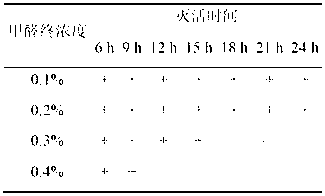
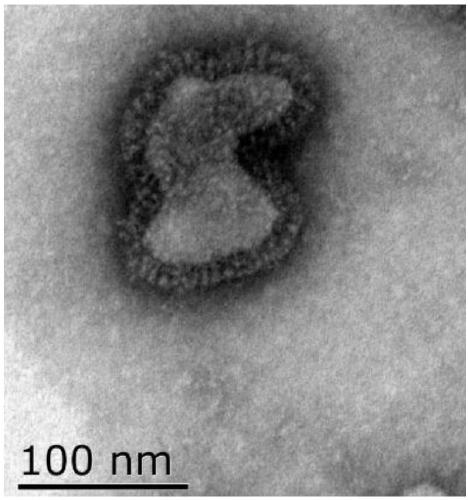
Patents
Literature
57results about How to "Stable biological properties" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
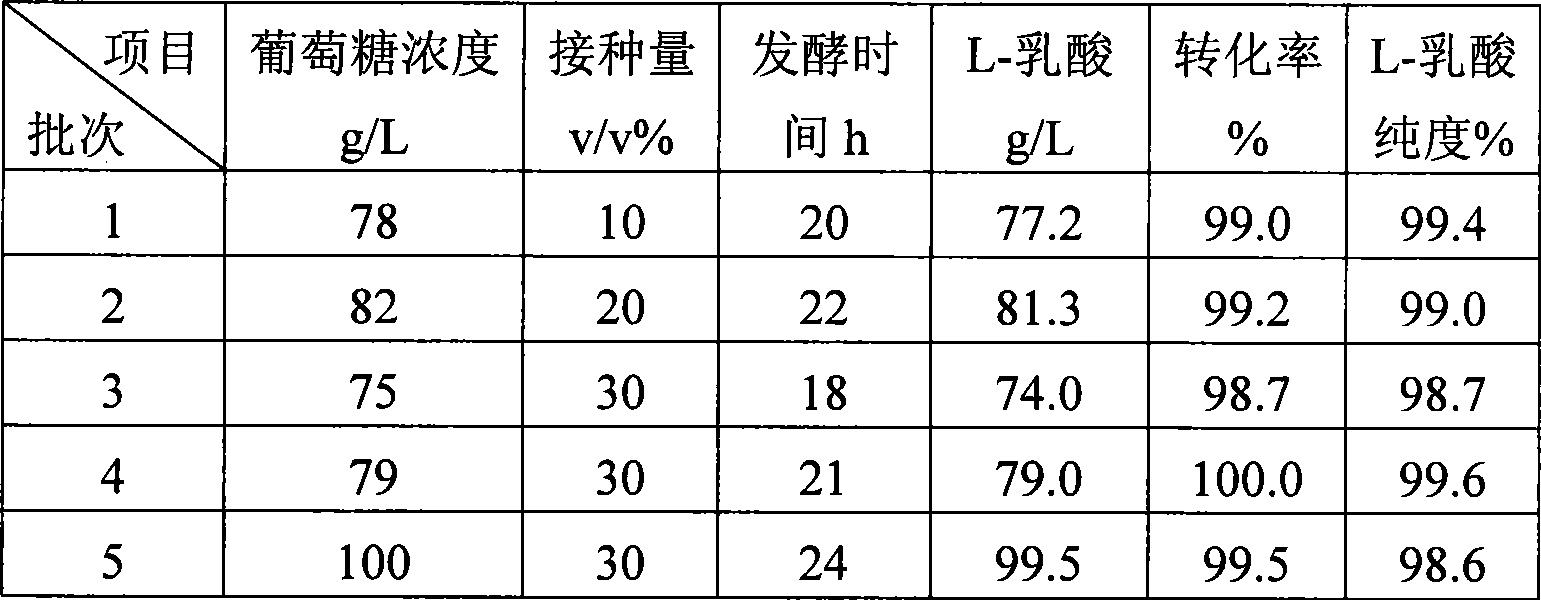
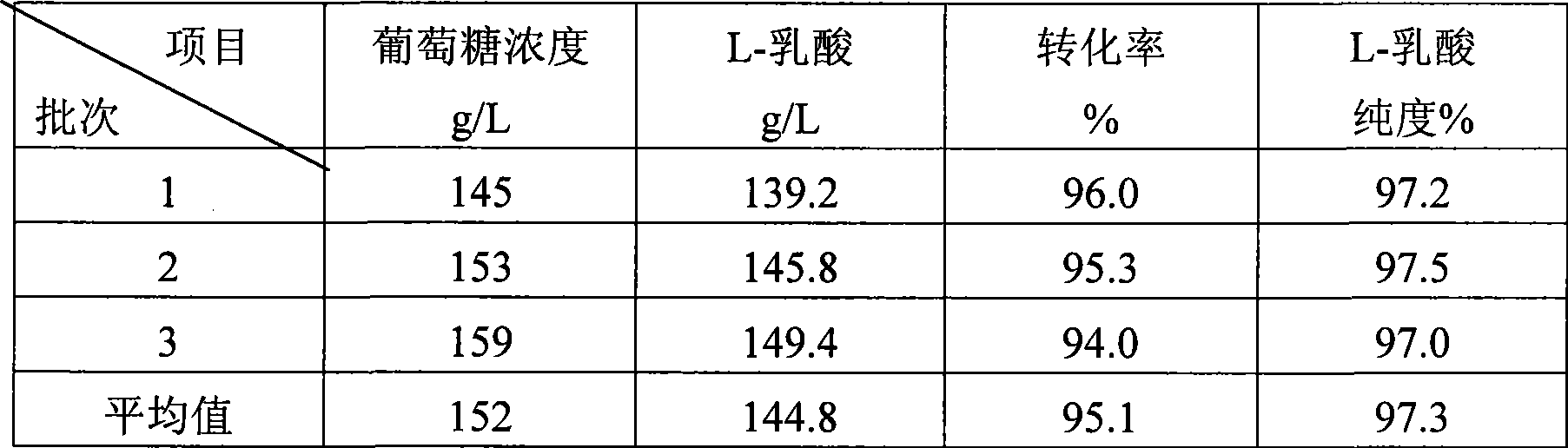
Method for producing L-lactic acid by Bacillus coagulans CGMCC No.2602
ActiveCN101544993AReduce fermentation costsSimple nutritional requirementsMicroorganism based processesFermentationMicroorganismGlucose polymers
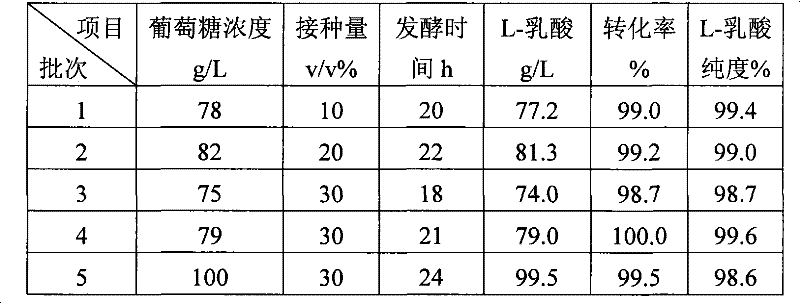
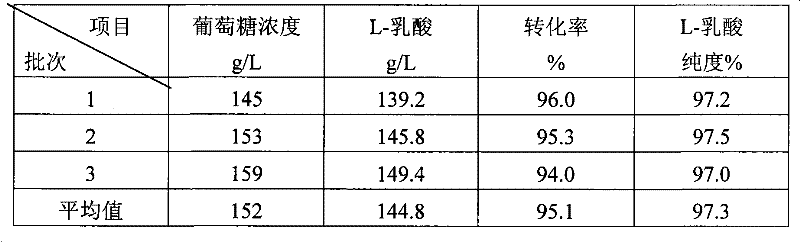
A method for producing L-lactic acid by Bacillus coagulans CGMCC No.2602 belongs to the technical field of microorganisms. In the invention, Bacillus coagulans CGMCC No.2602 is adopted, and under the condition of no oxygen supply, starchiness hydrolyzed sugar or dextrose is fermented by a semicontinuous intermittent fermentation way or an inter-sugar-compensating fermentation way to generate L-lactic acid with high optical purity. The invention has the advantages that the gemma property of the Bacillus coagulans is stable, and the L-lactic acid obtained by inoculating and fermenting the starchiness hydrolyzed sugar has high optical purity and rate of conversion of sugar and acid and short fermentation period; in addition, the semicontinuous intermittent fermentation way saves the time for preparing seeds by a continuous reladling and subculturing method, shortens the fermentation period, enhances the fermentation strength and obtains the L-lactic acid with both relatively high rate of conversion of sugar and acid and purity.
Owner:江苏省苏微微生物研究有限公司
Stem cell micro-needle patch for resisting wrinkles and removing freckles and preparation method thereof
InactiveCN104288129AStem Cell Essence InfiltrationInfiltrate smoothlyOrganic active ingredientsPeptide/protein ingredientsTransdermal patchEngineering
The invention discloses a stem cell micro-needle patch for resisting wrinkles and removing freckles and a preparation method thereof, and particularly relates to a micro-needle transdermal patch which is prepared of a anti-wrinkle and freckle removing essence and is injected into subcutaneous tissues so as to achieve cosmetic results, wherein the anti-wrinkle and freckle removing essence is prepared from stem cells, hyaluronic acid, epidermal growth factors, vitamin E and the like. The micro-needle patch has the advantages that the anti-wrinkle and freckle removing essence is introduced to a corium layer of skin by a microtubule, active ingredients permeate in the skin effectively and efficiently so that skin cells directly absorb all of the active ingredients in the anti-wrinkle and freckle removing essence, good effects are achieved, and finally the skin is vitalized from inside to outside so as to thoroughly relieve wrinkles and color spots from the skin. The stem cell micro-needle patch is used for resisting wrinkles and removing freckles and is safe, effective and fundamentally free of wounds.
