Porcine mycoplasma pneumoniae and application of porcine mycoplasma pneumoniae
A technology of mycoplasma hyopneumoniae and inactivated vaccine, which is applied in the field of mycoplasma hyopneumoniae, can solve the problems of reduced mucociliary function, hazards of pig farming, difficult to cure, etc., and achieve stable biological characteristics, good immunogenicity and good protective effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
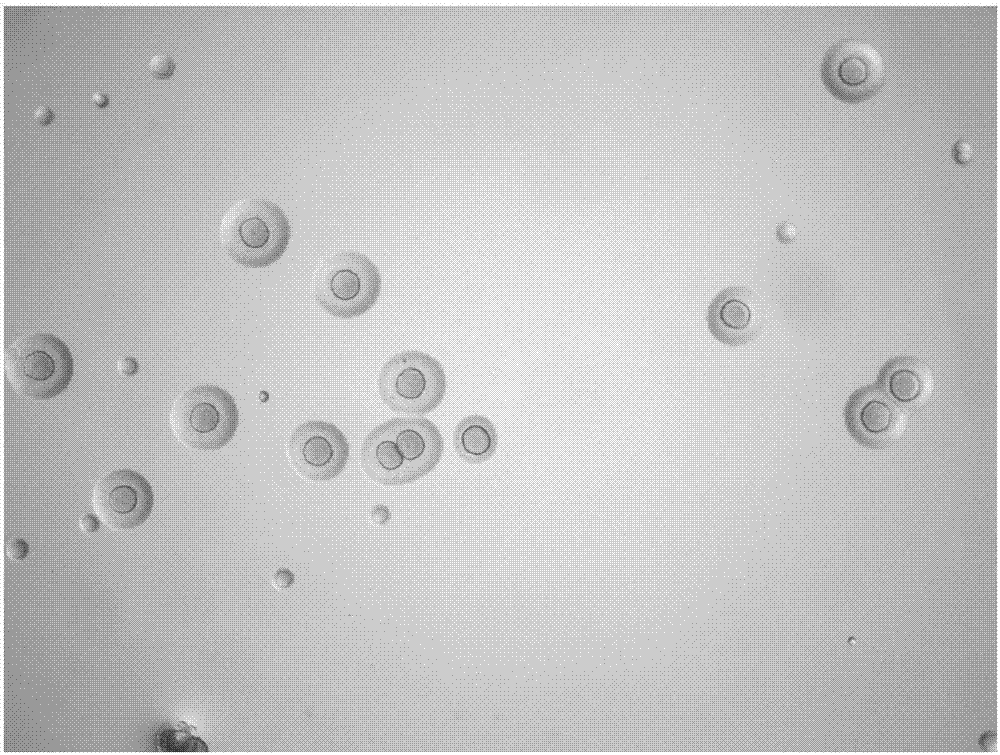
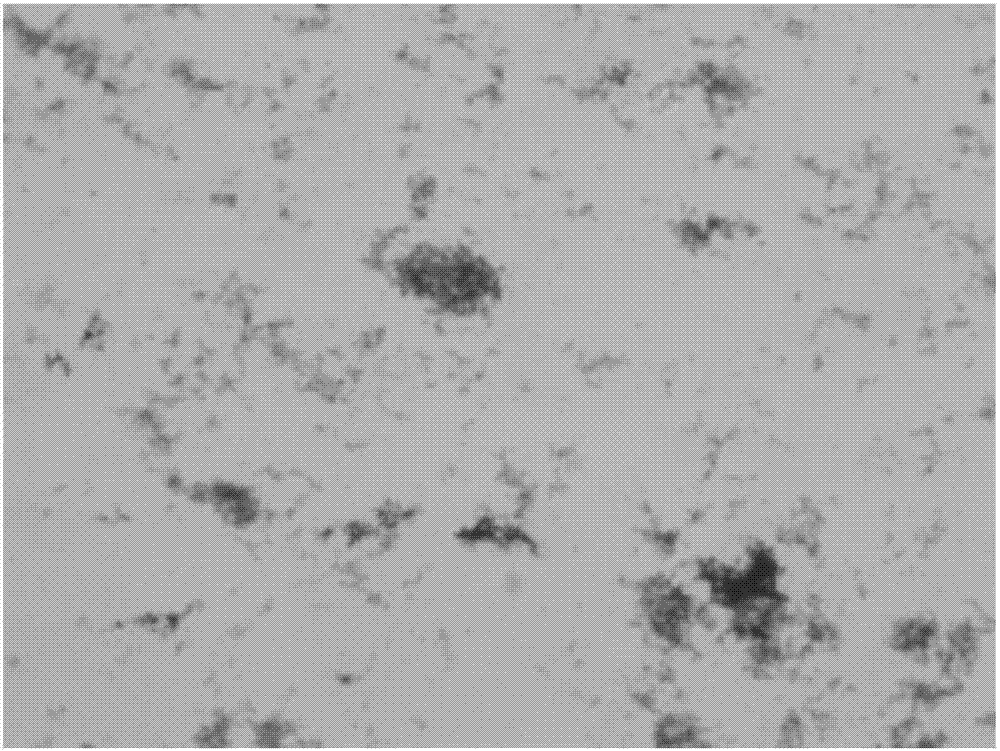
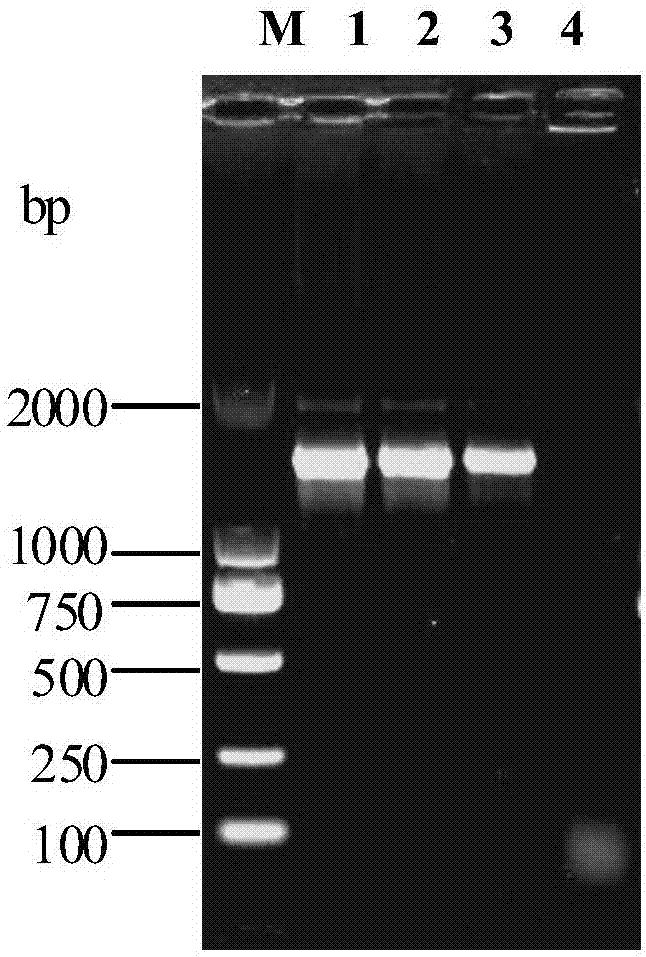
[0019] Embodiment 1, the separation and identification of Mycoplasma hyopneumoniae
[0020] 1.1 Disease data collection
[0021] The disease material came from the lungs of a 2-month-old nursery pig that had severe pneumonia and dyspnea in a large pig farm in Puyang City, Henan Province in April 2017.
[0022] 1.2 Preparation of medium
[0023] PPLO liquid medium: PPLO broth powder 10.5g, glucose 2.5g, yeast powder 2.5g, dissolved in 440mL ultrapure water, sterilized at 115°C for 15min, added MEM medium 5mL, horse serum 50mL, penicillin 80,000 units, Sterile mass fraction 10% arginine 10mL and 1% (w / v) phenol red 500μL. After the culture was sterilized at 115°C for 15 minutes, it was stored at 4°C for later use.
