Efficacy test method of mycoplasma gallisepticum inactivated vaccine and application thereof
A technology of Mycoplasma gallisepticum and inactivated vaccine, which is applied to measuring devices, instruments, scientific instruments, etc., can solve the problems of hidden dangers of virulent attacks on biological safety, errors in test results, easy to disperse poison, etc., and achieves small batch differences and results. Accurate, repeatable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Efficacy Testing Method of Mycoplasma Gallisepticum Inactivated Vaccine
[0022] 1. Vaccination
[0023] 20 40-day-old SPF chickens were used, 10 of which were injected subcutaneously at the back of the neck or intramuscularly with thighs to immunize 1 portion, and the other 10 were not immunized as controls. After 30 days, blood was collected, serum was separated, and ELISA antibody level or HI antibody titer was determined.
[0024] 2. Mycoplasma gallisepticum enzyme-linked immunosorbent assay (ELISA) and hemagglutination inhibition (HI) test
[0025] 1. Mycoplasma gallisepticum ELISA test
[0026] Purchase MG antibody ELISA (ELISA, MG Ab) kit: produced by IDEXX Company in the United States (reagents include: coated microplate, sample diluent, positive control, negative control, enzyme-labeled secondary antibody, substrate solution and stop solution) , Beijing IDEAS Century Yuanheng Co., Ltd.
[0027] The ELISA detection method was carried out according to the IDE...
Embodiment 2
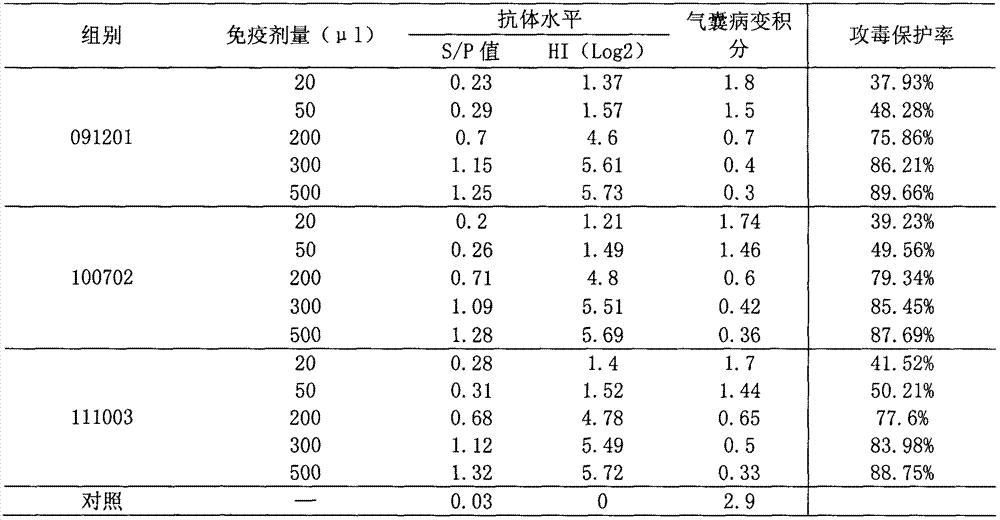
[0053]Parallel relationship between serum antibody titer and challenge protection rate of mycoplasma gallisepticum inactivated vaccine immunization test animals Use 3 batches of vaccines, each batch divides 60 40-day-old SPF chickens into 6 groups, 10 in each group, 50 of them were immunized by subcutaneous injection of 20 μl, 50 μl, 200 μl, 300 μl, 500 μl of the back of the neck or intramuscularly of the thigh, and the other 10 were not immunized as a control. After 30 days, blood was collected, serum was separated, and ELISA antibody level or HI antibody level was determined. At the same time, together with 10 control chickens, at 2-3m 3 Spray and challenge Mycoplasma gallisepticum R strain culture 500-600mL (each 1.0ml contains viable bacteria count 108-9CCU color change units) in a closed room (indoor temperature is 15°C-30°C, relative humidity is 50%-70%), The duration of spraying should not be less than 5 minutes, and the droplet size should be around 2.0 μm. Observe fo...
Embodiment 3
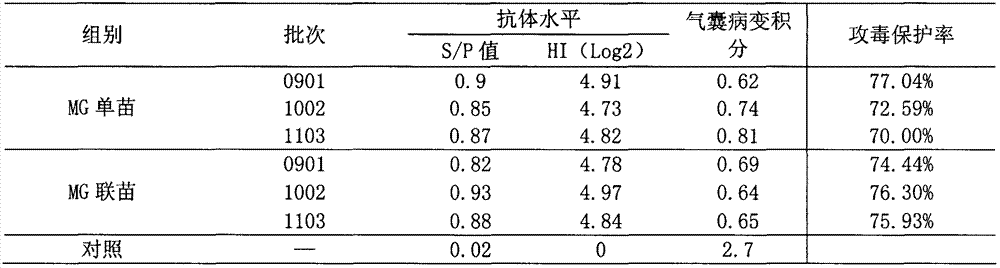
[0058] Comparison of serum antibody and challenge protection rate of Mycoplasma gallisepticum inactivated vaccine single vaccine and combined vaccine immunization experimental animals Three batches of vaccines were used for each single vaccine of Mycoplasma gallisepticum inactivated vaccine and combined vaccine of Mycoplasma gallisepticum inactivated vaccine. Ten 40-day-old SPF chickens were immunized with the same antigenic content of Mycoplasma gallisepticum inactivated vaccine single vaccine and Mycoplasma gallisepticum inactivated vaccine combined vaccine, and immunized with subcutaneous or thigh intramuscular injection at 0.2ml / feather, and another 10 Only non-immunization was used as a control. After 30 days, blood was collected, serum was separated, and ELISA antibody level or HI antibody level was determined. At the same time, together with 10 control chickens, at 2-3m 3 Spray and challenge Mycoplasma gallisepticum R strain culture 500-600mL (each 1.0ml contains 108-9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com