Owner:奥思达干细胞有限公司
Haemophilus parasuis and application thereof
InactiveCN104611274AImprove protectionStable biological propertiesAntibacterial agentsBacteriaDiseaseImmune effects
The invention aims to provide haemophilus parasuis and application thereof. The preservation number of haemophilus parasuis is CGMCC No. 10230. The haemophilus parasuis is used for preparing medicines for treating haemophilus parasuis. A haemophilus parasuis serum type 5 vaccine strain LX-5 has a stable biological characteristic, has relatively strong pathogenicity to a piglet, and has good immunogenicity. A propolis inactivated vaccine prepared by applying the haemophilus parasuis is safe and reliable and can generate an antibody in a relatively high level, is long in duration and has a good protective effect on the piglet counteracting toxic substances for homologous strains. The morbidity and the death rate of the immunized swinery are obviously reduced, and the immune effect of the vaccine reaches or is superior to existing commercialized vaccines in the market, so that prevalence and spread of the haemophilus parasuis can be effectively prevented, the economical loss caused by the disease is lowered, and the haemophilus parasuis has a broad application prospect.
Owner:QINGDAO AGRI UNIV
Serotype 5 haemophilus parasuis (HPs) vaccine strain
ActiveCN103194412AStable biological propertiesEpidemic preventionAntibacterial agentsBacteriaMortality rateHaemophilus Vaccines
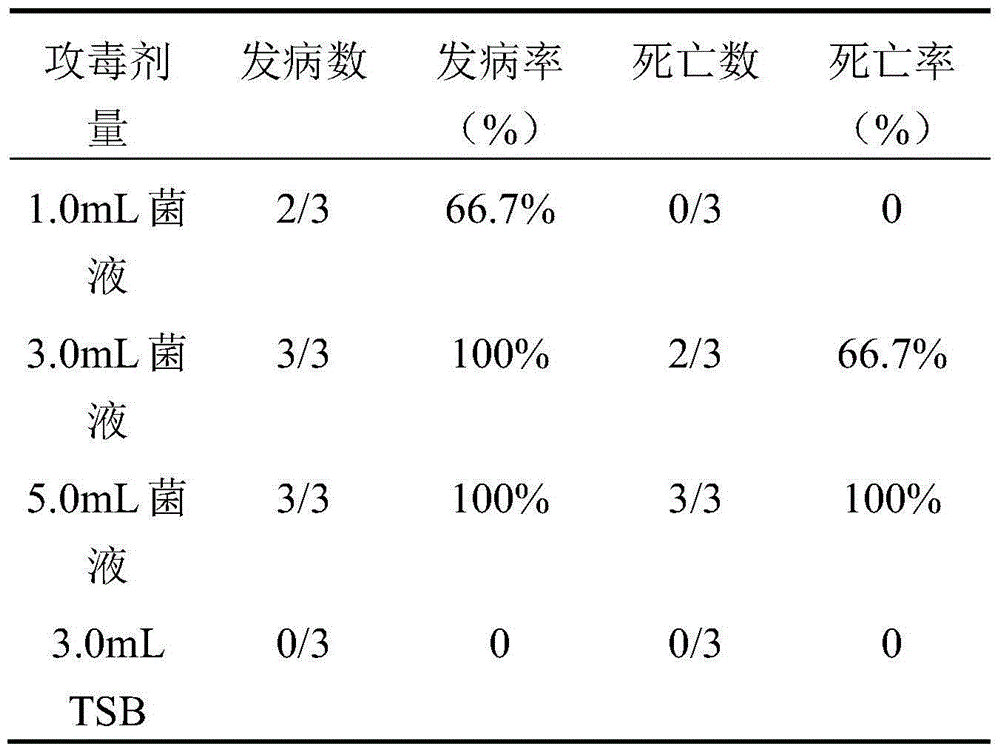
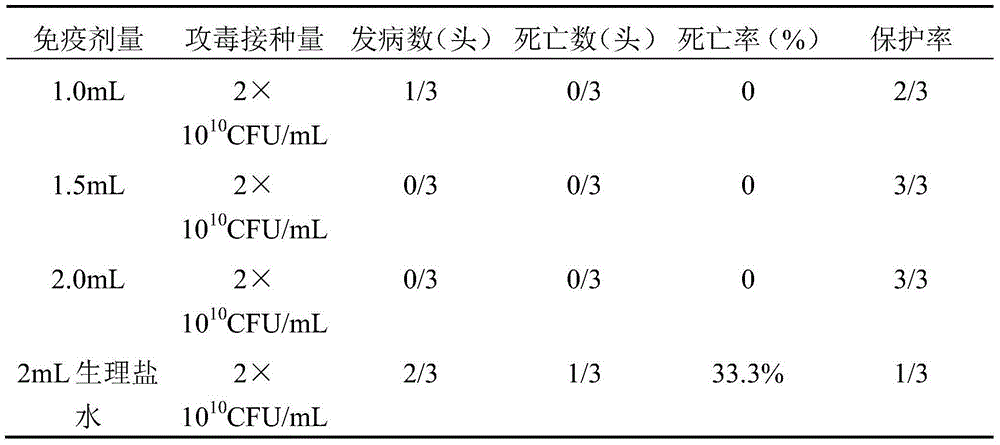
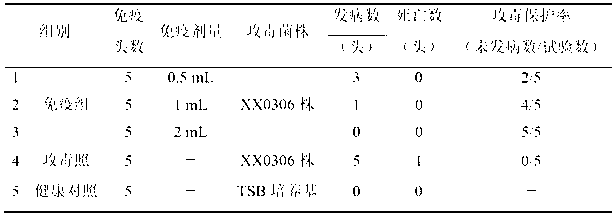
The invention relates to the field of vaccines of Haemophilus parasuis (HPs) in veterinary biological products and discloses a serotype 5 HPs vaccine strain. The class name of the vaccine strain is HPs. The strain number is XX0306. The vaccine strain has been collected in the China Center for Type Culture Collection, with collection number being CCTCC NO:M2013095 and collection date being March 21, 2013. The serotype 5 HPs strain XX0306 has stronger pathogenicity toward swine and has good immunogenicity. The inactivated vaccines prepared from the vaccine strain are safe and reliable and have quite good protective effects on the swine challenging homological strains. The morbidity and mortality of the immunized swinery are obviously reduced. Either the single vaccine or the combined vaccine has the immune effects equivalent or superior to the immune effects of the existing commercialized vaccines and can effectively prevent prevalence of HPs.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Amphiphilic graphene quantum dot material, preparation method thereof, fluorescent coding anti-counterfeiting ink based on the material and preparation method of the ink
ActiveCN110615428AInhibit side effectsSimple separation and purification processMaterial nanotechnologyGrapheneQuantum yieldCarbonization
The invention discloses an amphiphilic graphene quantum dot material, a preparation method thereof, fluorescent coding anti-counterfeiting ink based on the amphiphilic graphene quantum dot material and a preparation method of the fluorescent coding anti-counterfeiting ink. Hydrophobic citrate is used as a carbon source, hydrophilic amino alcohol is used as a nitrogen doping agent, and the amphiphilic graphene quantum dot material with hydrophobic ester groups and hydrophilic alcoholic hydroxyl edge groups is prepared through carbonization treatment. The prepared amphiphilic graphene quantum dot material has a high fluorescence quantum yield and good light, heat and chemical stability, and can be dispersed in various polar and non-polar solvents. Under certain conditions, the amphiphilic graphene quantum dot material can form an aggregate on the surfaces of a solution and a solid, and fluorescence emission of the amphiphilic graphene quantum dot material is changed from a single peak tomultiple peaks. The aggregation degree and aggregation state of the amphiphilic graphene quantum dot material are regulated and controlled, so that the fluorescence emission intensities of the amphiphilic graphene quantum dot material at different wavelengths are correspondingly changed, and the fluorescence coding marking based on the fluorescence intensity ratio is realized.
