Haemophilus parasuis and application thereof
A technology for Haemophilus suis and Haemophilus suis disease, which is applied to Haemophilus parasuis and its application fields, can solve the problems of weak immunogenicity and low immune cross-protection, and achieve the reduction of morbidity and mortality, Good protection effect and the effect of reducing economic loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: Separation, purification and identification of Haemophilus parasuis serotype 5 vaccine strain LX-5.
[0014] 1. Culture medium for production
[0015] Preparation of TSA solid medium: Accurately weigh 4g of TSA powder, dissolve in 100mL of distilled water, shake well, sterilize by high pressure steam at 121°C for 20min, cool to 50-60°C, add 5% mycoplasma-free fetal bovine serum and 0.005% NAD , mix evenly, pour it out, and store it in a 4°C refrigerator for later use.
[0016] Preparation of TSB liquid medium: Accurately weigh 3 g of TSB powder, dissolve in 100 mL of distilled water, shake well, and then sterilize by high-pressure steam at 121°C for 20 minutes. When cooled to 50-60°C, add 5% mycoplasma-free fetal bovine serum and 0.005% NAD , mix well and store in a 4°C refrigerator for later use.
[0017] 2. Isolation, purification and identification of Haemophilus parasuis
[0018] 2.1 Separation and purification
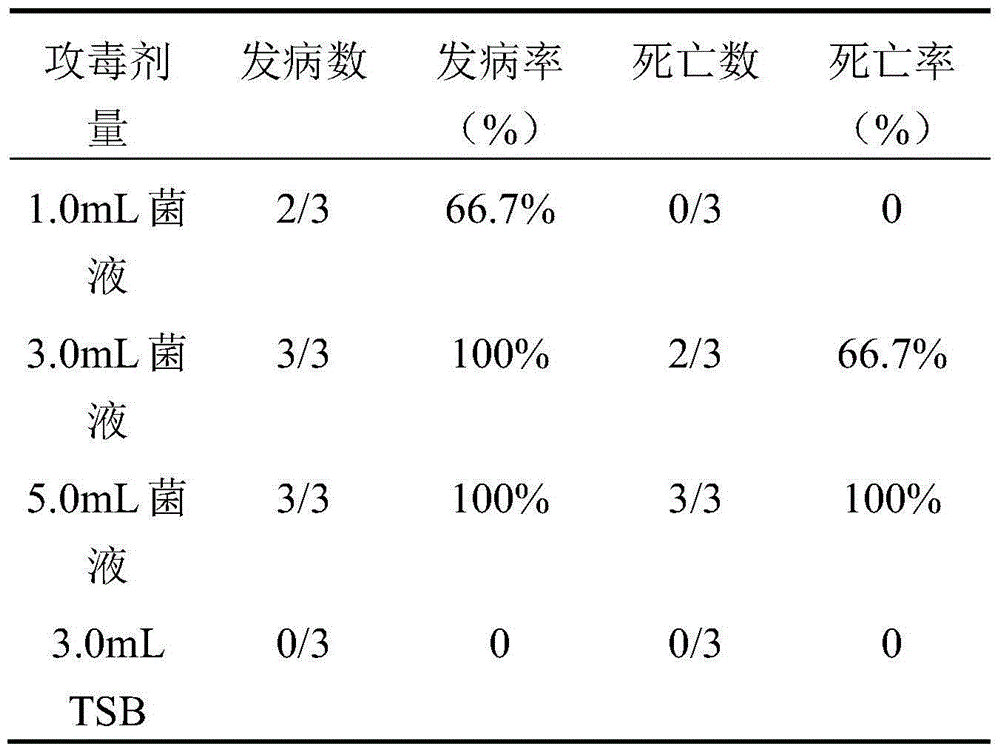
[0019] The strain was isolated from th...
Embodiment 2
[0042] Example 2: Application of HPs LX-5 strain in inactivated vaccine of Haemophilus parasuis.
[0043] 1 strain
[0044] The strain used for seedling production is Haemophilus parasuis serotype 5 LX-5 strain, and its preservation number is CGMCC No.10230. The LX-5 strain conforms to the standard strain morphology of Haemophilus parasuis, has stable ecological and cultural characteristics, strong toxicity to piglets, and good immunogenicity.
[0045] 2 Preparation of strains for production
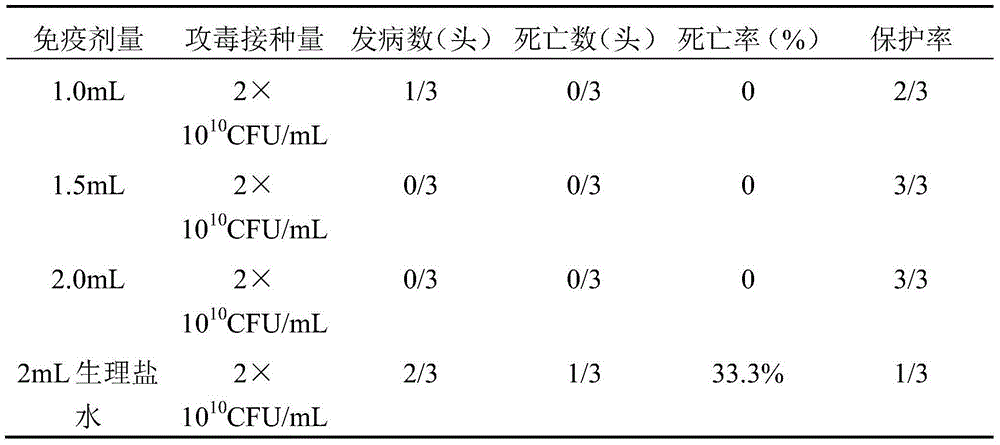
[0046] 2.1 First-level production seed culture Take the basal seeds of HPs LX-5 strain to resuscitate, inoculate in TSA solid medium supplemented with NAD and serum, culture at 37°C for 24-48 hours, continuously streak for 3 generations, pick smooth, moist, non-ferrous A single colony with transparent color and the size of a needle tip raised in the middle is inoculated in TSB liquid medium containing NAD and serum, cultured with shaking at 37°C and 180rpm for about 12 hours, then the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com