; and the nucleotide sequences of the primer 1, the primer 2, the primer 3 and the primer 4 are sequence 1, sequence 2, sequence 3 and sequence 4 sequentially in a sequence table. An experiment for the LAMP assay kit proves that the primers and the method only need anordinary water bath rather other an expensive PCR (Polymerase Chain Reaction) instrument but have higher sensitivity than PCR assay, moreover, a result does not need to be observed by way of gel electrophoresis, and the LAMP assay kit is easy and rapid to operate, and is particularly suitable for field assay in grass roots.
Owner:GUANGXI VETERINARY RES INST
Preparation method of recombinant antimicrobial peptide extracted from small intestine of pig and application thereof
ActiveCN103417574AHigh yieldLess quantityAntibacterial agentsPeptide/protein ingredientsUltrafiltrationAntimicrobial peptides
The invention discloses a preparation method of a recombinant antimicrobial peptide extracted from a small intestine of a pig and an application thereof. The preparation method comprises the following steps: mashing, homogenizing and repeatedly freezing and thawing the small intestine tissue of the pig with a meat grinder and a colloid mill, and preliminarily crushing cells; then performing the water boiling heat treatment, and extracting with acetic acid solution to obtain a large amount of antimicrobial peptides; centrifuging to enable the quantity of other proteins to be reduced greatly, filtering the supernate obtained through centrifuging by a tangential flow micro-filtration membrane and a tangential flow ultrafiltration membrane sequentially, adjusting the pH value, filtering the supernate by a tangential flow nanofiltration membrane, concentrating and dialyzing to desalt, and disinfecting after adjusting the osmotic pressure to obtain the recombinant antimicrobial peptide extracted from the small intestine of the pig. According to the invention, the application of the recombinant antimicrobial peptide adopts effects of the antimicrobial peptide on aspects of mucosal immunity, immunopotentiators, novel antibiotics and the like, and the mucosal immunity character of the antimicrobial peptide can play an important role in the prevention and control of transmissible gastroenteristis, epidemic diarrhea and mycoplasma gallisepticum; the antimicrobial peptide replaces traditional antibiotics, and is remarkable in effect.
Owner:派生特(福州)生物科技有限公司 +1
Mycoplasma gallisepticum formulation
InactiveUS20060257414A1Antibacterial agentsBacterial antigen ingredientsMycoplasma gallisepticumProtection sex
The present invention provides a protective formulation that prevents virulent Mycoplasma gallisepticum infection in birds of the order Galliformes. The formulation comprises a protective amount of live MG strain K5054 or derivatives thereof in a pharmaceutically acceptable carrier. A vaccine that prevents virulent Mycoplasma gallisepticum infection in birds of the order Galliformes is also presented. Methods for administering the formulation and vaccine are also presented.
Owner:UNIV OF GEORGIA RES FOUND INC
Mycoplasma gallisepticum formulation
InactiveUS7217420B2Antibacterial agentsBacterial antigen ingredientsProtection sexMycoplasma gallisepticum
The present invention provides a protective formulation that prevents virulent Mycoplasma gallisepticum infection in birds of the order Galliformes. The formulation comprises a protective amount of live MG strain K5054 or derivatives thereof in a pharmaceutically acceptable carrier. A vaccine that prevents virulent Mycoplasma gallisepticum infection in birds of the order Galliformes is also presented. Methods for administering the formulation and vaccine are also presented.
Owner:UNIV OF GEORGIA RES FOUND INC
Efficacy test method of mycoplasma gallisepticum inactivated vaccine and application thereof
The invention relates to the technical field of animal biological products and discloses an efficacy test method of mycoplasma gallisepticum inactivated vaccine. Simultaneously, the efficacy test method disclosed by the invention can also be applied to quality control of the vaccine, in particular comprising the determination of immune toxicity attack protection rate and vaccine quality standard, measurement of immunization deadline and clinical monitoring. Experiments prove that the animal toxicity attack test is replaced by a serology detection method (comprising ELISA (Enzyme-Linked Immuno Sorbent Assay) and HI (Hemagglutination Inbition)); the efficacy test method is simple, convenient, rapid, accurate in result and good in repeatability and specificity; due to the establishment of the efficacy test method, subjectivity for checking airbag pathological change integrals after attacking toxicity is reduced; toxicity dispersion is avoided; a basis for establishing a rational immune program is also provided; and the efficacy test method has general popularization significance.