Owner:XI AN JIAOTONG UNIV
Serotype 4 haemophilus parasuis (HPs) vaccine strain
ActiveCN103194413AStable biological propertiesEpidemic preventionAntibacterial agentsBacteriaMortality rateSerotype
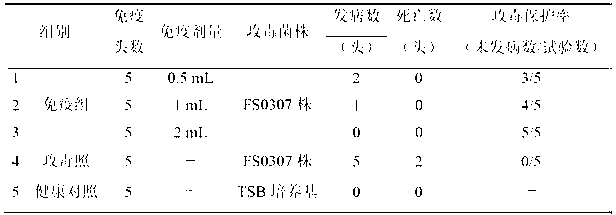
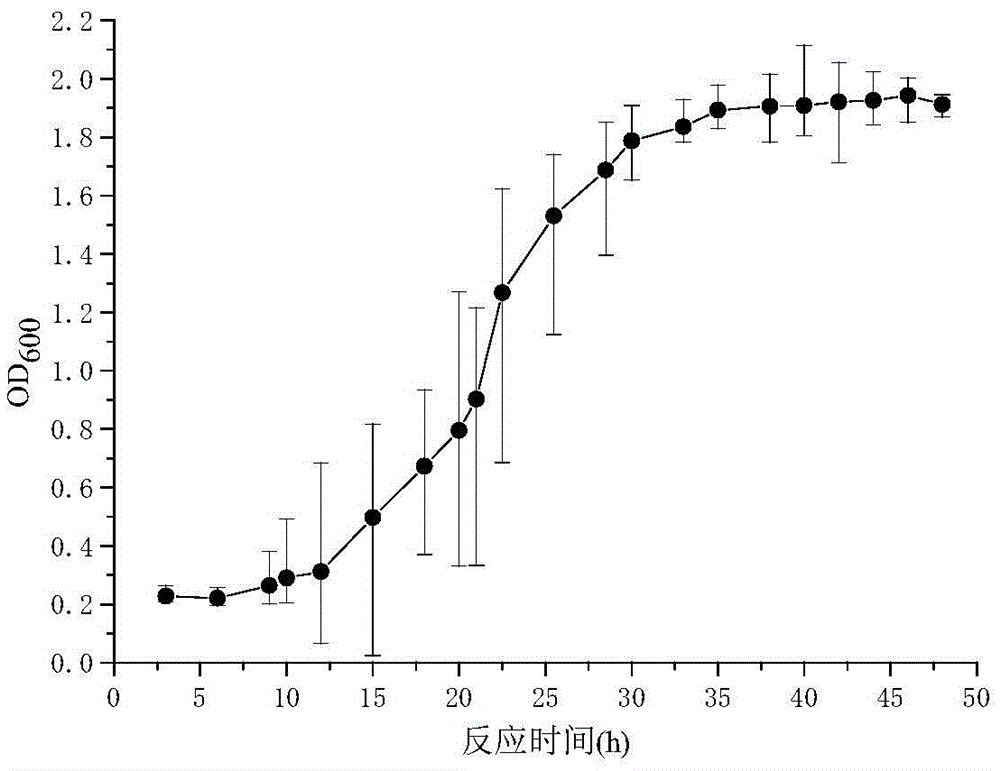
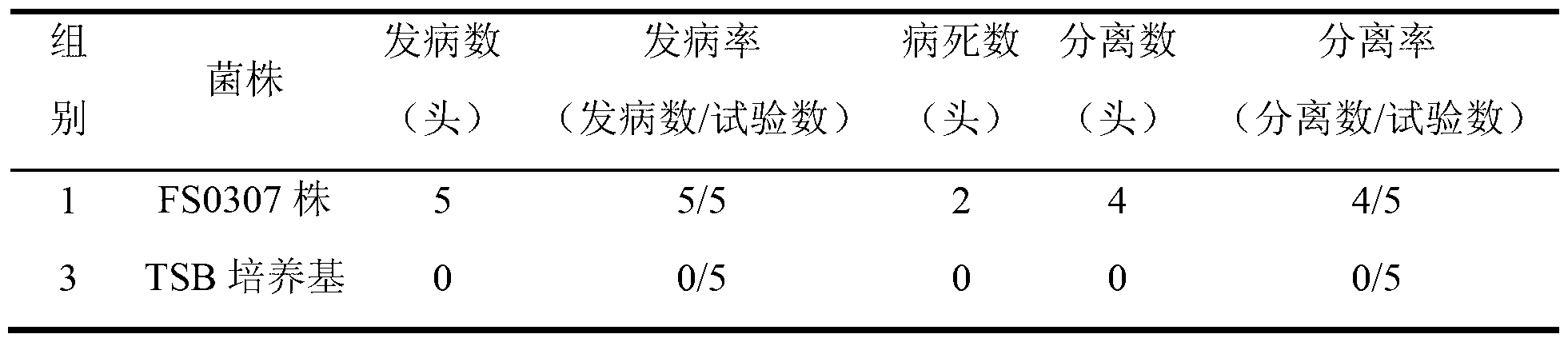
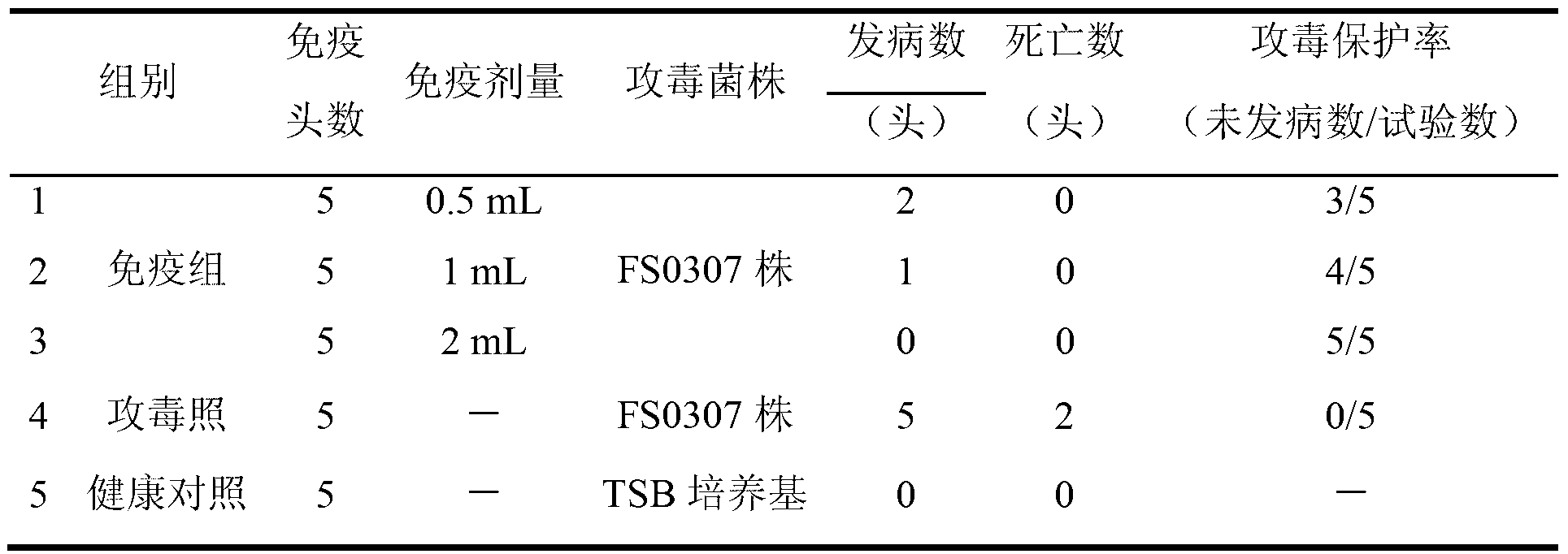
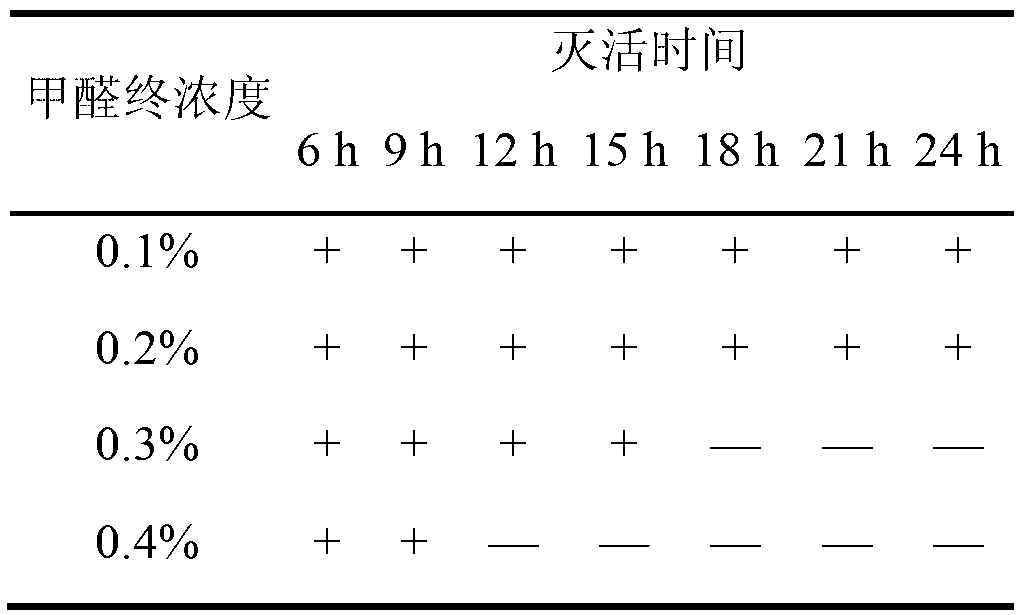
The invention relates to the field of vaccines of Haemophilus parasuis (HPs) in veterinary biological products and discloses a serotype 4 HPs vaccine strain. The class name of the vaccine strain is HPs. The strain number is FS0307. The vaccine strain has been collected in the China Center for Type Culture Collection, with collection number being CCTCC NO:M2013094 and collection date being March 21, 2013. The serotype 4 HPs strain FS0307 has stronger pathogenicity toward swine and has good immunogenicity. The inactivated vaccines prepared from the vaccine strain are safe and reliable and have quite good protective effects on the swine challenging homological strains. The morbidity and mortality of the immunized swinery are obviously reduced. Either the single vaccine or the combined vaccine has the immune effects equivalent or superior to the immune effects of the existing commercialized vaccines and can effectively prevent prevalence of HPs.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
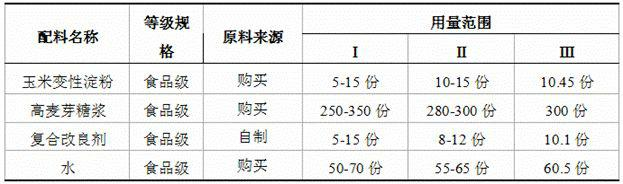
Special powder for producing high-quality mochi and making method of powder
The invention belongs to the technical field of food processing methods, in particular to a special powder for mochi and a making method of the powder. The powder special for mochi is processed by mixing glutinous rice flour, modified corn starch, high maltose syrup, a compound improver and water by certain weight percent and carrying out the following steps: (1) mixing; (2) steaming; (3) mixing the high maltose syrup with the material; (4) taking out the steamed powder; (5) cooling the steamed powder; and (6) sterilizing and packaging the steamed powder. The invention has the following characteristics: by using the method in which the raw materials and the process are simultaneously improved, the problems that the traditional mochi powder is easy to harden and go bad, has poor taste and short shelf life and is hard to preserve are solved; and the mochi products made of the mochi powder have good elasticity, strong pliability, tender taste and more than 6 months of shelf life.
