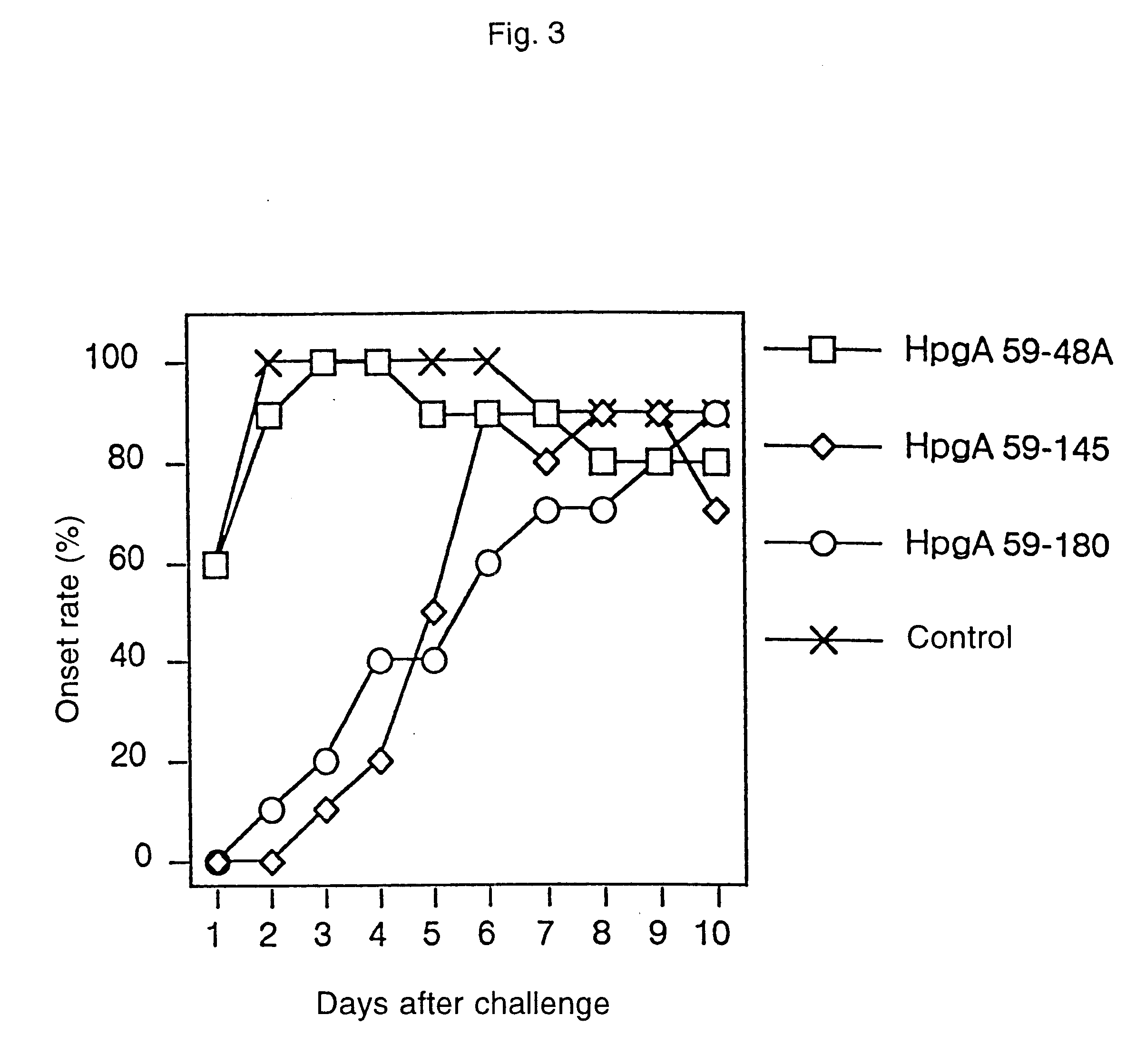
Patents
Literature
30 results about "Haemophilus paragallinarum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
GeXP rapid detection kit capable of simultaneously identifying nine pathogens of chicken respiratory tract diseases
ActiveCN102899424AStrong specificityImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationInfectious laryngotracheitisRespiratory tract disease
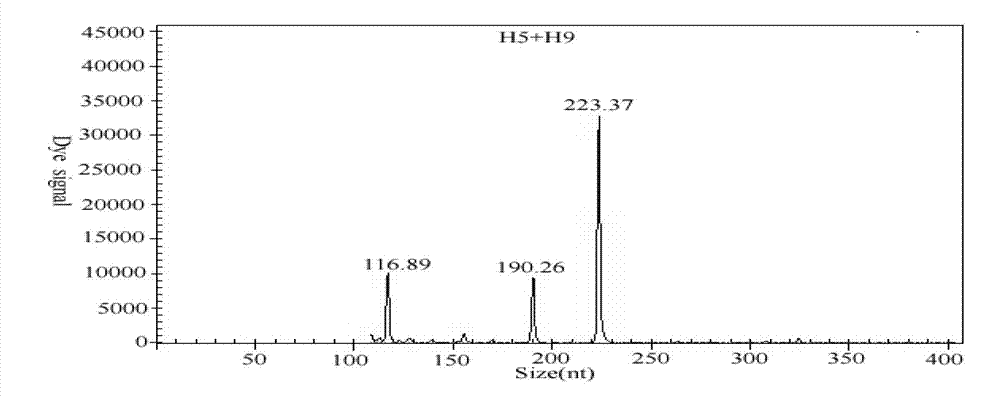
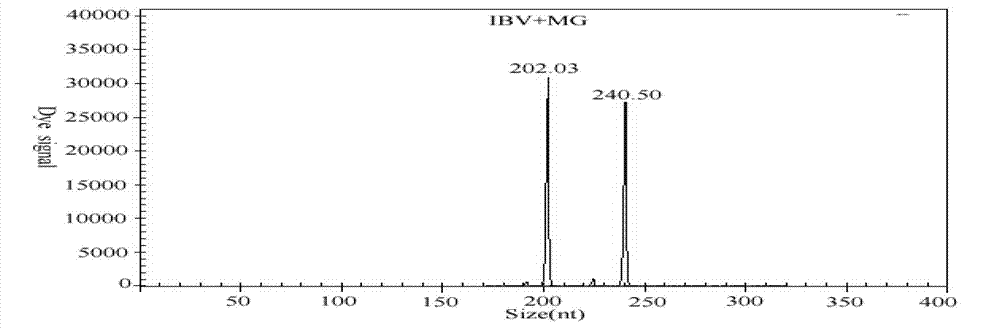
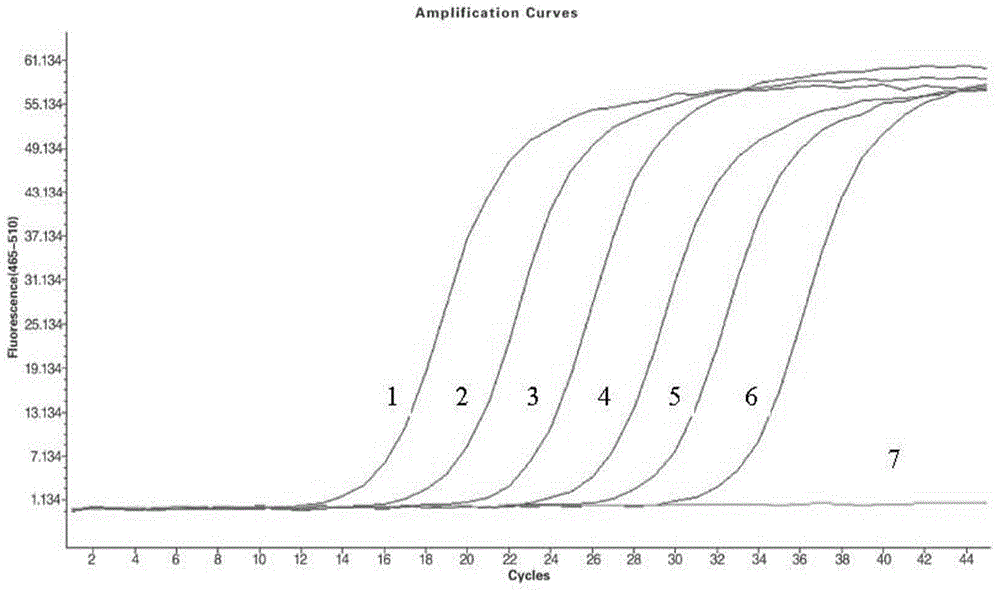
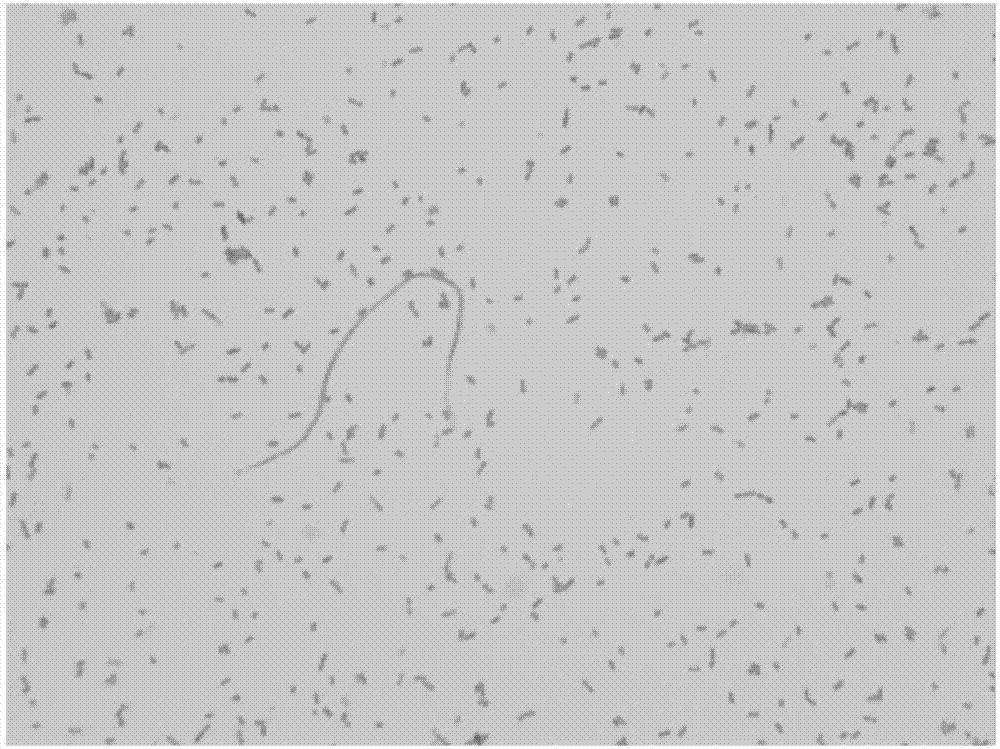
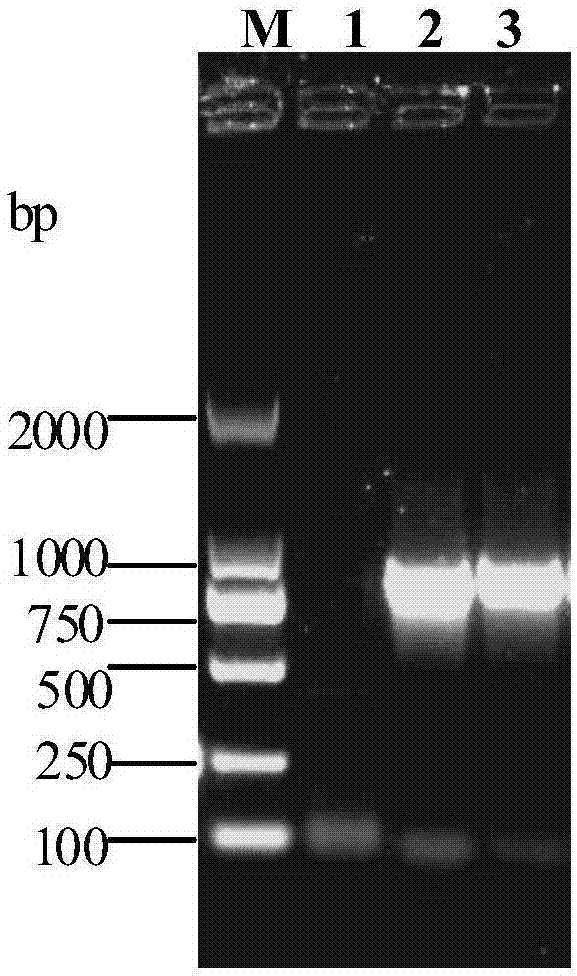
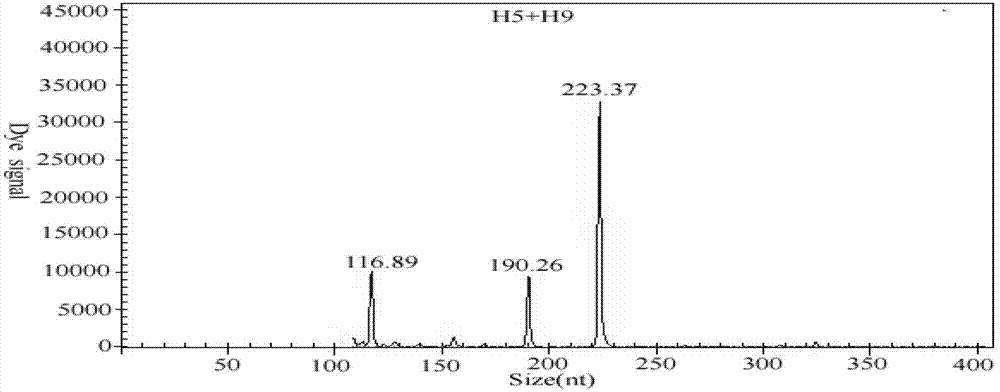
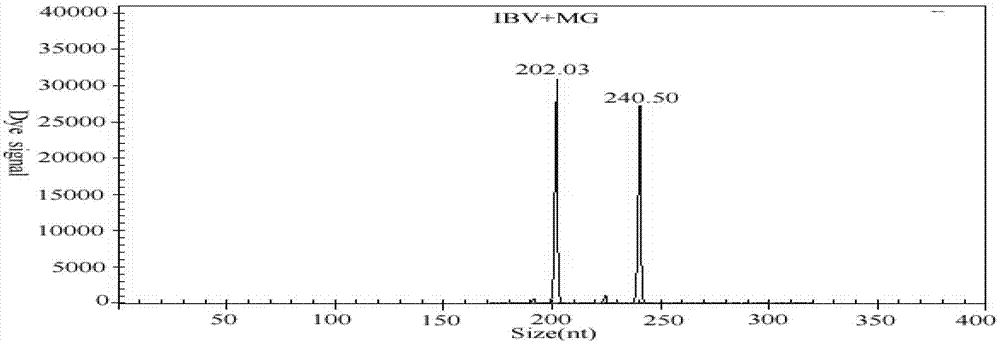
The invention discloses a GeXP rapid detection kit capable of simultaneously identifying nine pathogens of chicken respiratory tract diseases. The kit is used based on a GeXP system and comprises ten polymerase chain reaction (PCR) primer pairs; the kit is used for identifying and detecting avian influenza virus, H5, H7 and H9 subtype avian influenza virus, newcastle disease virus, infectious bronchitis, infectious laryngotracheitis, mycoplasma gallisepticum, bursa synovialis mycoplasma and haemophilus paragallinarum; and the kit is good in specificity, high in sensitivity and can detect 100 copy / mu l. Compared with an identifying result of the conventional experiment method of a pathogen separation and hemagglutination inhibition experiment or a serology experiment and the like, the GeXP rapid detection kit has the advantage that the coincidence rate reaches 100 percent. The kit is generally used for detecting the main chicken respiratory tract diseases and the pathogens thereof, so that a simple and high-flux detection kit and a detection system are provided, an actual requirement is met, and the application prospect is wide.