Owner:兆丰华生物科技(南京)有限公司
Lincomycin hydrochloride-spectinomycin sulfate injection and preparation method thereof
InactiveCN102793713AGood treatment effectHigh antibacterial activityAntibacterial agentsOrganic active ingredientsDiseaseSide effect
Owner:上海公谊药业有限公司
Mycoplasma gallisepticum formulation
ActiveUS20120021005A1Reduce sensitivitySsRNA viruses negative-senseAntibacterial agentsMedicineMycoplasma gallisepticum
The present invention provides a formulation that prevents virulent Mycoplasma galHsepticum infection in birds of the order GaIHf ormes. The formulation comprises live Mycoplasma galHsepticum strain K5831 or derivatives thereof in a pharmaceutically acceptable carrier. A vaccine that prevents virulent Mycoplasma galHsepticum infection in birds of the order Galliformes is also presented. Methods for administering the formulation and vaccine are also presented.
Owner:UNIV OF GEORGIA RES FOUND INC
Drug resistance judgment standard test method of mycoplasma gallisepticum to danofloxacin
ActiveCN110060784ADelay drug resistanceProtect and maintain effectivenessDrug referencesMycoplasma gallisepticumPulmonary circulation diseases
The invention discloses a drug resistance judgment standard test method of mycoplasma gallisepticum to danofloxacin, including the following steps: formulating a wild type critical value and a pharmacodynamic critical value of the danofloxacin to the mycoplasma gallisepticum, formulating a dosing scheme of the danofloxacin to the mycoplasma gallisepticum, formulating a clinical critical value of the danofloxacin to the mycoplasma gallisepticum, and obtaining a drug resistance judgment standard of the danofloxacin to the mycoplasma gallisepticum through a tree diagram which is made through a CLSI susceptibility breakpoint. With the adoption of the drug resistance judgment standard test method of mycoplasma gallisepticum to danofloxacin, the drug resistance judgment standard of the mycoplasma gallisepticum to the danofloxacin can be formulated; stable medication data support can be provided for scientific cultivation; clinical medication can be guided more scientifically; chronic respiratory diseases such as nasosinusitis, trachitis and air sacculitis of chickens can be effectively treated; reference is provided for clinical medication; drug resistance of mycoplasma gallisepticum todanofloxacin can be effectively relieved to a certain extent; and effectiveness of danofloxacin is protected and maintained.
Owner:HUAZHONG AGRI UNIV
Mycoplasma gallisepticum high density fermentation culture technology
ActiveCN108949606AImprove high densityHigh densityBacteriaMicroorganism based processesAntigenMicroorganism
The invention discloses a mycoplasma gallisepticum high density fermentation culture technology, and belongs to the field of agriculture microbes. A chicken bursa mycoplasma fermentation high densityantigen is obtained through following steps: resuscitating freeze-dried strains in a mycoplasma gallisepticum culture medium, and then carrying out inoculum enlargement and fermentation culture. During the fermentation process, a mycoplasma gallisepticum culture medium is added, the pH is adjusted, industrial amplify production is carried out, enough nutrients are provided for mycoplasma gallisepticum, the production cost is reduced, the antigen content is high, and the technology is suitable for large scale application and has a wide application prospect.
Owner:兆丰华生物科技(南京)有限公司
Novel gene engineering subunit vaccine for mycoplasma gallisepticum
ActiveCN109999191ANot pathogenicReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsNucleotideVaccine Production
The invention provides an immunological composition and a subunit vaccine. The immunological composition comprises a protein which is selected from one or an arbitrary combination of two or more of mycoplasma gallisepticum-associated proteins encoded with nucleic acid molecules of SEQ ID NO: 1 or 3 or 5 or 7 or 9 or nucleic acid molecules which are 95% or above identical to the nucleotide sequenceof SEQ ID NO: 1 or 3 or 5 or 7 or 9. The vaccine adopts eukaryotic expression, the antigenicity and immunogenicity of the product are similar to those of a natural protein, the expression level is high, the immunogenicity is strong, the protective effect is good, and the vaccine has no pathogenicity to chickens; besides, large-scale serum-free suspension culture preparation of the vaccine can berealized through a bioreactor, and meanwhile the vaccine production cost is greatly reduced.