Owner:东莞市圣心食品有限公司
Bacillus marinus capable of inducing disease resistance and stress tolerance of plant
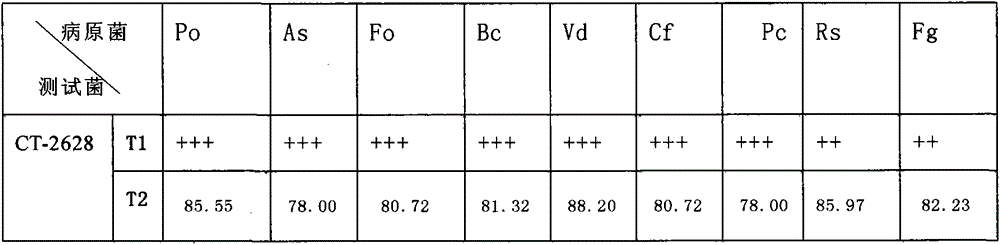
InactiveCN105018367ANo pollution in the processStable biological propertiesBiocideBacteriaEcological environmentBeef extract
The invention discloses bacillus marinus capable of inducing disease resistance and stress tolerance of a plant. The bacillus marinus CT2628 (Bacillus sp.) is obtained from an ocean ecological environment through artificial induced mutation breeding and culture condition optimization; the proper artificial culture and fermentation condition is as follows: a proper fermentation culture solution of the CT2628 with the collection number of CGMCC No.8921 is prepared according to the following formula: 1-3g of a beef extract, 2-3g of peptone, 1-3g of yeast powder, 1-3g of glucose, 20-25g of NaCl, 0.3-0.5g of KCl, 1.9-2.5g of MgCl2.7H2O, 0.01-0.02g of FePO4 and 1000ml of water and has the pH value of 7.0-8.0. The invention discloses bacillus marinus capable of inducing disease resistance, salt resistance and cold resistance of the plant and application of the bacillus marinus to agriculture.
Owner:ZHEJIANG FORESTRY UNIVERSITY +1
Method for constructing oral squamous cell carcinoma animal model
InactiveCN101469320AStable biological propertiesLittle difference in biological traitsEducational modelsTumor/cancer cellsSquamous CarcinomasChemical carcinogens
The invention relates to a method for constructing animal models, in particular to a method for constructing oral mucosa carcinoma animal models. The method adopts the technical proposal that the method for constructing the oral mucosa carcinoma animal models comprises the following steps: firstly, culturing a tumor tissue and tumor cells; and identifying biological characteristics of in vitro growth of the cells. The method for constructing the oral mucosa carcinoma animal models has the advantages that: firstly, Rca-B cell lines and Rca-T cell lines constructed by the method all come from single cloned tumor cells induced by SD pure mouse chemical carcinogens, and have the same genetic background and stable biological characteristics; and secondly, the characteristics of the cell lines such as hypodermal tumor formation in a nude mouse and high experimental liver transfer rate provide two practical animal models for study on prevention and treatment of oral mucosa epicytoma.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Application of serotype 5 haemophilus parasuis (HPs) vaccine strain
ActiveCN103191421AStable biological propertiesEpidemic preventionAntibacterial agentsAntibody medical ingredientsMortality rateHaemophilus Vaccines
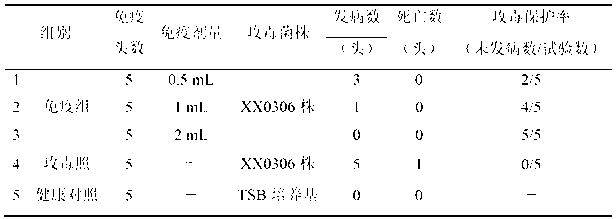
The invention relates to the field of vaccines of Haemophilus parasuis (HPs) in veterinary biological products and discloses application of a serotype 5 HPs vaccine strain. The class name of the vaccine strain is HPs. The strain number is XX0306. The vaccine strain has been collected in the China Center for Type Culture Collection, with collection number being CCTCC No:M2013095 and collection date being March 21, 2013. The serotype 5 HPs strain XX0306 has stronger pathogenicity toward swine and has good immunogenicity. The inactivated vaccines prepared from the vaccine strain are safe and reliable and have quite good protective effects on the swine challenging homological strains. The morbidity and mortality of the immunized swinery are obviously reduced. Either the single vaccine or the combined vaccine has the immune effects equivalent or superior to the immune effects of the existing commercialized vaccines and can effectively prevent prevalence of HPs.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
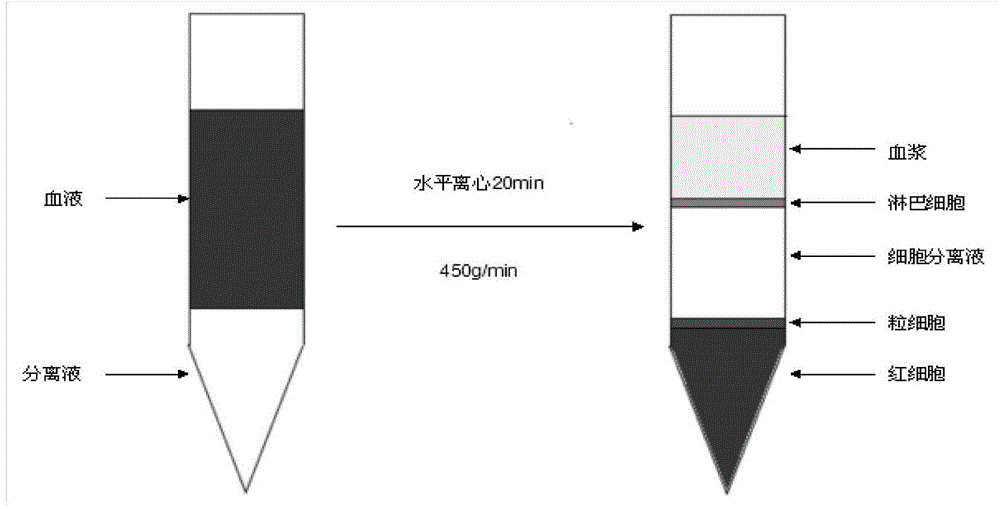
Method for enabling mononuclear cell of blood to perform reversion differentiation for producing human blood derived auto-retina stem cells as well as kit and application
ActiveCN107326009ARich sourcesConvenient sourceCulture processNervous system cellsCulture fluidDNA fragmentation
The invention belongs to the technical field of biology, in particular relates to the technical field of biomedicine and in particular relates to a cell culture method for enabling human somatic cells to perform reversion differentiation for producing human blood derived auto-retina stem cells as well as a kit and application. The preparation method comprises the following steps: taking somatic cells as raw cells, sequentially culturing the raw cells through a cell culture solution A1, a cell culture solution A2 and a cell culture solution A3, thereby obtaining the blood derived auto-retina stem cells. The human blood cells serve as the raw cells, and the raw cells are subjected to reversion differentiation so as to produce the human blood derived auto-retina stem cells. A cell culture solution formula composed of small molecular substances is used, and the human blood cells are rapidly subjected to reversion differentiation so as to produce the human blood derived auto-retina stem cells under the conditions that the human somatic chromosome DNA sequence is not changed and any foreign gene or DNA fragment is not inserted. The production speed, yield and purity in the invention are obviously superior to those in the prior art.
Owner:深圳百年干细胞技术研究院有限公司
Double-antibody sandwich ELISA antigen detection kit for Porcine deltacoronavirus (PDCoV) N protein
InactiveCN110361542AStable biological propertiesHigh linearity coefficientBiological material analysisBiological testingAntigenCapture antibody
The invention belongs to the field of biotechnology detection and particularly relates to a double-antibody sandwich ELISA antigen detection kit for Porcine deltacoronavirus (PDCoV) N protein. The kitcomprises a monoclonal antibody PDCoV-N-1A2 ELISA plate, positive and negative controls, an HRP labeled detection antibody PDCoV-N-5C5, a sample diluent, a color developing solution and a washing solution, wherein the antibodies PDCoV-N-1A2 and PDCoV-N-5C5 are secreted by hybridoma cell strains PDCoV-N-1A2 and PDCoV-N-1A2, respectively. The kit of the invention can specifically detect PDCoV N protein by the double-antibody sandwich ELSIA method established by using 1A2 as a capture antibody and using hybridization 5C5 (HRP coupled) as a detection antibody, and provides a new detection means for the clinical diagnosis and epidemiological investigation of PDCoV infection.