Owner:GUANGXI VETERINARY RES INST
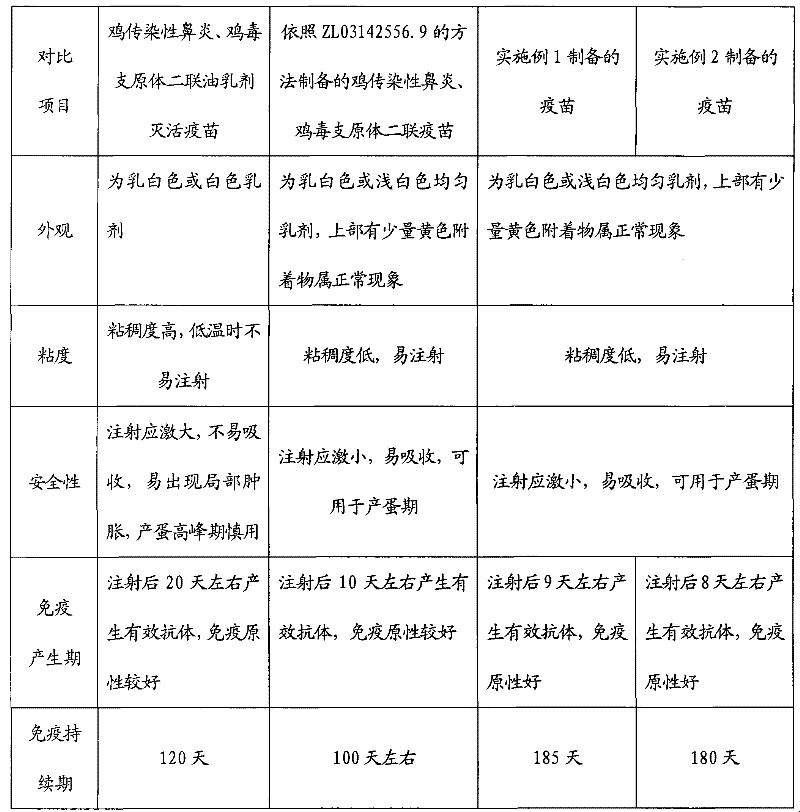
Preparation method of chicken infectious rhinitis and mycoplasma gallisepticum bivalent lipid inactivated vaccine
InactiveCN102406925AReduce manufacturing costIncrease production costAntibacterial agentsBacterial antigen ingredientsImmune effectsMycoplasma gallisepticum antigen
The invention provides a preparation method of a chicken infectious rhinitis and mycoplasma gallisepticum bivalent lipid inactivated vaccine, belonging to the technical field of biological products for animals. The preparation method is implemented by the following steps of: preparing a type A haemophilus paragallinarum inactivated antigen bacterial liquid, a type C haemophilus paragallinarum inactivated antigen bacterial liquid and a mycoplasma gallisepticum antigen liquid; uniformly mixing the type A haemophilus paragallinarum inactivated antigen bacterial liquid with the type C haemophilus paragallinarum inactivated antigen bacterial liquid in the volume ratio of 1:1 to obtain a mixed haemophilus paragallinarum inactivated antigen bacterial liquid; uniformly mixing the mixed haemophilus paragallinarum inactivated antigen bacterial liquid with the mycoplasma gallisepticum antigen liquid in the ratio of 1:1-1:1.5, pouring into an emulsifying tank and stirring; slowly adding poplar bark lipid till the final volume percentage concentration of the poplar bark lipid in the mixture is 2.0-3.8 percent; and continually stirring and emulsifying for 30-60 minutes. The vaccine has the advantages of easiness for absorbing, no toxic or side effect, short immunogenic time, long immune duration, good immune effect and capability of effectively preventing chicken infectious rhinitis and mycoplasma gallisepticum.