Owner:苏州沃美生物有限公司
Medicament for preventing and controlling mycoplasma galliseplicum disease
InactiveCN101342301ARigorous formulaClear division of laborUnknown materialsAntiparasitic agentsDiseaseAdditive ingredient
The present invention discloses a medicine which is used for preventing and treating mycoplasma gallisepticum. The medicine comprises the ingredients of the following weight portions: 5 to 15 portions of dyer woad root, 5 to 15 portions of cordate houttuynia, 5 to 15 portions of dyer woad leaf, 5 to 15 portions of honeysuckle, 5 to 15 portions of radix scutellariae, 5 to 15 portions of forsythia, 3 to 10 portions of Chinese ephedra, 3 to 10 portions of almond, 3 to 10 portions of balloon-flower root, 10 to 30 portions of plaster, 3 to 10 portions of rhizoma anemarrhenae, 2 to 6 portions of fried draba nemorosa linnei, 2 to 6 portions of fried perilla, 2 to 6 portions of white fructus tribuli, 2 to 6 portions of chrysanthemum, 2 to 6 portions of rhubarb, 2 to 6 portions of gardenia, 2 to 6 portions of powder of gall bladder, and 2 to 6 portions of licorice. The herbs are insolated, weighed, crushed, filtered by a sieve with 60 to 80 meshes, and mixed well, so as to prepare the medicine. When in clinical use, the medicine is dipped for 30 to 60 minutes in boiling water, so that the effective ingredients can be fully dissolved and separated to facilitate the absorption in the body of the sick chicken. Simultaneously, a small amount of sugar and Vc are added to improve the medicinal effects. The medicine has high cure rate, no adverse reaction, no drug residues in the body, and nearly no drug resistance. The production of the medicine has the advantages of easily available raw materials, simple preparation, convenient use, and capability of wide promotion and application in the chicken breeding industry.
Owner:HEBEI AGRICULTURAL UNIV.
Citric acid and compound citric acid application in pharmacy
ActiveCN101530405AControl epidemicAvoid residueAntibacterial agentsSulfur/selenium/tellurium active ingredientsDiseaseBacterial disease
The invention relates to a new usage of citric acid and compound citric acid in the field of pharmacy. The product can prevent and control viral diseases and bacterial diseases of livestock and poultry; wherein, the compound citric acid comprises the following components according to weight portion: 93-98 portions of citric acid, 0.5-4 portions of gualfenesin and 1.5-3 portions of etamsylate. Proved by in vivo and vitro experiments, the compound citric acid can play a role in preventing and controlling fowl mycoplasma gallisepticum, avian influenza (H9 subtype), bursal virus disease and other diseases, and can effectively kill animal pathogenic bacterias such as cattle colon bacillus, mycoplasma gallisepticum, chicken colibacillosis, etc.
Owner:四川通达动物保健科技有限公司
Multiplex PCR detection primer set and kit used for mycoplasma gallisepticum, mycoplasma gallisepticum and parabacterium bacillus and application of kit
ActiveCN110452999AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesNucleotideMycoplasma gallisepticum
The invention provides a multiplex PCR detection primer set and kit used for mycoplasma gallisepticum, mycoplasma gallisepticum and parabacterium bacillus and application of the kit, and belongs to the technical field of molecular detection. The primer set comprises MS-F, MS-R, MG-F, MG-R, APG-F and APG-R; the nucleotide sequences of MG-F, MG-R, MS-F, MS-R, APG-F and APG-R are shown in SEQ ID NO:1-SEQ ID NO:6 respectively. It is verified that the primer set has high specificity and sensitivity.