Owner:YANGZHOU UNIV
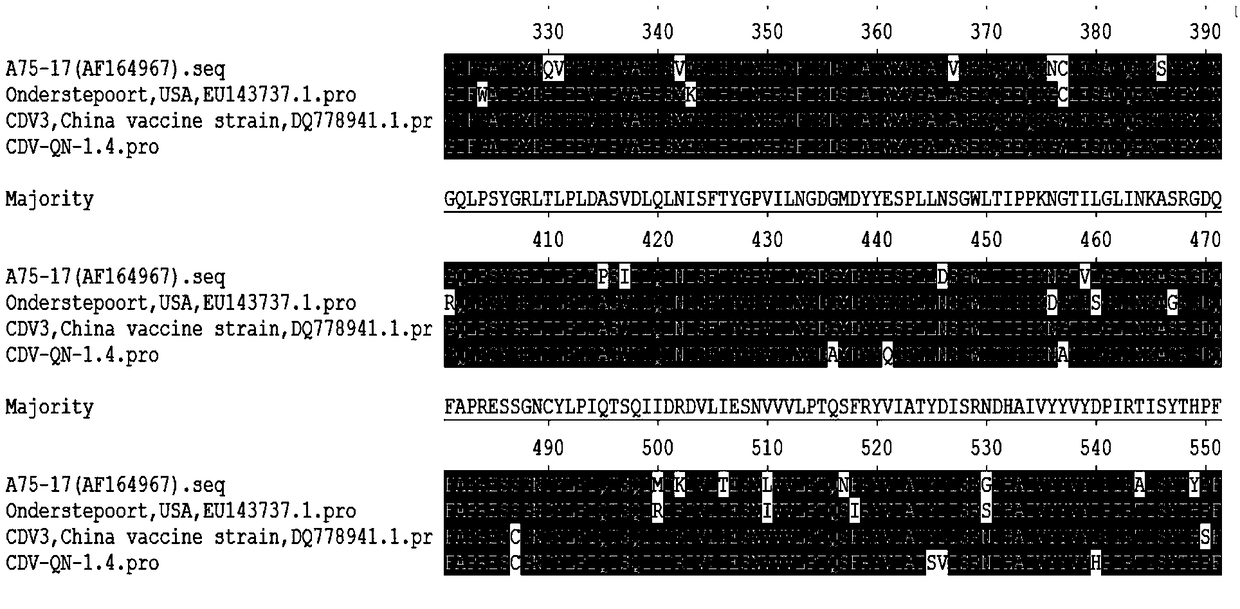
Canine distemper parvovirus bigeminy subunit vaccine
ActiveCN108704128AStable biological propertiesImproving immunogenicitySsRNA viruses negative-senseViral antigen ingredientsDiseaseCanine parvovirus
The invention provides a canine distemper parvovirus bigeminy subunit vaccine which comprises a vaccine adjuvant and an antigen. The antigen is H protein and vp2 proteain with the amino acid sequencebeing SEQ ID NO:2. The canine distemper parvovirus bigeminy subunit vaccine has the stable biological characteristics, good immunogenicity, safety and reliability and has the very good protection effect on canine distemper highly virulent GN strain challenge and canine parvovirus QN strain challenge, prevalence and propagation of canine distemper and parvoviruses can be effectively prevented, economic losses caused by the two diseases can be lowered, and the vaccine has a wide application prospect.
Owner:QINGDAO AGRI UNIV
Porcine mycoplasma pneumoniae and application of porcine mycoplasma pneumoniae
ActiveCN107488612AHigh proliferative titerStable biological propertiesAntibacterial agentsBacterial antigen ingredientsImmunogenicityMicrobiological culture
The invention discloses porcine mycoplasma pneumoniae and an application of the porcine mycoplasma pneumoniae. The porcine mycoplasma pneumoniae is mycoplasma bovis HNMhy1, has a collection number of CGMCC (China General Microbiological Culture Collection Center) NO: 13858 and is collected in China General Microbiological Culture Collection Center in Beijing, China on May 26, 2017. The porcine mycoplasma pneumoniae strain HNMhy1 is separated from a nursery pig having typical dyspnea and lung consolidation, has stable biological characteristics, and higher pathogenicity on the nursery pig, can cause a typical deep breathing symptom of the nursery pig and has good immunogenicity. A vaccine prepared from the porcine mycoplasma pneumoniae strain HNMhy1 is safe and reliable and has a better protection effect on the porcine primary atypical pneumonia.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
Mycoplasma synoviae
PendingCN105733987AStable biological propertiesToxicAntibacterial agentsBacterial antigen ingredientsPost immunizationToxicity
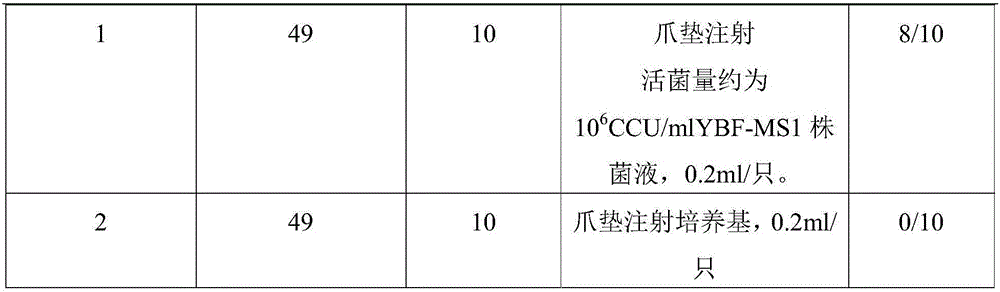
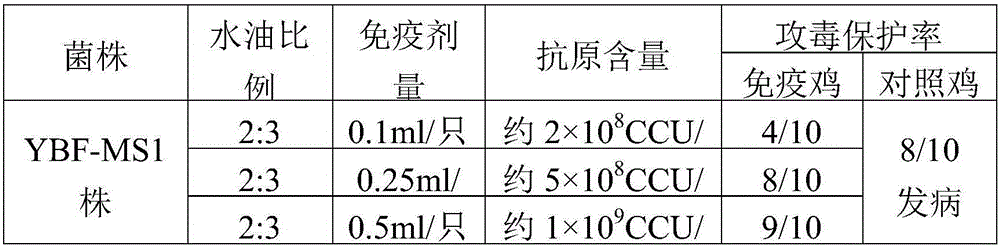
The invention provides mycoplasma synoviae.A preservation number of the mycoplasma synoviae is CCTCC M 2016080.The mycoplasma synoviae has the advantages that a mycoplasma synoviae YBF-MS1 strain is stable in biological characteristics, high in toxicity and excellent in immunogenicity; oil adjuvant inactivated vaccine prepared from the mycoplasma synoviae is safe and reliable, is long in persistent period and has cross protection properties, high-level antibodies can be generated by the oil adjuvant inactivated vaccine, the post-immunization mycoplasma synoviae morbidity can be obviously reduced, and accordingly epidemic mycoplasma synoviae can be effectively prevented; mycoplasma synoviae vaccine is not available on market in China, the mycoplasma synoviae inactivated vaccine prepared from the mycoplasma synoviae YBF-MS1 strain is a key for preventing and controlling diseases, and market gaps can be filled.
Owner:YEBIO BIOENG OF QINGDAO
A Deodorizing and Deodorizing Strain qdn01 and Its Application in Biological Deodorization
ActiveCN104152375BStable biological propertiesFast reproductive capacityBacteriaMicroorganism based processesSequence analysisFeces
Owner:QIQIHAR UNIVERSITY +1
Hypsizygus marmoreus strain and culture method thereof
InactiveCN108260469AImprove uniformityExcellent morphologyFungiMicroorganism based processesBiotechnologyMicrobiology
The invention relates to the technical field of edible fungi, and in particular relates to a hypsizygus marmoreus strain and a culture method thereof. The hypsizygus marmoreus strain WZ-29 has a preservation number of GDMCC NO: 60276. Hypsizygus marmoreus grown from the hypsizygus marmoreus strain WZ-29 disclosed in the invention has extremely high homogeneity and excellent morphological characteristics, so that packaging and sale of the product and establishment of an edible fungus brand are facilitated. In addition, the strain has a short culture and cultivation period and stable biologicalcharacteristics, and greatly improves the production efficiency and stability of a plant; and yield per unit is high, every bottle is basically consistent when mass production is performed, and the strain has broad application prospects.
Owner:韶关市星河生物科技有限公司
Application of serum 4 type haemophilus parasuis vaccine strain
ActiveCN103182077AStable biological propertiesEpidemic preventionAntibacterial agentsAntibody medical ingredientsSerum igeHaemophilus
The invention relates to the field of haemophilus parasuis vaccine strains in veterinarian biological products and discloses an application of a serum 4 type haemophilus parasuis strain. The serum 4 type haemophilus parasuis strain is classified and named as haemophilus parasuis with the strain number of FS0307 and is preserved in China Center for Type Culture Collection with the preservation number of CCTCC NO:M2013094 and the preservation date of March 21, 2013. The serum 4 type haemophilus parasuis strain FS0307 has strong pathogenicity to pigs and good immunogenicity; an inactivated vaccine prepared by applying the serum 4 type haemophilus parasuis strain is safe and reliable and has a good protection effect to pigs attacked by toxicity of homogenous strains; and morbidity and mortality of the pigs after immunization are obviously reduced. Whether a single vaccine or a combined vaccine, immunity of the serum 4 type haemophilus parasuis strain achieves or is better than that of the existing commercialization vaccine on the market, and prevalence of haemophilus parasuis can be effectively prevented.