Owner:SHANDONG BINZHOU BOLAIWEI BIOTECH
Preparation method of chicken infectious rhinitis lipid inactivated vaccine
InactiveCN102406934AReduce dosageReduce manufacturing costAntibacterial agentsSenses disorderAntigenImmune effects
The invention provides a preparation method of a chicken infectious rhinitis lipid inactivated vaccine, belonging to the technical field of biological products for animals. The preparation method is implemented by the following steps of: preparing a type A haemophilus paragallinarum inactivated antigen bacterial liquid and a type C haemophilus paragallinarum inactivated antigen bacterial liquid; uniformly mixing the type A haemophilus paragallinarum inactivated antigen bacterial liquid with the type C haemophilus paragallinarum inactivated antigen bacterial liquid in the volume ratio of 1:1, and pouring into an emulsifying tank; stirring with a mixer; slowly adding poplar bark lipid at a low speed till the final volume percentage concentration of the poplar bark lipid in the mixture is 2.0-3.8 percent; and continually stirring and emulsifying for 30-60 minutes to obtain the vaccine, wherein the final bacterium content of a type A strain and the final bacterium content of a type C strain in the vaccine are 1,000,000,000 cfu / ml. The vaccine has the advantages of easiness for absorbing, no toxic or side effect, short immunogenic time, long immune duration, good immune effect and capability of effectively preventing chicken infectious rhinitis and mycoplasma gallisepticum.
Owner:SHANDONG BINZHOU BOLAIWEI BIOTECH
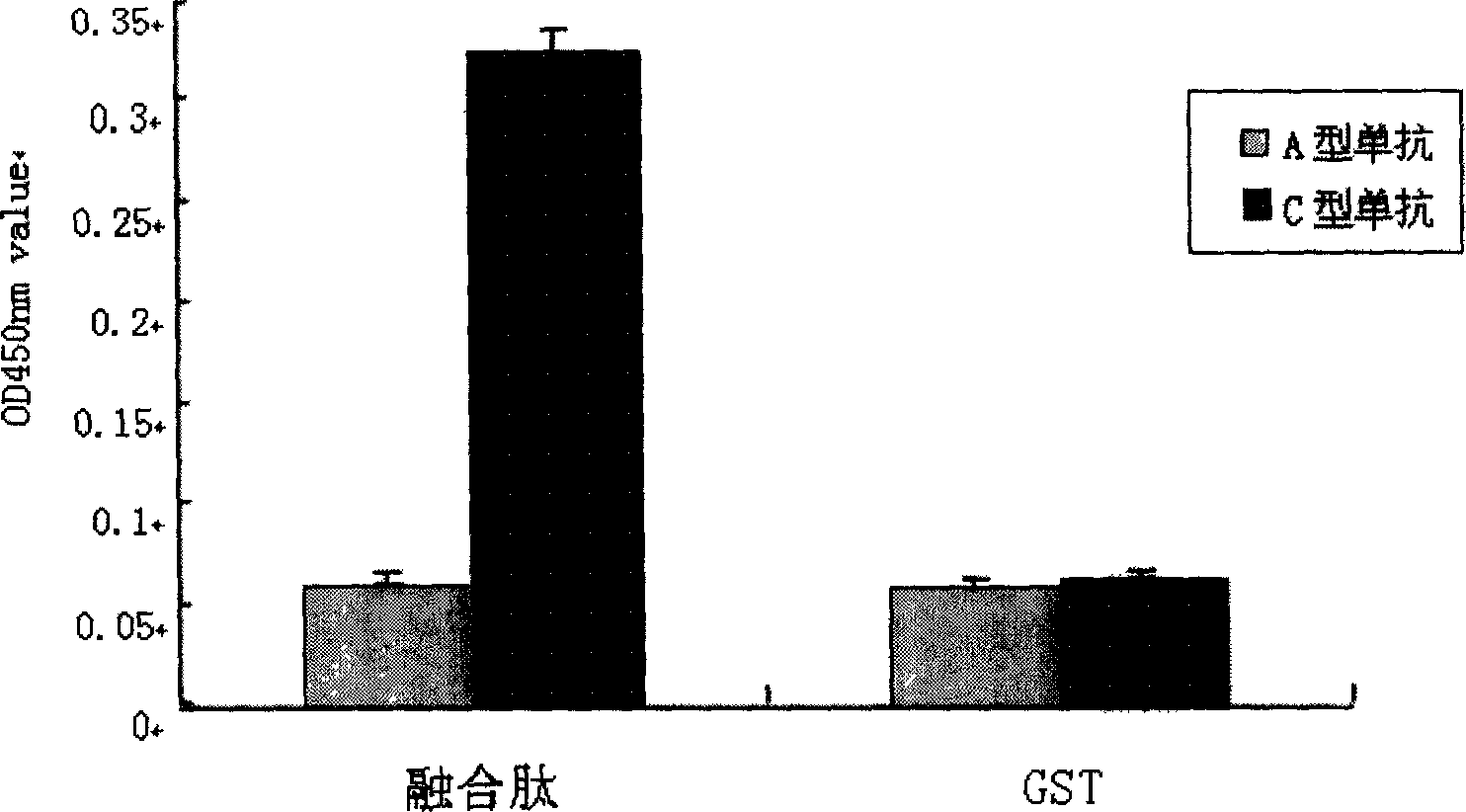
Polypeptide for Haemophilus paragallinarum and process for preparing the same
InactiveUS6919080B2Safe and effectiveLess adverse side effectAntibacterial agentsBacteriaFowlHaemophilus
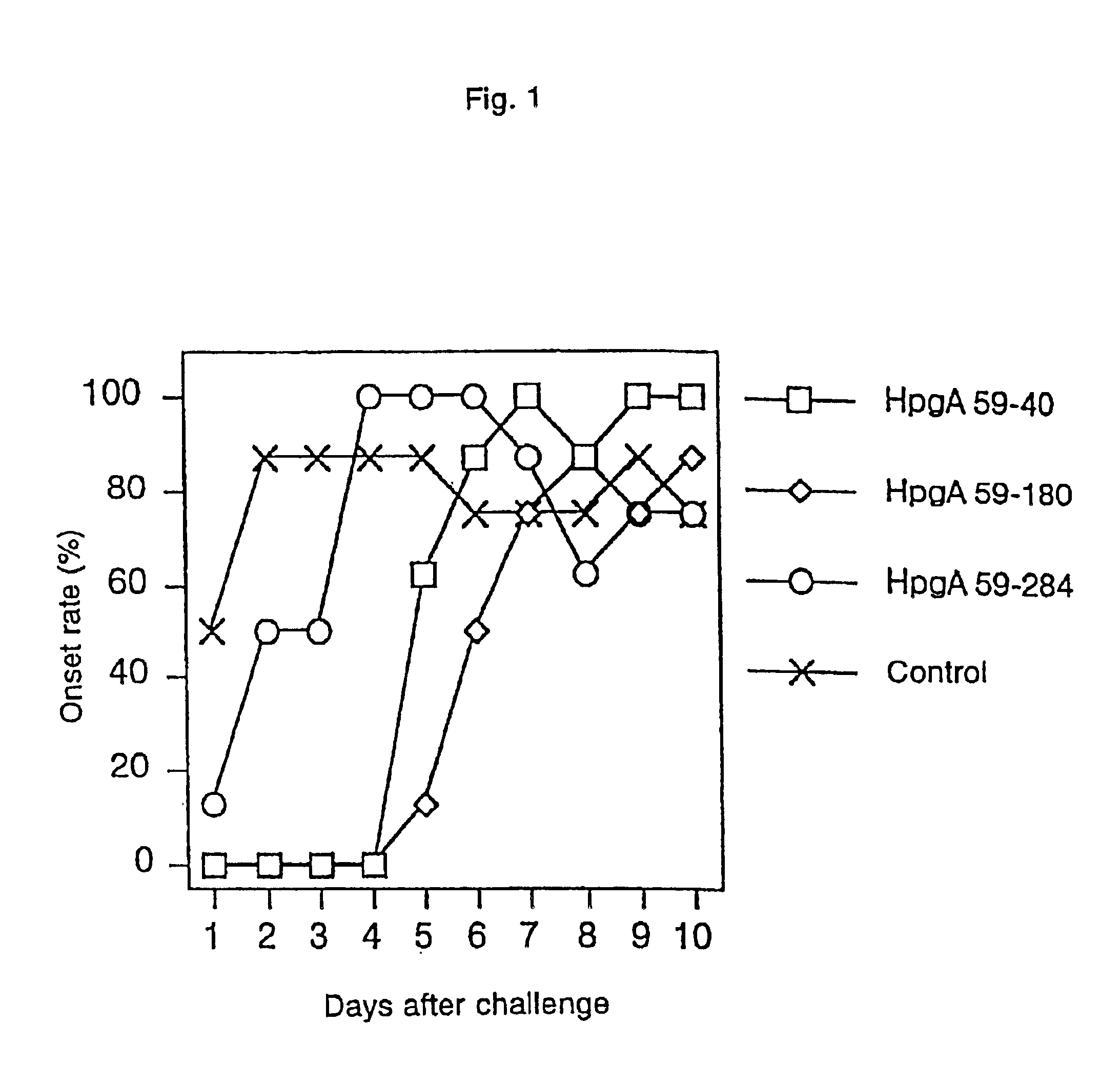
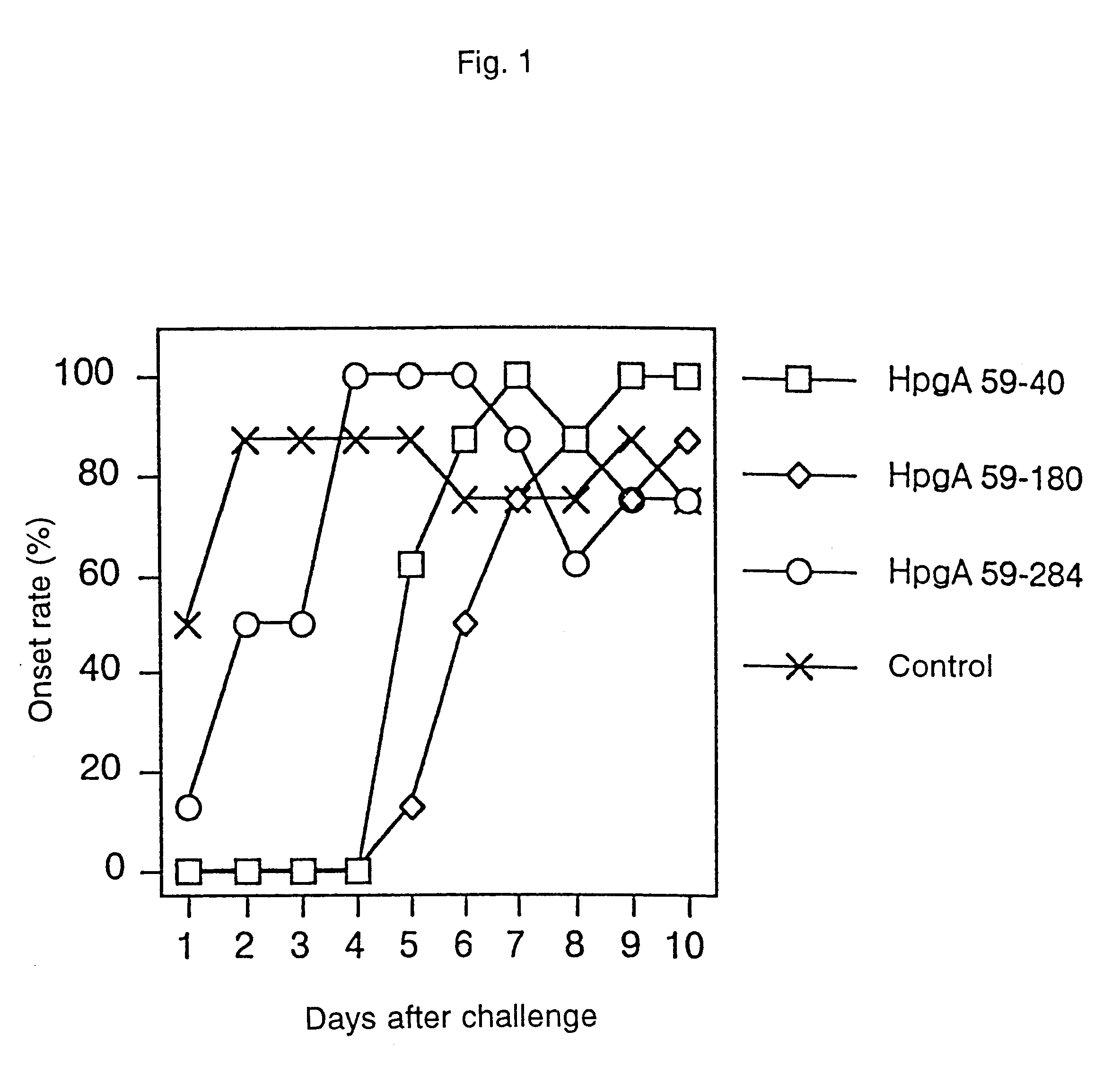
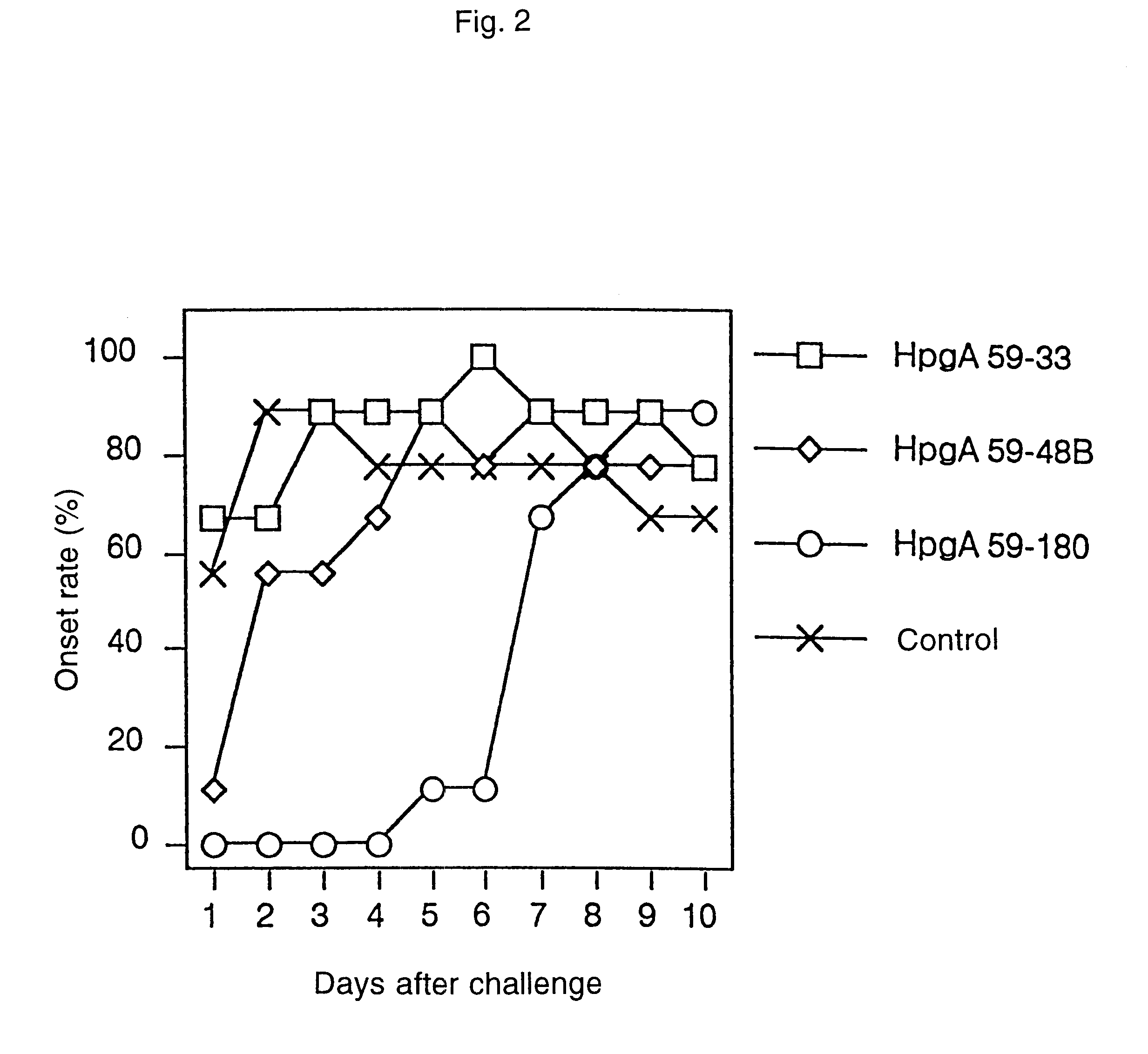
A novel peptide obtained from Haemophilus paragallinarum has been found useful for preventing avian infectious coryza. This polypeptide induces production of hemagglutination-inhibition antibody and prevents infection and onset of avian infectious coryza. The invention further provides a gene coding for the polypeptide, a recombinant vector for expression of this gene, a host transformed with this vector, a process for preparing the polypeptide in a host, a vaccine for avian infectious coryza comprising the polypeptide as an active ingredient, a monoclonal antibody obtained using the polypeptide as an immunogen, and a diagnostic agent and a therapeutic agent for avian infectious coryza using the peptide and the antibody.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST
Haemophilus parasuis detection kit and detection method thereof
ActiveCN104711359AHigh sensitivityAccurate distinctionMicrobiological testing/measurementMicroorganism based processesPasteurellaBacilli
The invention relates to a haemophilus parasuis detection kit and a detection method thereof, and belongs to the technical field of molecular biology. The detection kit comprises a primer pair, PCR Mix, a position control and dd H2O. The haemophilus parasuis detection kit disclosed by the invention has the primer pair designed according to an mviN gene sequence in a high conserved domain, is good in specificity, and can accurately distinguish the haemophilus parasuis strain LC from haemophilus paragallinarum, actinobacillus pleuropneumoniae, pasteurella muhocida, arcanobacterium pyogenes, staphylococcus aureus and streptococcus suis; the detection kit and the detection method provided by the invention are high in sensitivity, short in consumed time, accurate in detection, and important in significance of monitoring haemophilus parasuis reproduction, disease occurrence and prevalence as well as timely control of the disease.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Indirect ELISA (Enzyme-linked Immuno Sorbent Assay) kit for detecting type A haemophilus paragallinarum antibody as well as detection method and application thereof
InactiveCN107219364AEasy to manufactureEasy to purifyBiological material analysisColor/spectral properties measurementsElisa kitAntiendomysial antibodies
The invention discloses an indirect ELISA kit for detecting Haemophilus paragallinarum type A antibody, a detection method and an application thereof, and relates to the technical field of biological detection. The ELISA kit of the present invention includes: coated microplate, washing solution, serum diluent, substrate chromogenic solution, rabbit anti-chicken enzyme-labeled secondary antibody, stop solution, type A Hpg standard positive serum and type A Hpg standard negative serum Serum; wherein, the coated microtiter plate uses the recombinant hemagglutinin protein of Haemophilus paragallinarum type A as the coated antigen. The ELISA kit of the present invention is used to detect the antibody of Haemophilus paragallinarum type A, and has the advantages of high efficiency, good sensitivity, specificity and repeatability, and the kit is easy to operate, fast and low in cost, and is suitable for clinical applications and promote.
