Preparation method and application of haemophilus paragallinarum trivalent genetic engineering subunit vaccine
A vaccine and gene technology, applied in the field of preparation of Haemophilus paragallinarum trivalent genetic engineering subunit vaccine, can solve the problems affecting vaccine safety, cumbersome purification process, serious side effects, etc., achieve superior immune effect and ensure immune effect , the effect of increasing the antibody level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Construction of recombinant baculovirus rBac-rApg-ABC
[0025] 1. Design the gene fragment encoding the fusion protein rApg-ABC, and submit it to Nanjing GenScript for sequence optimization according to the codon preference of insect cells, and synthesize the optimized sequence (SEQ ID NO:2) onto the pFastBac I transfer vector , to obtain the pFastBac-rApg-ABC transfer vector.
[0026] 2. Construction of recombinant baculovirus:
[0027] The transfer vector pFastBac-rApg-ABC was transferred into Escherichia coli DH10Bac competent cells, and positive clones were selected for PCR identification with M13 primers. M13-F: TGTAAAACGACGGCCAGT; M13-R: CAGGAAACAGCTATGAC. The PCR reaction system was (total volume 25 μL): 0.5 μL DNA template, 0.5 μL each of M13-F and M13-R, 12.5 μL DNA polymerase and 11 μL sterile water. The PCR reaction conditions are: 95°C, 5min; 30 cycles of 95°C for 30s, 65°C for 30s, 72°C for 90s; 72°C for 10min. 1% agarose gel electrophores...
Embodiment 2
[0029] Embodiment 2: Preparation of recombinant rApg-ABC protein