Owner:INNER MONGOLIA AUTONOMOUS REGION ACAD OF AGRI & ANIMAL HUSBANDRY SCI
Mycoplasma gallisepticum detection method
InactiveCN106282300APromote growth and proliferationEasy to implementMicrobiological testing/measurementMycoplasma gallisepticumGlucose polymers
The technical problem to be solved in the present invention is to rapidly and efficiently proliferate Mycoplasma gallisepticum so as to be used for rapid detection of Mycoplasma gallisepticum pathogen. In order to achieve the purpose, the prevent invention is achieved by adopting the following technical scheme that the Mycoplasma gallisepticum detection method is characterized in that a Mycoplasma gallisepticum liquid culture medium is prepared, wherein yeast extract powder, glucose and trypticase are concurrently used in a soybean papain digest, such that the actual passage time shortening effect is more than 10% of the passage time shortening effect of the only use of the traditional yeast extract powder, the shortest passage time can be as fast as 12-16 h, and the passage time is 18 h in most cases. According to the present invention, the Mycoplasma gallisepticum liquid culture medium is adopted as the main proliferation base material of the formula so as to rapidly detect the Mycoplasma gallisepticum pathogen, such that the effective reference is provided for the definite diagnosis of the disease and the epidemic prevention work of the chicken flocks.
Owner:LINYI UNIVERSITY
Fluid culture medium and method for isolated-culturing mycoplasma gallisepticum using same
The invention discloses a fluid culture medium. The fluid culture medium is prepared by mixing a basic culture medium and an auxiliary culture medium. The basic culture medium contains the following components by mass concentration (g / L): 3-7 g / L of sodium chloride, 0.3-0.6 g / L of potassium chloride, 1.5-3.5 g / L of sodium phosphate dibasic dodecahydrate, 0.1-0.4 g / L of magnesium sulfate heptahydrate, 0.1-0.2 g / L of potassium dihydrogen phosphate, 10-15 g / L of glucose, 6-8 g / L of lactoalbumin hydrolysate and 6-7 g / L of yeast extract powder; and the auxiliary culture medium contains the following components by volume concentration (mL / L): 40-80 mL / L of fetal calf serum, 1-2 mL / L of penicillin, 2-3 mL / L of phenol red, 40-80 mL / L of a DMEM high glucose culture medium, 8-10 mL / L of arginine, 8-10 mL / L of cysteine and 8-10 mL / L of coenzyme I. The invention further discloses a preparation method for the culture medium and a method for isolated-culturing mycoplasma gallisepticum using the culture medium. The fluid culture medium is capable of providing the more excellent growing conditions, accelerating metabolism, advantageously shortening isolation time of the mycoplasma gallisepticum and improving an isolating rate.
Owner:HUAZHONG AGRI UNIV
New application of p-hydroxyl cinnamic acid
ActiveCN101780064AAvoid residueEnsure safetyAntibacterial agentsOrganic active ingredientsDiseaseEscherichia coli
The invention discloses a new application of p-hydroxyl cinnamic acid in preparing a medicament for preventing and curing viral diseases or bacterial diseases of livestock and poultries. The product can prevent and cure viral and bacterial diseases of the livestock and the poultries and improve the production performance of the livestock and the poultries. Proved by in vivo and in vitro experiments, the p-hydroxyl cinnamic acid has the effects of preventing and curing diseases caused by mycoplasma gallisepticums, cow escherichia coli, and the like. Proved by in vivo experiments, the p-hydroxyl cinnamic acid has the effects of preventing and curing Fabricius bursal viral diseases of the livestock and the poultries, avian mycoplasmosis and avian influenza H9 and H5 subtype virus infections.