Owner:YANGLING VOCATIONAL & TECHN COLLEGE +1
Kit for PCR (Polymerase Chain Reaction) detection of haemophilus paragallinarum
The invention relates to a kit and more particularly relates to a kit for PCR (Polymerase Chain Reaction) detection of haemophilus paragallinarum. The kit comprises 20 microliters of PCR buffer solution, 25 microliters of ultrapure water, 5 microliters of Marker DL2000, 3 microliters of 15pmol / microliter upper primers, 3 microliters of 15pmol / microliter lower primers, 3 microliters of polymerase, 3 microliters of bromophenol blue, 15 microliters of TE buffer solution, 5 microliters of negative control and 5 microliters of positive control. The kit has the beneficial effects that the kit has the advantage of rapid detection of a PCR detection technology and the kit is low in construction cost; the special primers are adopted so as to meet the detection of a PCR product of a gene segment which is 0.00953ng at the minimum; the detection sensitivity is high.
Owner:SHANDONG BINZHOU BOLAIWEI BIOTECH
Haemophilus paragallinarum, inactivated vaccine and preparation method of inactivated vaccine
InactiveCN104212736ANo adverse reactionAddress effectivenessAntibacterial agentsBacteriaLaboratory cultureHaemophilus paragallinarum
The invention discloses haemophilus paragallinarum, an activated vaccine, and a preparation method of the activated vaccine. The provided haemophilus paragallinarum is actually the HN-VA strain of haemophilus paragallinarum, and the HN-VA strain has been preserved in the China General Microbiological Culture Collection Center (CGMCC), the preservation address is No.1 West Beichen Road, Chaoyang District, Beijing, and the preservation number is CGMCC No.7211. For the first time, the HN-VA strain of haemophilus paragallinarum is adopted to prepare an activated vaccine for preventing chicken infectious rhinitis. The research and preparation of the inactivated vaccine effectively solve the problems that the chicken infectious rhinitis is hard to prevent, and the happening rate and death rate of the disease are high; moreover the inactivated vaccine will not generate any adverse reaction to chicken, the experimental safety is high, a high-titer neutralizing antibody will generate in the chicken after vaccination, and the immunity effect is good.
Owner:ZHENGZHOU HOUYI PHARMA
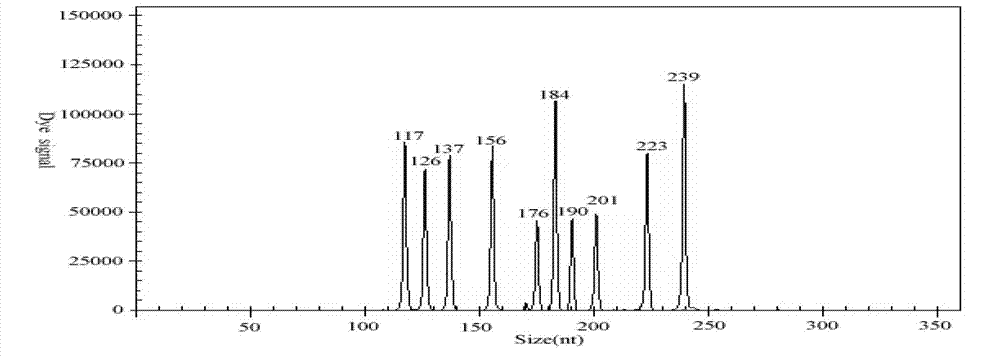
Haemophilus paragallinarum fluorogenic quantitative PCR detection method
InactiveCN105002284AEasy to operateNo further action requiredMicrobiological testing/measurementHaemophilusBiology
The invention discloses a haemophilus paragallinarum fluorogenic quantitative PCR detection method. According to the haemophilus paragallinarum fluorogenic quantitative PCR detection method, a hagA gene with the high degree of specificity and conservative property is selected as an amplification target gene, SYBR Green I fluorochrome is taken as a detection target, through the continuous optimization of fluorogenic quantitative PCR reaction conditions, the preparation of a standard substance and the establishment of a standard curve, the haemophilus paragallinarum fluorogenic quantitative PCR detection method of the SYBR Green I fluorochrome jogged with DNA, and the DNA of a haemophilus paragallinarum hagA genetic template can be quantified accurately. The haemophilus paragallinarum fluorogenic quantitative PCR detection method has the advantages of being strong in specificity, high in sensibility, simple in operation, rapid in detection, accurate in quantification and the like, the haemophilus paragallinarum fluorogenic quantitative PCR detection method can be used for clinic rapid diagnosis of haemophilus paragallinarum and the quantitative analysis of the degree of infection by the haemophilus paragallinarum, and the haemophilus paragallinarum fluorogenic quantitative PCR detection method is suitable for popularization and application.
Owner:SHANDONG BINZHOU ANIMAL SCI & VETERINARY MEDICINE ACADEMY
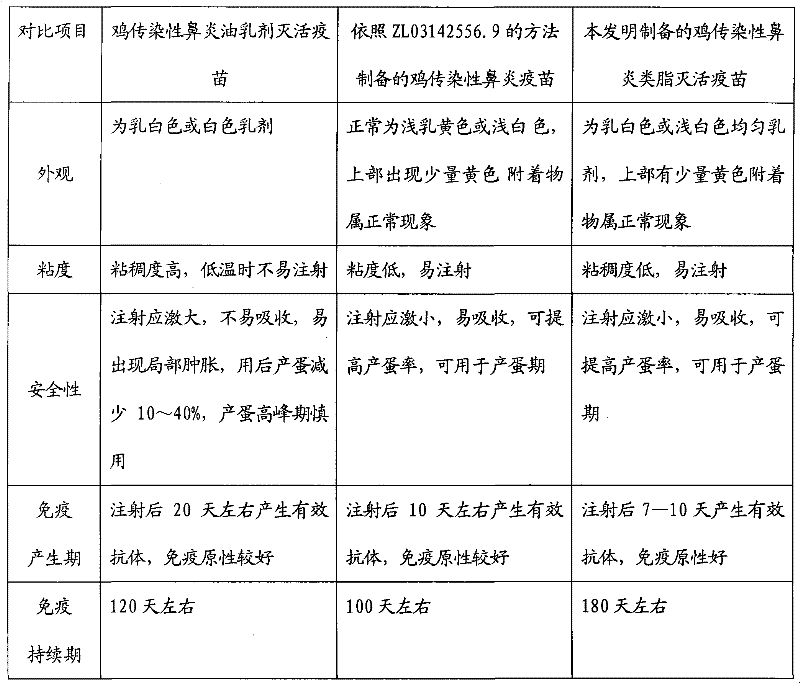
Inactivated vaccine for infectious coryza of chickens
InactiveCN102380092AImprove securityNo side effectsAntibacterial agentsAntibody medical ingredientsInfectious RhinitisShake up
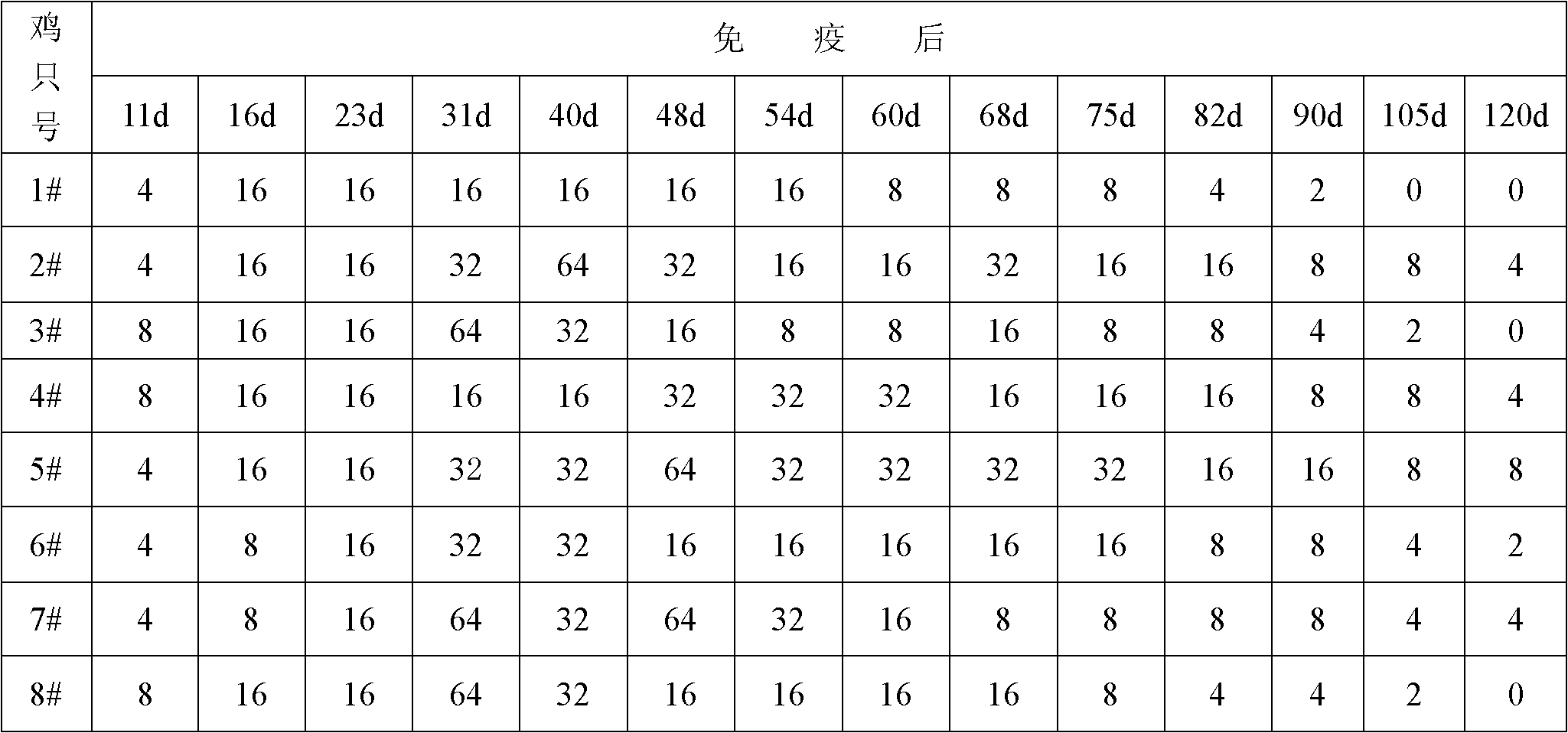
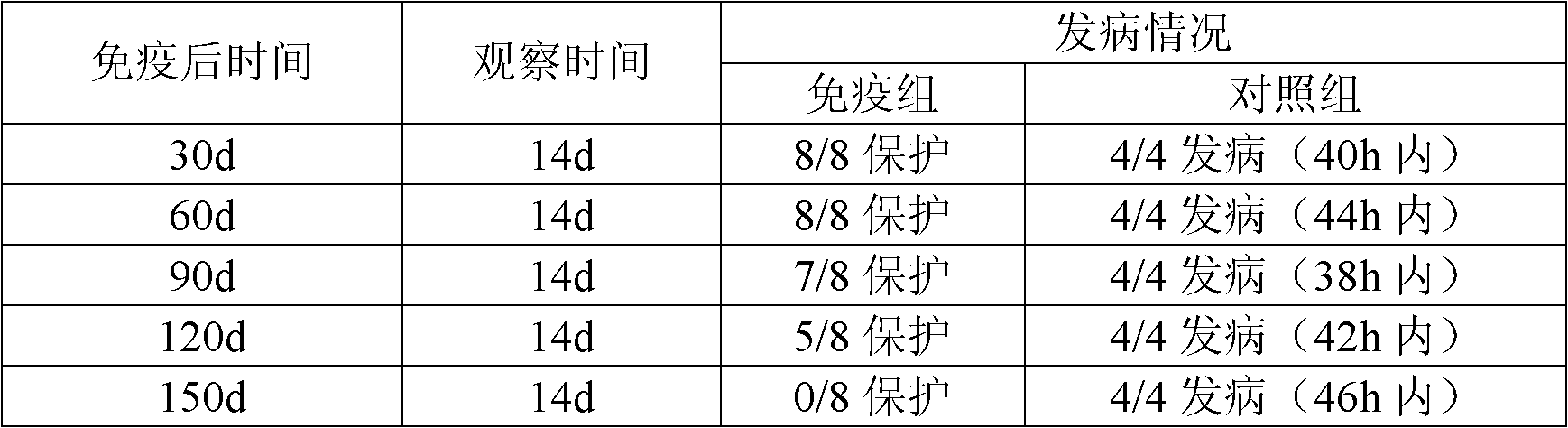
The invention relates to an inactivated vaccine for infectious coryza of chickens. The preparation method of the inactivated vaccine for infectious coryza of chickens comprises the following steps: using sterilized PBS (phosphate-buffered saline) to dilute inactivated haemophilus paragallinarum A type C-hpg-8 strain concentrated bacteria liquid prepared by the conventional method by a certain proportion, then mixing with vaccine adjuvants taking carbomer as a main component according to the proportion, stirring and shaking up so as to prepare the inactivated vaccine for infectious coryza of chickens. After a chicken is immunized by the inactivated vaccine for infectious coryza of chickens, the following advantages can be realized: the antibody titer generated by the inoculated chicken is high, the protection period of the antibody is long, and the protection rate against strong virus is high and the like.
Owner:YEBIO BIOENG OF QINGDAO
Antigen simulating epipeptide of C type para chicken blood phili bacillus and its use
InactiveCN1724560AEasy to operateAntibacterial agentsBacterial antigen ingredientsInfectious RhinitisTyrosine
The invention relates to the antigen imitated epitope peptide of Haemophilus paragallinarum serovar C in China and its application, wherein its amino acid sequence is represented by the general formula of YX1PX2QWWX3X4X5X6X7, wherein Y represents tyrosine residue, P represents proline residue, Q represents glutamine residue, W represents tryptophan residue, X1-X7 represents any amino acids. The peptide can be used for providing an easily-manipulated serological type differential diagnosis method for chicken infectious rhinitis.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES +1
Preparation method of inactivated vaccine of infectious coryza of chicken
ActiveCN101648013AEasy to operateIncreased number of cultured bacteriaAntibacterial agentsBacterial antigen ingredientsHaemophilusFermentation