[0030] 1. Amplification of recombinant baculovirus: inoculate insect cell sf9 with recombinant baculovirus rBac-rApg-ABC, culture at 27°C for 4 days, collect the culture, centrifuge and take the supernatant to obtain the f2 generation recombinant baculovirus.
[0031] 2. Identification of expressed proteins
[0032] (1) The f2 generation recombinant baculovirus was inoculated into insect cell sf9 at an inoculum amount of MOI=5-10, cultured at 27° C. for 4 days, the culture was collected, and the supernatant was collected by centrifugation to obtain the recombinant rApg-ABC protein.
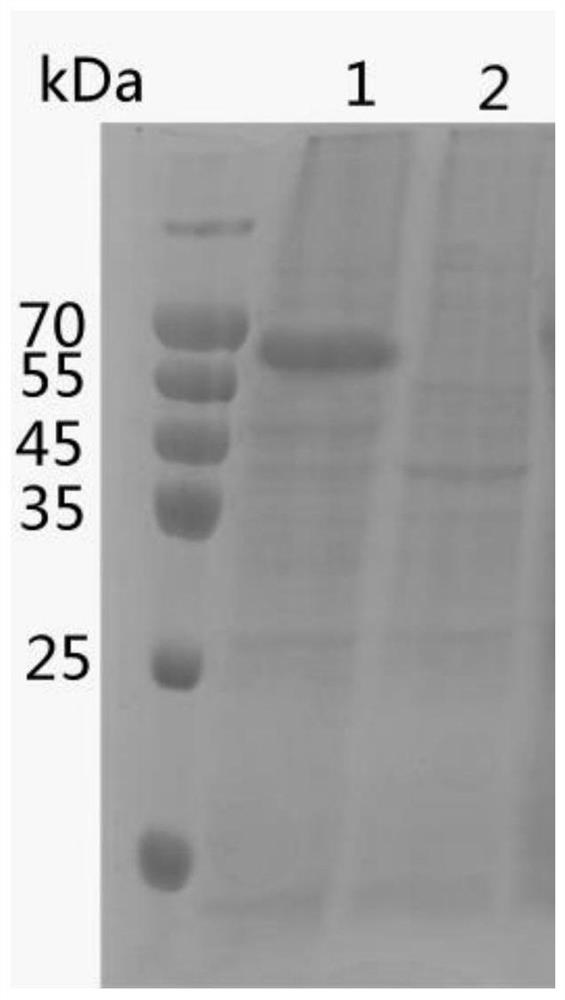
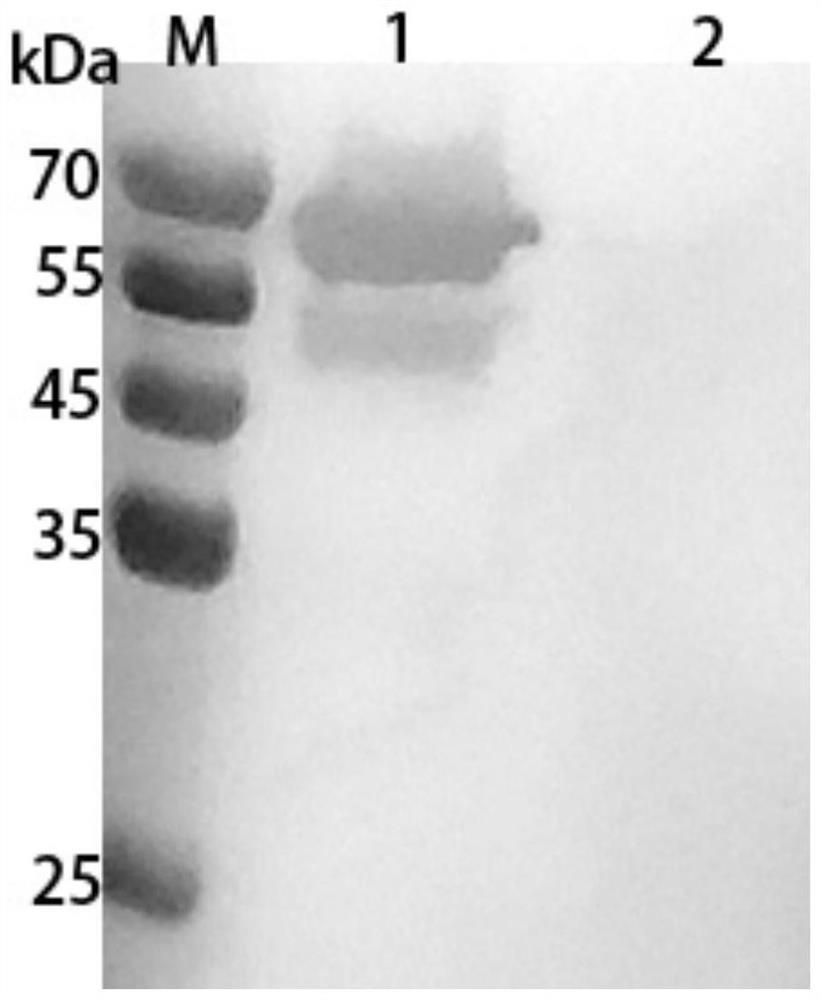
[0033] (2) SDS-PAGE identification: The above supernatant was subjected to SDS-PAGE electrophoresis; after electrophoresis, after staining and decolorization, it was found that there was a band at about 66kDa, and its molecular weight was consistent with the theoretical size, indicating that the expression was successful.
[0034]...
Embodiment 3
[0038] The preparation of embodiment 3 Haemophilus paragallinarum trivalent genetic engineering subunit vaccine
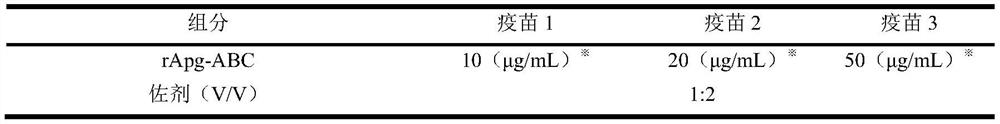
[0039] Take the rApg-ABC recombinant protein obtained in Example 2, add an adjuvant to emulsify, mix well, and store at 4°C. See Table 1 for specific vaccine ratios.
[0040] Table 1 Composition ratio of duck Tembusu virus genetically engineered subunit vaccine
[0041]
[0042] Note: ※ represents the protein concentration in each ml of vaccine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com