Owner:CHINA AGRI UNIV
Pleuromutilin compounds with thioether side chains as well as preparation method and application of pleuromutilin compounds
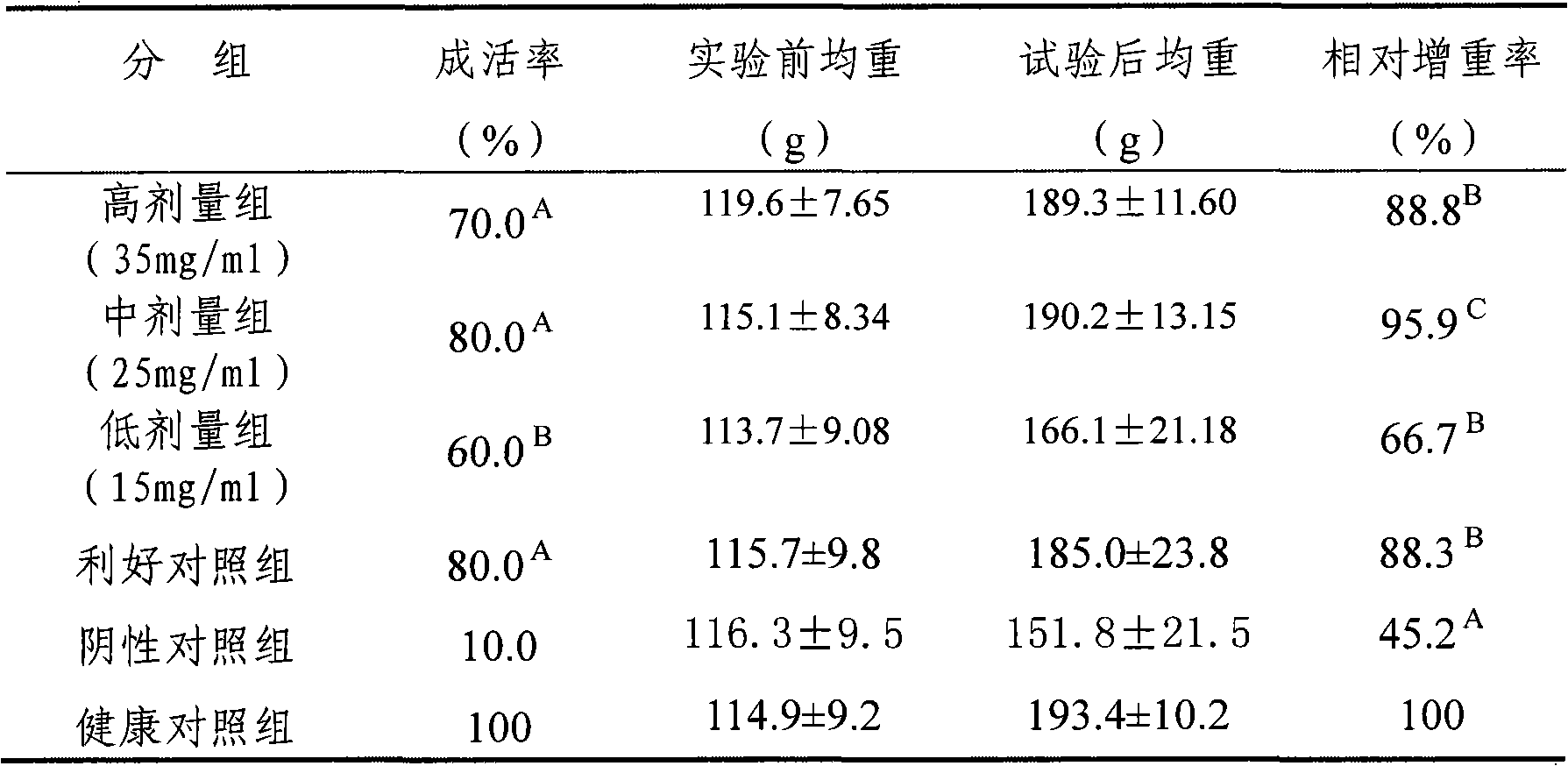
ActiveCN106008395AGood in vitro anti-mycoplasma activityAntibacterial agentsOrganic active ingredientsHydrogenHalogen
The invention discloses pleuromutilin compounds with thioether side chains as well as a preparation method and an application of the pleuromutilin compounds and belongs to the field of medicinal chemistry. The compounds have the structure shown in the formula 2 or in the formula 3, wherein R1, R2 and R3 are independently selected from hydrogen, methoxyl, methyl, hydroxy, nitryl, halogen and trichloromethyl; R is ethyl or vinyl. The pleuromutilin compounds with the thioether side chains have good activity for inhibiting mycoplasma gallisepticum and are particularly suitable for being taken as a novel antimycoplasma drug to be used for preventing and treating mycoplasma infection diseases of human beings or animals.