The invention discloses a preparation method of an inactivated vaccine of infectious coryza of chicken, which comprises the following steps: firstly, diluting haemophilus paragallinarum strains, inoculating a chicken agar plate, culturing at 37 DEG C for 22-24h; selecting a typical colony to inoculate a chicken agar slant culture-medium, culturing to obtain first class slant strains; secondly, getting the first class slant strains to inoculate into a chicken soup culture medium, vibrating and culturing at 37 DEG C for 12h, obtaining second class strains; thirdly, inoculating the second class strains into a semisynthetic culture medium, culturing by a biological fermentation cylinder; and inactivating to obtain the inactivated vaccine. Aiming at defects of complex process, high production cost, low yield and the like existing in the prior preparation method of the inactivated vaccine of infectious coryza of chicken, the research work of an improved production process is developed, and the important breakout on the culture method is obtained. The method has simple and feasible operation; compared with the prior art, the invention greatly improves the culture strains, shortens the culture time by 10-12h, and effectively lowers the production cost.
Owner:哈药集团生物疫苗有限公司
Haemophilus paragallinarum B strain fermentation medium, preparation method thereof and application thereof
ActiveCN106190909ATo promote metabolismShorten the adaptation periodBacteriaMicroorganism based processesAdditive ingredientHAEMOPHILUS B
The invention discloses a haemophilus paragallinarum B strain fermentation medium, a preparation method thereof and application thereof, and belongs to the field of agricultural microorganism. The haemophilus paragallinarum B strain fermentation medium is prepared from 5 to 100 g / L of casein peptone, 0.5 to 80 g / L of yeast powder, 0.5 to 80 g / L of polyvalent peptone, 0.5 to 20 g / L of sodium chloride, 0.5 to 20 g / L of sodium glutamate, 0.5 to 30 g / L of glucose, 0.5 to 30 g / L of fish meal peptone, 5 to 100 mL / L of chicken serum, 0.5 to 50 mL / L of microelement solution and 1 to 30 mL / L of NAD (Nicotinamide Adenine Dinucleotide) solution. The haemophilus paragallinarum B strain fermentation medium disclosed by the invention contains all nutritional ingredients which are needed for growth of haemophilus paragallinarum B strains, growth and metabolism of the strains are facilitated, the adaption period of thallus is shortened, the adaptive capacity of the thallus after inoculation is strong, the fermentation period is short, the viable bacteria quantity during harvesting is high, and the haemophilus paragallinarum B strain fermentation medium is suitable for large-scale application and has a wide prospect.
Owner:山东滨州沃华生物工程有限公司
Chinese medicinal granule for treating infectious rhinitis of avian
The invention relates to a traditional Chinese medicine compound granule for treating avian infectious rhinitis, including five medicines of siberian cocklour fruit, honeysuckle flower, wild chrysanthemum, biond magnolia flower and India madder root. The invention is mainly used for treating infectious rhinitis which is caused by haemophilus paragallinarum and other respiratory tract pathogens and is accompanied with nasal and sinus mucosa acute catarrhal inflammation and catarrhal conjunctivitis lesions, while the infectious rhinitis has the symptoms of flowing thin and clear liquid of the nasal cavity, sneezing, eyelid edema, appetite suppression, poor growth of chicks, reduced egg yield of laying hens and so on. The medicine can be prepared into the soluable granule by the combination of the special physiological structure of short reproductive tracts of birds which can not digest the wood fiber, the invention can be dissolved rapidly in the fodder drinking water, and the invention can achieve the purpose of rapid onset of action after the drinking water is absorbed by sick chickens.
Owner:TIANJIN RINGPU BIO TECH
Polypeptide originating in haemophilus paragallinarum and process for producing the same
InactiveUS6544519B1Safer and effective vaccineLess adverse side effectAntibacterial agentsBacteriaDiagnostic agentHaemophilus
A novel peptide obtained from Haemophilus paragallinarum has been found useful for preventing avian infectious coryza. This polypeptide induces production of hemagglutination-inhibition antibody and prevents infection and onset of avian infectious coryza. The invention further provides a gene coding for the polypeptide, a recombinant vector for expression of this gene, a host transformed with this vector, a process for preparing the polypeptide in a host, a vaccine for avian infectious coryza comprising the polypeptide as an active ingredient, a monoclonal antibody obtained using the polypeptide as an immunogen, and a diagnostic agent and a therapeutic agent for avian infectious coryza using the peptide and the antibody.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST
Haemophilus paragallinarum and application thereof
InactiveCN107354114AHigh proliferative titerStable biological propertiesAntibacterial agentsBacteriaFacial swellingInfectious Rhinitis
The invention discloses a haemophilus paragallinarum and application thereof. The haemophilus paragallinarum is haemophilus paragallinarum HNHpg1, the preservation number is CGMCC NO:13859, the preservation date is May 26th, 2017, the preservation place is China General Microbiological Culture Collection Center and the preservation address is Beijing, China. The haemophilus paragallinarum HNHpg1 is separated from secreta of sinus infraorbitalis of a laying hen suffering from typical respiratory symptoms and head and facial swelling, has stable biological characteristics, has stronger pathogenicity on chicks, can result in morbidity and death of the chicks and has good immunogenicity. A vaccine prepared by utilizing the haemophilus paragallinarum HNHpg1 disclosed by the invention is safe and reliable and has a better protective effect on chicken infectious rhinitis.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
GeXP rapid detection kit capable of simultaneously identifying nine pathogens of chicken respiratory tract diseases
ActiveCN102899424BStrong specificityImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationInfectious laryngotracheitisRespiratory tract disease
The invention discloses a GeXP rapid detection kit capable of simultaneously identifying nine pathogens of chicken respiratory tract diseases. The kit is used based on a GeXP system and comprises ten polymerase chain reaction (PCR) primer pairs; the kit is used for identifying and detecting avian influenza virus, H5, H7 and H9 subtype avian influenza virus, newcastle disease virus, infectious bronchitis, infectious laryngotracheitis, mycoplasma gallisepticum, bursa synovialis mycoplasma and haemophilus paragallinarum; and the kit is good in specificity, high in sensitivity and can detect 100 copy / mu l. Compared with an identifying result of the conventional experiment method of a pathogen separation and hemagglutination inhibition experiment or a serology experiment and the like, the GeXP rapid detection kit has the advantage that the coincidence rate reaches 100 percent. The kit is generally used for detecting the main chicken respiratory tract diseases and the pathogens thereof, so that a simple and high-flux detection kit and a detection system are provided, an actual requirement is met, and the application prospect is wide.