Owner:JIANGSU ACAD OF AGRI SCI
Caprine parainfluenza virus type 3 JS14-2 strain and application thereof
ActiveCN108949700AImproving immunogenicityEpidemic preventionSsRNA viruses negative-senseMicrobiological testing/measurementAdjuvantImmunogenicity
The invention belongs to the field of veterinary biological products and discloses a caprine parainfluenza virus type 3 JS14-2 strain and an application thereof in preparation of inactivated vaccines.The inactivated vaccines are prepared and obtained by the steps of: separating and purifying to obtain the caprine parainfluenza virus type 3 JS14-2 strain, carrying out centrifugation and formaldehyde inactivation on virus liquid and adding an adjuvant for emulsification. The invention provides a virus strain which can be used for preparing caprine parainfluenza virus type 3 inactivated vaccines. The strain belongs to domestic prevalent strains, and is strong in virulence and good in antigenicity; the strain has better growth characteristic on MDBK (Madin-Darby Bovine Kidney) cells, and canobtain stable high titer (more than or equal to 107 TCID50 / mL, and the hemagglutination valence is more than or equal to 28); the vaccine preparation method is perfect, and the immunogenicity and theimmune protective effect are good; the used immunizing dosage is low, the safety is high, the immune duration is long, the production and use cost is greatly saved, and the benefit for effectively controlling the caprine parainfluenza virus type 3 in clinical practices is achieved.
Owner:JIANGSU ACAD OF AGRI SCI
Haemophilus paragallinarum and application thereof
InactiveCN107354114AHigh proliferative titerStable biological propertiesAntibacterial agentsBacteriaFacial swellingInfectious Rhinitis
The invention discloses a haemophilus paragallinarum and application thereof. The haemophilus paragallinarum is haemophilus paragallinarum HNHpg1, the preservation number is CGMCC NO:13859, the preservation date is May 26th, 2017, the preservation place is China General Microbiological Culture Collection Center and the preservation address is Beijing, China. The haemophilus paragallinarum HNHpg1 is separated from secreta of sinus infraorbitalis of a laying hen suffering from typical respiratory symptoms and head and facial swelling, has stable biological characteristics, has stronger pathogenicity on chicks, can result in morbidity and death of the chicks and has good immunogenicity. A vaccine prepared by utilizing the haemophilus paragallinarum HNHpg1 disclosed by the invention is safe and reliable and has a better protective effect on chicken infectious rhinitis.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
Spontaneous lymphocyte highly metastatic human ovarian sarcomatoid carcinoma cell line
InactiveCN101845418AClear genetic backgroundStable biological propertiesMicrobiological testing/measurementMicroorganism based processesBALB/cIntraperitoneal route
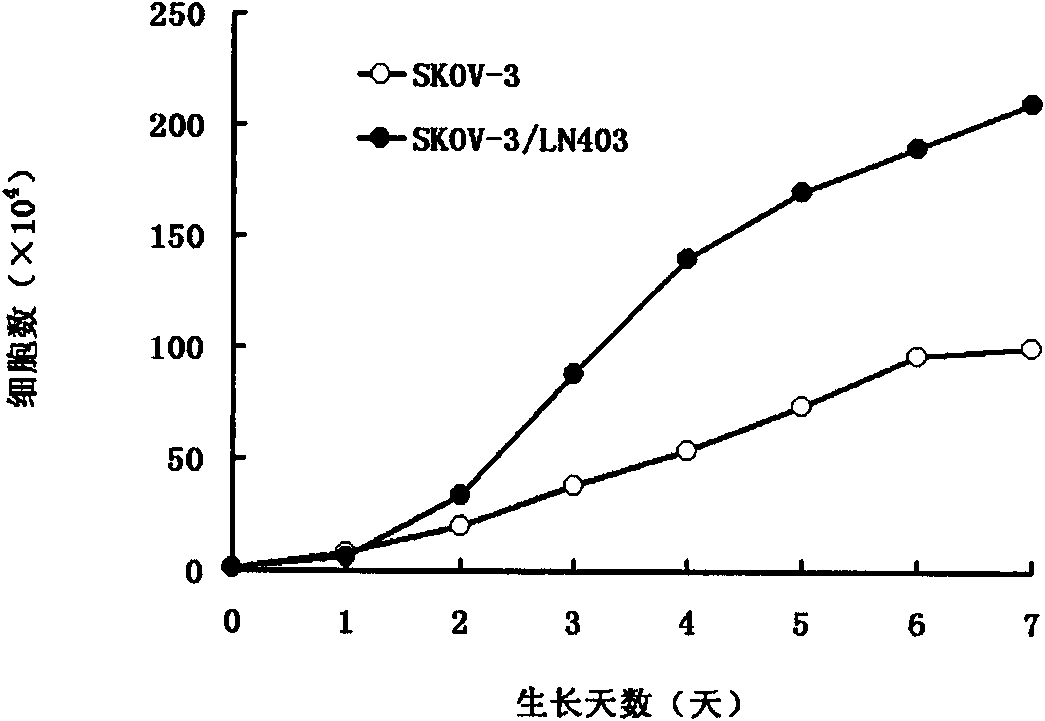
The invention belongs to the fields of biotechnology and microorganism animal cell lines, and in particular relates to a spontaneous lymphocyte highly metastatic human ovarian sarcomatoid carcinoma cell line, and an establishment method and application thereof. In the invention, the human ovarian sarcomatoid carcinoma cell line SKOV-3 (ATCC code: HTB-77) is taken as a parent cell; and the spontaneous lymphocyte highly metastatic human ovarian sarcomatoid carcinoma cell line SKOV-3 / LN403 is obtained by separating and through strategies, such as BALB / c nude mouse intraperitoneal injection, retroperitoneal lymph node continuous screening, amplification in vitro DNA, passage and the like, and the preservation number is CGMCC No.2940. The cell line and an animal model established thereby can simulate the actual clinical situation of a patient with ovarian cancer so as to provide an ideal experiment tool for researching a biological behavior and a regulation and control mechanism for ovarian cancer lymph node metastasis, and screening clinical anti-metastasis medicaments.
Owner:THE OBSTETRICS & GYNECOLOGY HOSPITAL OF FUDAN UNIV
Method of preparing porcine circovirus 2 type sensitive cell line, cell line prepared therethrough, and application of the cell line
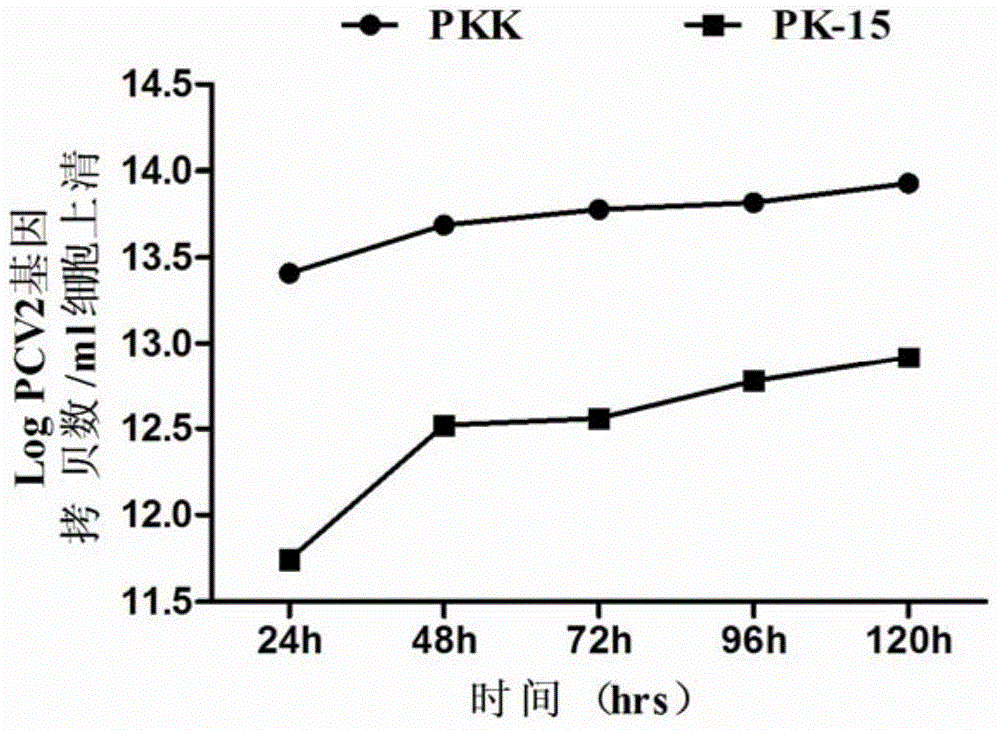
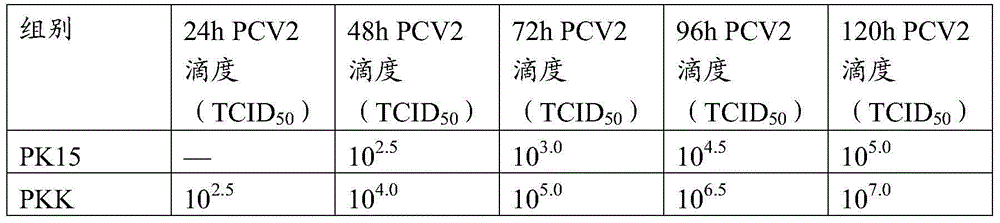
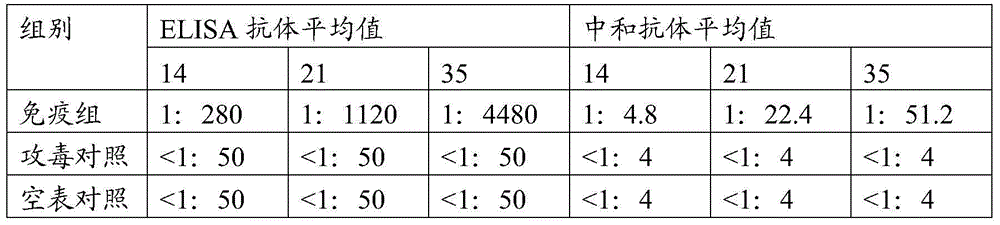
InactiveCN105316295AIncreased sensitivityStable biological propertiesViral antigen ingredientsInactivation/attenuationBiological propertyPorcine circovirus
The invention belongs to veterinary biological products and more particularly relates to a method of preparing a porcine circovirus 2 type sensitive cell line, the cell line prepared therethrough, and an application of the cell line, and also provides a method of preparation of a high-titer porcine circovirus 2 type inactivated vaccine with the porcine circovirus 2 type sensitive cell line. The porcine circovirus 2 type sensitive cell line is uniform in shape, is stable in cell growth speed and biological characters, can generate high-titer 2-type porcine circovirus and can be used in researching and application of inactivated vaccines and relative diagnosis reagents of the 2-type porcine circovirus.