Owner:SOUTH CHINA AGRI UNIV
Premixed feed for preventing Mycoplasma gallisepricum, and preparation method thereof
InactiveCN105343420AEliminate hidden dangersNo residual toxic side effectsAntibacterial agentsOrganic active ingredientsSide effectVitamin C
The invention relates to a premixed feed for preventing Mycoplasma gallisepricum, and a preparation method thereof. The premixed feed comprises, by weight, 5-7 parts of Radix Scutellariae, 4-6 parts of Amur Corktree Bark, 6-10 parts of Rhizoma Dioscoreae Bulbiferae, 6-10 parts of honeysuckle flower, 6-10 parts of Fructus Gardeniae, 4-6 parts of radix bupleuri, 25-35 parts of Folium Isatidis, 6-10 parts of Divaricate Saposhnikovia Root, 4-6 parts of realgar, 4-6 parts of alum, 8-12 parts of licorice root, and 1-3 parts of vitamin C. The premixed feed is composed of above traditional Chinese medicines, and can well prevent and treat the Mycoplasma gallisepricum. The performances of the premixed feed are better than effects of antibiotics on chickens. The performances of Chinese herbal medicines in the above composition make Mycoplasma gallisepticum viruses be outward drained from the chickens through defecation channels. The premixed feed also allows young protomers in the invisible stage of chickens to be killed and then to be drained through the defecation channels in order to eliminate hidden Mycoplasma gallisepricum troubles. The premixed feed adapts to all fertility stages of fryers, and has no residual and no toxic or side effects on the chickens.
Owner:HENGYANG FUKUANG FEED ADDITIVE CO LTD
New application of p-hydroxyl cinnamic acid
ActiveCN101780064BAvoid residueEnsure safetyAntibacterial agentsOrganic active ingredientsEscherichia coliDisease
The invention discloses a new application of p-hydroxyl cinnamic acid in preparing a medicament for preventing and curing viral diseases or bacterial diseases of livestock and poultries. The product can prevent and cure viral and bacterial diseases of the livestock and the poultries and improve the production performance of the livestock and the poultries. Proved by in vivo and in vitro experiments, the p-hydroxyl cinnamic acid has the effects of preventing and curing diseases caused by mycoplasma gallisepticums, cow escherichia coli, and the like. Proved by in vivo experiments, the p-hydroxyl cinnamic acid has the effects of preventing and curing Fabricius bursal viral diseases of the livestock and the poultries, avian mycoplasmosis and avian influenza H9 and H5 subtype virus infections.
Owner:CHINA AGRI UNIV
New uses of p-hydroxycinnamic acid
ActiveCN102293764AAvoid residueEnsure safetyAntibacterial agentsOrganic active ingredientsDiseaseBird flu
The invention discloses new application of p-hydroxycinnamic acid to preparation of medicines for preventing and treating viral disease or bacterial disease of livestock and poultry. The product can prevent and treat viral disease or bacterial disease of livestock and poultry and can improve production performance of livestock and poultry. In vivo and in vitro tests show that the p-hydroxycinnamic acid can prevent and treat diseases caused by mycoplasma gallisepticum, bovine colibacillosis and the like. The in vivo test shows that the p-hydroxycinnamic acid can prevent and treat poultry bursafabricius viral disease, avian mycoplasmosis and bird flu H9 and H5 subtype virus infection.
Owner:CHINA AGRI UNIV
Lactobacillus plantarum and microecological preparation, preparation method and application thereof
ActiveCN111635873AEnhance immune functionImprove thymus indexAntibacterial agentsBacteriaBiotechnologyDisease
The invention provides lactobacillus plantarum and a microecological preparation, a preparation method and application thereof. The lactobacillus plantarum is named as Lactobacillus Plantarum BLCC2-0125, and is preserved in the China Center for Type Culture Collection in Wuhan City on April 24th, 2020, and the preservation number is CCTCC NO: M 2020078. The lactobacillus plantarum can improve thebody immunity, has a prevention effect on low-pathogenicity avian influenza, and can prevent and treat mycoplasma gallisepticum disease.
Owner:山东宝来利来生物工程股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com