Owner:GUANGXI VETERINARY RES INST
Antigen analogue epi-peptide of para bacillus fowl blood phili and its use
InactiveCN1597693AEasy to operateGood economic benefits and market prospectsBacteria peptidesBiological testingSerotypePhenylalanine
An antigen mimicking epitope peptide of chicken parahemophilus has its amino acid sequence: CSWYFPX1X2C. It can be used to prepare the diagnosing reagent and vaccine of chicken's infectious rhinitis.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA +1
Culture medium efficiently culturing chicken coryza haemophilus paragallinarum and preparation method and application of culture medium
InactiveCN107325993ACultivate extensivelyPromote growthBacteriaMicroorganism based processesSerum igeNosematosis
The invention relates to a culture medium efficiently culturing chicken coryza haemophilus paragallinarum and a preparation method and application of the culture medium. The culture medium is scientific and reasonable in formula, chick embryo allantoic fluid replaces serum, the cost can be greatly lowered, growth of the culture medium culturing the chicken coryza haemophilus paragallinarum is facilitated, compared with other culture media sold on the market, the cost is lowered by 20%-30%, the number of growing chicken coryza haemophilus paragallinarum thalli is increased by 5%-500%, and the culture medium can be widely used for culturing the chicken coryza haemophilus paragallinarum.
Owner:CHONGQING ACAD OF ANIMAL SCI
Antigen simulating epipeptide of A type parachicken blood phili bacillus and its use
InactiveCN1724559AReduce chemical residueReduce the impactAntibacterial agentsBacterial antigen ingredientsAspartic acid residueEpitope
The invention relates to the antigen imitated epitope peptide of Haemophilus paragallinarum serovar A in China and its application, wherein its amino acid sequence is represented by the general formula of X1X2X3X4AX5DPVX6X7X8, wherein A represents alanine residue, D represents aspartate residue, P represents proline residue, V represents valine residue, X1-X8 represents any amino acids. The invention also discloses the use of the epitope peptide of Haemophilus paragallinarum serovar A -type in China as active ingredient in the preparation of vaccine for treating chicken infectious rhinitis.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES +1
Chicken feed additive, applications thereof and chicken feed
ActiveCN109170174AReasonable formulaLow costAntibacterial agentsOrganic active ingredientsBiotechnologyEscherichia coli
The invention relates to a chicken feed additive, applications thereof and a chicken feed, and belongs to the technical field of feeds. The chicken feed additive comprises the following raw materials:20-40 parts by weight of modified chitosan, 5-7 parts by weight of sodium diacetate, 2-2.5 parts by weight of chlorogenic acid, 3-4 parts by weight of grapefruit peel, 4-6 parts by weight of Indian mock strawberries and 8-12 parts by weight of schefflera octophylla. The modified chitosan is obtained by modifying chitosan by serine. The chicken feed additive is reasonable in formula, safe, non-toxic, and low in cost, and can effectively kill a plurality of pathogenic bacteria in chicken eyes in addition to aspergillus, and reduce the prevalence of chicken eye diseases. The additive can be usedfor preparing the chicken feed for treating chicken eye diseases, and can reduce the prevalence of chickens by killing aspergillus, salmonella, haemophilus paragallinarum and escherichia coli. The chicken feed containing the chicken feed additive is safe to eat and conducive to improving the breeding efficiency.
Owner:安吉福寿农业开发有限公司
A detection kit for Haemophilus parasuis and its detection method
ActiveCN104711359BHigh sensitivityAccurate distinctionMicrobiological testing/measurementMicroorganism based processesStaphylococcus aureusPosition control
The invention relates to a haemophilus parasuis detection kit and a detection method thereof, and belongs to the technical field of molecular biology. The detection kit comprises a primer pair, PCR Mix, a position control and dd H2O. The haemophilus parasuis detection kit disclosed by the invention has the primer pair designed according to an mviN gene sequence in a high conserved domain, is good in specificity, and can accurately distinguish the haemophilus parasuis strain LC from haemophilus paragallinarum, actinobacillus pleuropneumoniae, pasteurella muhocida, arcanobacterium pyogenes, staphylococcus aureus and streptococcus suis; the detection kit and the detection method provided by the invention are high in sensitivity, short in consumed time, accurate in detection, and important in significance of monitoring haemophilus parasuis reproduction, disease occurrence and prevalence as well as timely control of the disease.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Antigen simulating epipeptide of A type parachicken blood phili bacillus and its use
InactiveCN1304419CReduce chemical residueSimplify the seedling making processAntibacterial agentsBacterial antigen ingredientsAspartic acid residueInfectious Rhinitis
The invention relates to the antigen imitated epitope peptide of Haemophilus paragallinarum serovar A in China and its application, wherein its amino acid sequence is represented by the general formula of X1X2X3X4AX5DPVX6X7X8, wherein A represents alanine residue, D represents aspartate residue, P represents proline residue, V represents valine residue, X1-X8 represents any amino acids. The invention also discloses the use of the epitope peptide of Haemophilus paragallinarum serovar A -type in China as active ingredient in the preparation of vaccine for treating chicken infectious rhinitis.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES +1
Antigen simulating epipeptide of C type para chicken blood phili bacillus and its use
InactiveCN1304420CEasy to operateAntibacterial agentsBacterial antigen ingredientsInfectious RhinitisTyrosine
The invention relates to the antigen imitated epitope peptide of Haemophilus paragallinarum serovar C in China and its application, wherein its amino acid sequence is represented by the general formula of YX1PX2QWWX3X4X5X6X7, wherein Y represents tyrosine residue, P represents proline residue, Q represents glutamine residue, W represents tryptophan residue, X1-X7 represents any amino acids. The peptide can be used for providing an easily-manipulated serological type differential diagnosis method for chicken infectious rhinitis.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES +1
Kit for detecting chicken coryza haemophilus paragallinarum on basis of loop-mediated isothermal amplification and detection method of kit
InactiveCN107326096AImprove thermal stabilityPromote amplificationMicrobiological testing/measurementAstragalus polysaccharideBetaine
The invention relates to a kit for detecting chicken coryza haemophilus paragallinarum on the basis of loop-mediated isothermal amplification and a detection method of the kit. By adding dextran and astragalus polysaccharide in a loop-mediated isothermal amplification system to reach the final concentration being 0.1-0.5 mol / L (on the basis of glucose), the stability of polymerase is improved, polymerase and betaine which are high in price in an ordinary LAMP kit can be partially replaced, amplification of high content GC fragments can be well promoted, the detection reaction time can be shortened to 20-30 minutes, and the detection period can be shortened while the detection cost is lowered; an important meaning to loop-mediated isothermal amplification on chicken coryza haemophilus paragallinarum detection is achieved.
Owner:CHONGQING ACAD OF ANIMAL SCI
Emodin methyl ether microemulsion and application thereof
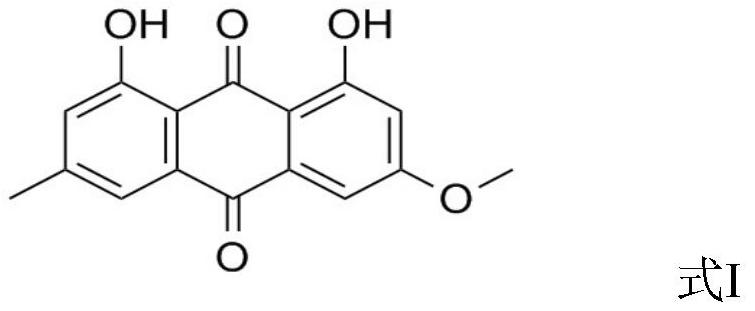
PendingCN114831937AHelp healthy developmentAntibacterial agentsOrganic active ingredientsBiotechnologyRespiratory tract disease
The invention discloses an emodin methyl ether microemulsion and an application thereof. The emodin methyl ether microemulsion is a microemulsion system composed of an organic solvent and a surfactant. Preferably, the formula of the microemulsion system comprises one or more of N, N-dimethylformamide or N, N-dimethylacetamide, ethanol or Tween-20. In-vitro experiments prove that the physcion can be used for killing clinical drug-resistant bacillus cereus, enterococcus faecalis, bacillus gallisepticum, ornithobacterium rhinotracheitis, haemophilus paragallinarum, klebsiella, chlamydia psittaci, mycoplasma gallisepticum and mycoplasma synoviae. Meanwhile, animal experiments prove that the emodin methyl ether microemulsion is suitable for preventing and treating respiratory tract diseases caused by avian rhinotracheitis, haemophilus paragallinarum, klebsiella, chlamydia psittaci and mycoplasma gallisepticum clinically, liver rupture caused by bacillus cereus, enterococcus faecalis and chicken bacilli, and the like. The bovine mastitis and endometritis caused by klebsiella and bacillus cereus can be treated.