[0024] PPLO solid medium: PPLO liquid medium added with 1.5% (w / v) agar powder. After the culture was sterilized at 115°C for 15 minutes, it was stored at 4°C for later use.
[0025] 1.3 Isolation and culture of mycoplasma
[0026] Take an appropriate amount of...
Embodiment 2
[0042] Embodiment 2, the preparation of vaccine, safety and efficacy test
[0043] 2.1 Preparation of vaccine
[0044] Inoculate the Mycoplasma hyopneumoniae strain HNMhy1 into PPLO liquid medium and place it at 37°C, 5% CO 2 Cultivate in an incubator, collect the culture after 3 days, measure the concentration, and adjust the number of bacteria of Mycoplasma hyopneumoniae in the culture to 2×10 9 CFU / mL, add 2mL formaldehyde solution (mass fraction: 37%) for every 1000mL culture, inactivate at 37°C for 12h to prepare the vaccine stock solution; then add aluminum hydroxide adjuvant (Sigma, F5881) equal to the volume of the vaccine stock solution , mixed to make Mycoplasma hyopneumoniae inactivated vaccine, the bacteria number of Mycoplasma hyopneumoniae in the vaccine is about 1×10 9 CFU / mL.
[0045] 2.2 Vaccine safety test:
[0046] Ten 15-day-old healthy piglets were selected, all of which were negative for Mycoplasma hyopneumoniae ELISA antibodies. Divided into 2 group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com