Owner:PU LIKE BIO ENG
Marine bacillus with multiple functions on plants and its application
InactiveCN104928200BIncrease moisture contentReduced symptoms of salt damageBiocidePlant growth regulatorsBiotechnologyYeast
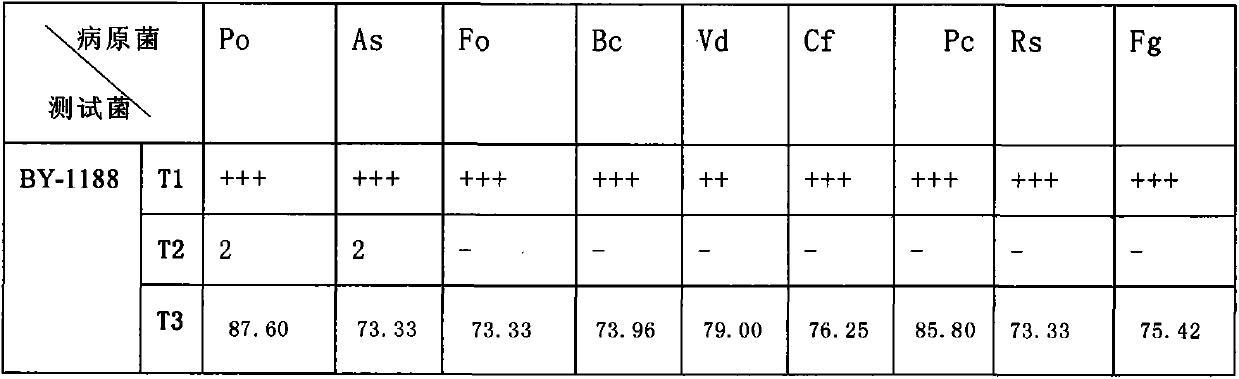
The invention discloses a marine bacillus having multiple functions on plants. The marine bacillus BY-1188 strain (Bacillus marinus) is obtained from a marine habitat, and through artificial mutation breeding and optimization of culture conditions, its preservation number is: CGMCC No. The BY-1188 strain of 6187, the strain has been tested by medium optimization, and its suitable fermentation medium formula is: peptone 2-5g, yeast powder 1-3g, NaCl, 25-30g, KCl0.3-0.5g, MgCl2 7H2O1.9‑2.5g, FePO4O.01‑0.02g, water 1000ml, pH7.0‑7.5, the above is culture solution, add agar 16‑19g to make solid medium. The invention has the advantages of stable biological characteristics and pesticide activity, and no pollution to the environment.
Owner:QINGDAO UNIV OF SCI & TECH
Induced pluripotent stem cell line for reducing cell immunogenicity and construction method thereof
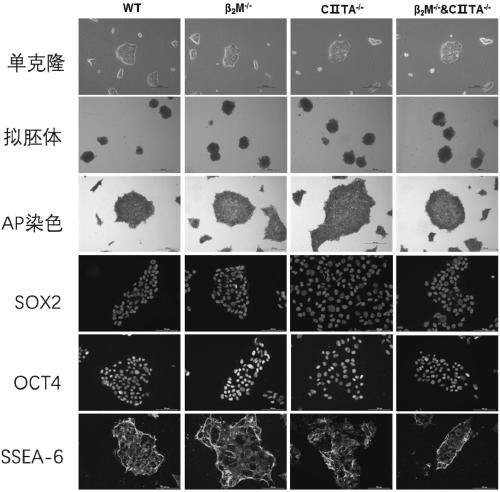
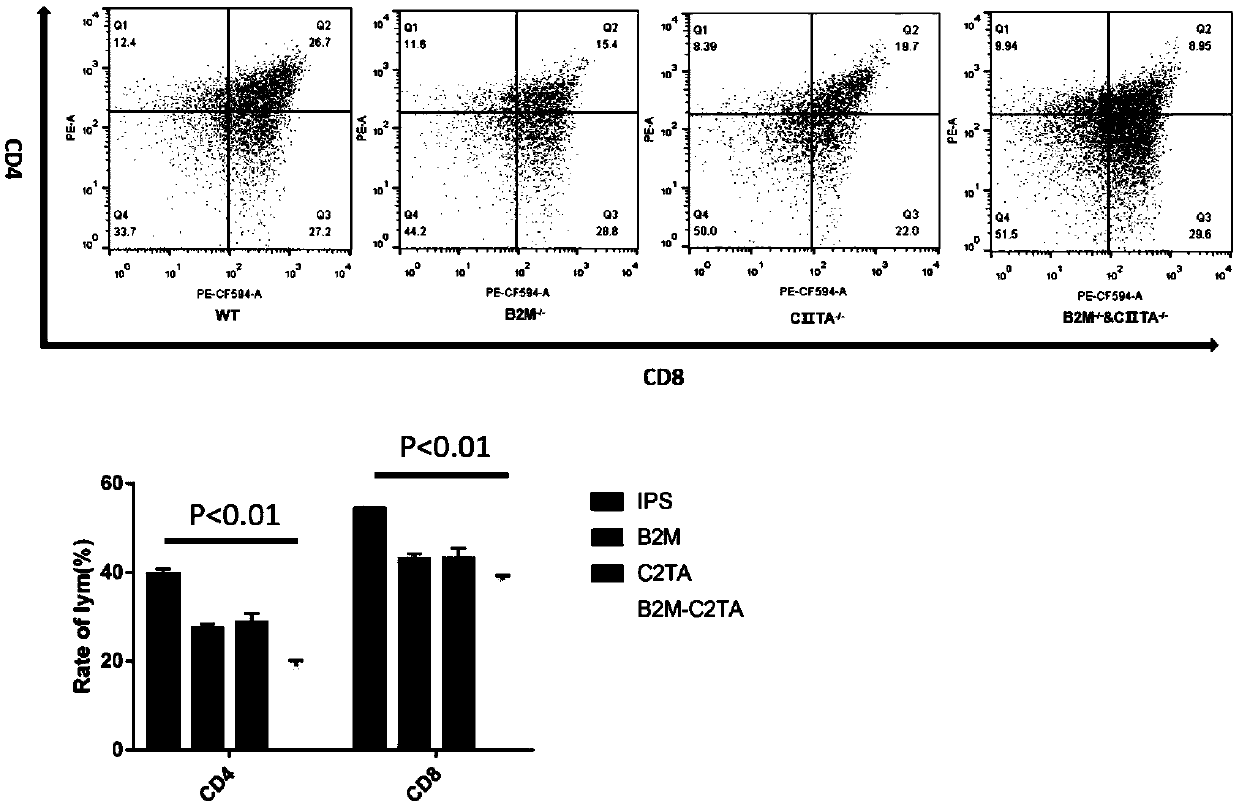
PendingCN111424016AClear genetic backgroundStable biological propertiesStable introduction of DNANucleic acid vectorReprogrammingImmunogenicity
The invention belongs to the field of stem cell regeneration medicine and transformation medicine, and relates to an induced pluripotent stem cell line for reducing cell immunogenicity and a method thereof. According to the invention, a human induced pluripotent stem cell line with knocking out of a beta-2 microglobulin ([beta]2m) gene (hiPSCs-[beta]<2>M<- / ->), a human induced pluripotent stem cell line with knocking out of a MHC class II transactivator (C[II]TA) gene (hiPSCs-C[II]TA<- / ->), and a human induced pluripotent stem cell line with simultaneous knocking out of the [beta]2m gene and the C[II]TA gene (hiPSCs-[beta]<2>M<- / ->&C[II]TA<- / ->) are obtained through a human cell reprogramming method. The cell line provided by the invention is a human-derived cell and has similar biologicalcharacteristics with human embryonic stem cells, and immunogenicity of human induced pluripotent stem cells can be obviously reduced after relevant immune genes are knocked out. The invention breaksaway from political and ethical disputes around use problems of human embryonic stem cells, and provides a new thought and method for treating cardiovascular diseases by stem cell transplantation.