Owner:CHINA AGRI UNIV
Application of dextran-combined astragalus polysaccharide in detection of haemophilus paragallinarum causing infectious rhinitis of chicken as well as kit and detection method of dextran-combined astragalus polysaccharide
InactiveCN107385087AImprove thermal stabilityPromote amplificationMicrobiological testing/measurementMicroorganism based processesAstragalus polysaccharideBetaine
The invention relates to application of dextran-combined astragalus polysaccharide in detection of haemophilus paragallinarum causing infectious rhinitis of chicken as well as a kit and a detection method of the dextran-combined astragalus polysaccharide. By adding dextran and astragalus polysaccharide in a loop-mediated isothermal amplification system until the final concentration is 0.1-0.5mol / L (calculated by glucose), the stability of polymerase is improved, expensive 'polymerase' and 'glycine betaine' in a common LAMP kit can be partly replaced, a high-content GC fragment can be promoted to amplify very well, the detection reaction time can be reduced to 20-30 minutes, the detection cost can be reduced, meanwhile, the detection period is shortened, and the dextran-combined astragalus polysaccharide has important significance for loop-mediated isothermal amplification in the detection of the haemophilus paragallinarum causing the infectious rhinitis of chicken.
Owner:CHONGQING ACAD OF ANIMAL SCI
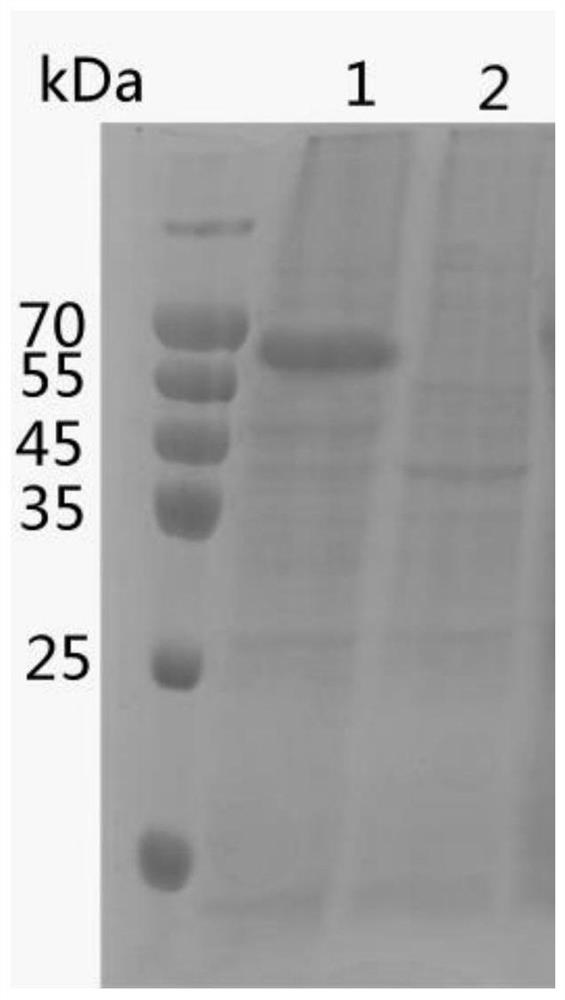
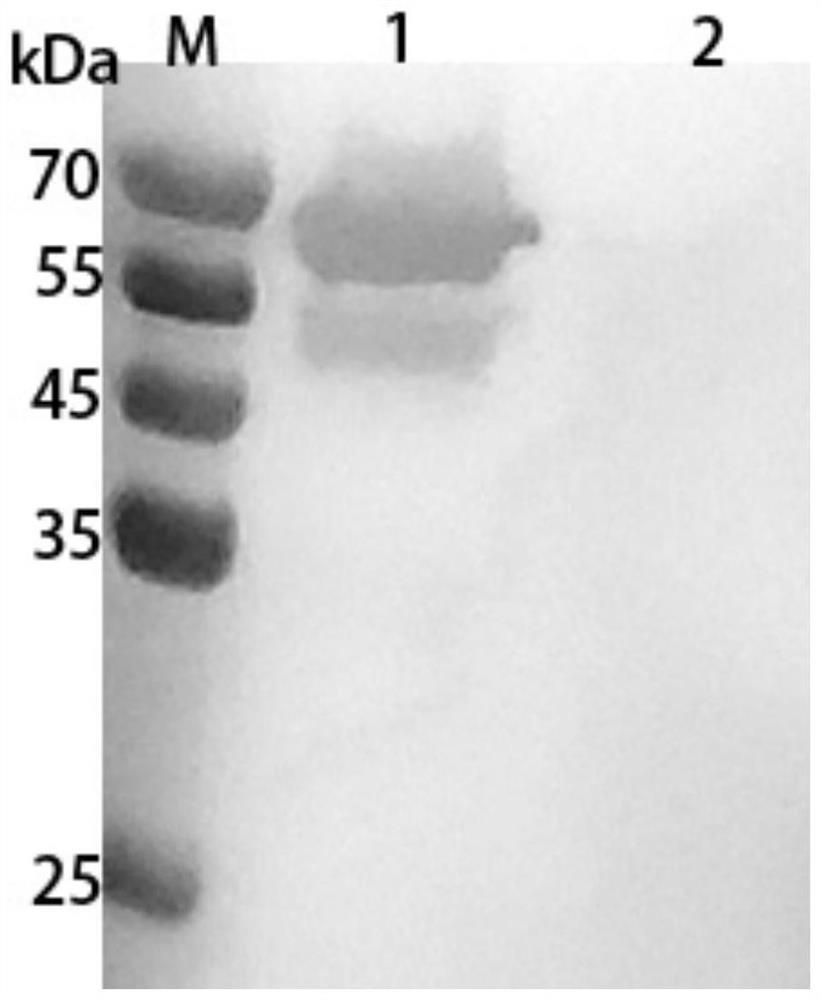
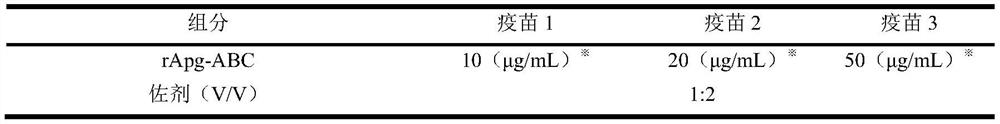
Preparation method and application of haemophilus paragallinarum trivalent genetic engineering subunit vaccine
ActiveCN114262719AImprove immunityPerfect post-modification functionAntibacterial agentsBacterial antigen ingredientsAntigenAdjuvant
The invention discloses a preparation method and application of a haemophilus paragallinarum trivalent genetic engineering subunit vaccine, and belongs to the field of veterinary biological products. According to the invention, the fusion protein rApg-ABC capable of simultaneously preparing three serotypes of haemophilus paragallinarum A, B and C is constructed and expressed. According to the invention, an rApg-ABC fusion protein gene is connected to a pFastBac1 vector, and the rApg-ABC fusion protein gene is transformed into a DH10Bac competent cell to obtain a recombinant rod grain. And transfecting the recombinant baculovirus to sf9 cells, culturing to obtain recombinant baculovirus, inoculating the recombinant baculovirus into suspended insect cells HF to efficiently express antigen protein, extracting, purifying, carrying out BEI inactivation, adding an adjuvant, and emulsifying to obtain the vaccine. The preparation method is simple, can prepare a large amount of antigen protein, is short in time consumption and high in expression quantity, greatly reduces the production cost, and is beneficial to large-scale production.
Owner:扬州优邦生物药品有限公司
Preparation method of inactivated vaccine of infectious coryza of chicken
ActiveCN101648013BEasy to operateIncreased number of cultured bacteriaAntibacterial agentsBacterial antigen ingredientsHaemophilusFermentation
The invention discloses a preparation method of an inactivated vaccine of infectious coryza of chicken, which comprises the following steps: firstly, diluting haemophilus paragallinarum strains, inoculating a chicken agar plate, culturing at 37 DEG C for 22-24h; selecting a typical colony to inoculate a chicken agar slant culture-medium, culturing to obtain first class slant strains; secondly, getting the first class slant strains to inoculate into a chicken soup culture medium, vibrating and culturing at 37 DEG C for 12h, obtaining second class strains; thirdly, inoculating the second class strains into a semisynthetic culture medium, culturing by a biological fermentation cylinder; and inactivating to obtain the inactivated vaccine. Aiming at defects of complex process, high production cost, low yield and the like existing in the prior preparation method of the inactivated vaccine of infectious coryza of chicken, the research work of an improved production process is developed, and the important breakout on the culture method is obtained. The method has simple and feasible operation; compared with the prior art, the invention greatly improves the culture strains, shortens the culture time by 10-12h, and effectively lowers the production cost.
Owner:哈药集团生物疫苗有限公司
Haemophilus paragallinarum type b strain fermentation medium, its preparation method and application
ActiveCN106190909BTo promote metabolismShorten the adaptation periodBacteriaMicroorganism based processesFermentationHAEMOPHILUS B
The invention discloses a haemophilus paragallinarum B strain fermentation medium, a preparation method thereof and application thereof, and belongs to the field of agricultural microorganism. The haemophilus paragallinarum B strain fermentation medium is prepared from 5 to 100 g / L of casein peptone, 0.5 to 80 g / L of yeast powder, 0.5 to 80 g / L of polyvalent peptone, 0.5 to 20 g / L of sodium chloride, 0.5 to 20 g / L of sodium glutamate, 0.5 to 30 g / L of glucose, 0.5 to 30 g / L of fish meal peptone, 5 to 100 mL / L of chicken serum, 0.5 to 50 mL / L of microelement solution and 1 to 30 mL / L of NAD (Nicotinamide Adenine Dinucleotide) solution. The haemophilus paragallinarum B strain fermentation medium disclosed by the invention contains all nutritional ingredients which are needed for growth of haemophilus paragallinarum B strains, growth and metabolism of the strains are facilitated, the adaption period of thallus is shortened, the adaptive capacity of the thallus after inoculation is strong, the fermentation period is short, the viable bacteria quantity during harvesting is high, and the haemophilus paragallinarum B strain fermentation medium is suitable for large-scale application and has a wide prospect.
Owner:山东滨州沃华生物工程有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com