Owner:FUDAN UNIV
Mycoplasma columbinum and application thereof
ActiveCN107446858AHigh proliferative titerStrong pathogenicityAntibacterial agentsBacterial antigen ingredientsMicroorganismBiological property
The invention discloses mycoplasma columbinum and application thereof. The mycoplasma columbinum is named mycoplasma columbinum HNMcol, the preservation number is CGMCC (China General Microbiological Culture Collection Center) NO: 14300, the preservation date is June 22, 2016, the preservation unit is CGMCC, and the preservation address is Beijing, China. The mycoplasma columbinum HNMcol is separated from adult race pigeons suffering from typical dyspnea and nasosinusitis, has stable biological properties, has stronger pathogenicity on pigeons, is capable of causing symptoms of typical nasosinusitis, dyspnea and death of the pigeons, and has good immunogenicity. A vaccine prepared by utilizing the mycoplasma columbinum HNMcol is safe and reliable and has better protective effect on a chronic respiratory disease of the pigeons.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
Special powder for producing high-quality mochi and making method of powder
InactiveCN102008046BRaw materials are simplePoor water retentionFood preparationPliabilityFood processing
The invention belongs to the technical field of food processing methods, in particular to a special powder for mochi and a making method of the powder. The powder special for mochi is processed by mixing glutinous rice flour, modified corn starch, high maltose syrup, a compound improver and water by certain weight percent and carrying out the following steps: (1) mixing; (2) steaming; (3) mixing the high maltose syrup with the material; (4) taking out the steamed powder; (5) cooling the steamed powder; and (6) sterilizing and packaging the steamed powder. The invention has the following characteristics: by using the method in which the raw materials and the process are simultaneously improved, the problems that the traditional mochi powder is easy to harden and go bad, has poor taste and short shelf life and is hard to preserve are solved; and the mochi products made of the mochi powder have good elasticity, strong pliability, tender taste and more than 6 months of shelf life.
Owner:东莞市圣心食品有限公司
Serum 18 type riemerella anatipestifer and application
ActiveCN112646750AImprove protectionReduce morbidityAntibacterial agentsBacterial antigen ingredientsDiseaseOil adjuvant
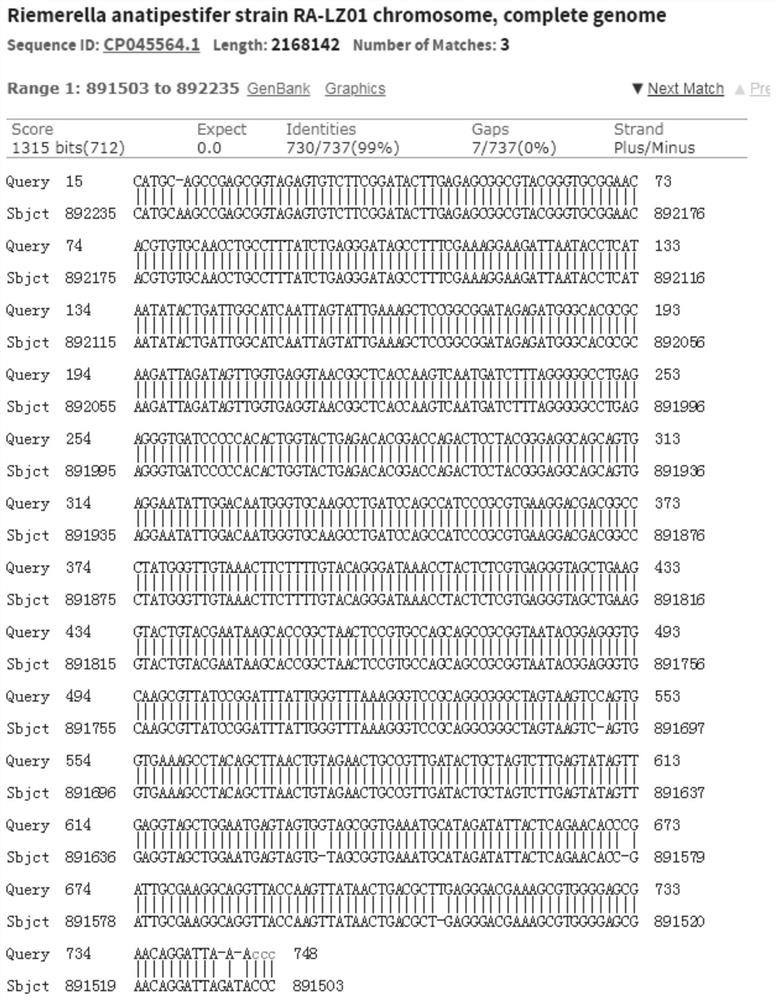
The invention provides serum 18 type riemerella anatipestifer and application. The strain is named as riemerella anatipestifer YJ821-18, has a preservation number of CCTCC NO: M2020528, and is preserved in CCTCC on September 21, 2020. The strain has stable biological characteristics, relatively strong pathogenicity to ducklings and good immunogenicity. An oil adjuvant inactivated vaccine prepared from the strain is safe and reliable, can generate relatively high-level antibodies, is long in lasting period and has a very good protection effect on ducks attacked by homologous strains, the morbidity and mortality of immunized ducks are obviously reduced, and the prevalence of riemerella anatipestifer diseases can be effectively prevented.
Owner:重庆永健生物技术有限责任公司
Method for producing L-lactic acid by Bacillus coagulans CGMCC No.2602
ActiveCN101544993BStrong environmental resistanceEasy to saveMicroorganism based processesFermentationMicroorganismGlucose polymers
A method for producing L-lactic acid by Bacillus coagulans CGMCC No.2602 belongs to the technical field of microorganisms. In the invention, Bacillus coagulans CGMCC No.2602 is adopted, and under the condition of no oxygen supply, starchiness hydrolyzed sugar or dextrose is fermented by a semicontinuous intermittent fermentation way or an inter-sugar-compensating fermentation way to generate L-lactic acid with high optical purity. The invention has the advantages that the gemma property of the Bacillus coagulans is stable, and the L-lactic acid obtained by inoculating and fermenting the starchiness hydrolyzed sugar has high optical purity and rate of conversion of sugar and acid and short fermentation period; in addition, the semicontinuous intermittent fermentation way saves the time forpreparing seeds by a continuous reladling and subculturing method, shortens the fermentation period, enhances the fermentation strength and obtains the L-lactic acid with both relatively high rate ofconversion of sugar and acid and purity.
Owner:江苏省苏微微生物研究有限公司
Method for culturing cord blood type embryonic stem cells as well as identification and application
ActiveCN104651306APromote growthGrowth BenefitsBlood/immune system cellsBiological testingCord blood stem cellLaminin
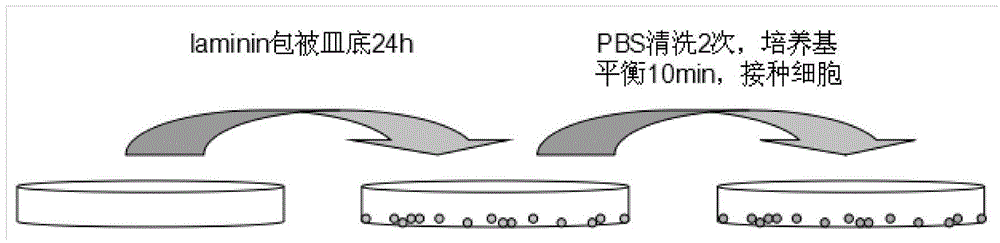
The invention discloses a method for culturing cord blood type embryonic stem cells as well as identification and application, relating to a method for culturing stem cells as well as identification and application. The culture method comprises: 1, collection of cord blood; 2, separation of the cord blood; 3, coating of a cord blood type embryonic stem cell culture dish; and 4, culture amplification of the cord blood type embryonic stem cells. The cord blood type embryonic stem cells are used for assisting recovery of injured lymphocytes. The formula of the culture medium adopts multiple cell factors to stably maintain the biological characteristic of cells and accelerate cell proliferation. The culture dish is coated by adopting laminin, and the laminin is beneficial to wall attachment of cells, so that the wall attachment time of the cells is effectively shortened, the adhesion degree of the cells to the bottom of the dish is improved and the cells are attached to the wall more firmly.
Owner:天晴干细胞股份有限公司
Human breast cancer cell line with high lung metastasis
InactiveCN101748097AClear genetic backgroundStable biological propertiesTissue cultureLymphatic SpreadAnti invasion
The invention belongs to the field of microbial and animal cell lines, and particularly relates to a human breast cancer cell line MDA-MB-231 / 08-001 with spontaneous high lung metastasis potentiality and an establishment method thereof. The cells of the cell line are humanized cells that have 92 expression genes different from those of parent cells, higher in-vitro growth capacity, multiplication capacity, anti-invasion capacity, tumor forming property and lung metastasis than the parent cells; and in 4 weeks after the cells are inoculated, the in-situ tumor volume of a scid mouse is 4,224.67+ / -1,038.95mm<3>, the weight is 2.09+ / -0.51g, the spontaneous lung metastasis is 100 percent, and the number in a metastasis lesion is 41 in each lung. The cell line and an established animal model can simulate the whole process from the growth of the tumor cells at implantation position to the formation of the metastasis lesion at a target organ, which is quite consistent with the clinical practical condition, and can be conveniently used for the research of breast cancer lung metastasis mechanisms and related medicaments.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com