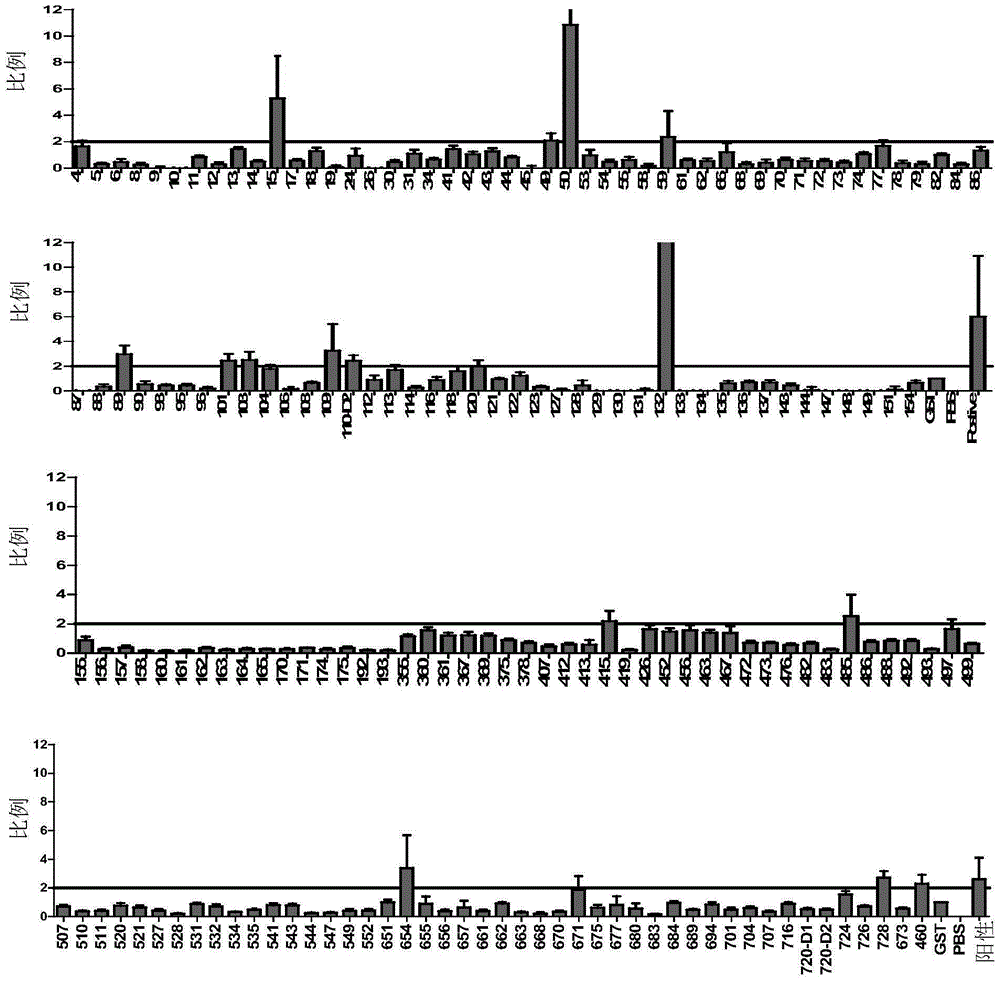
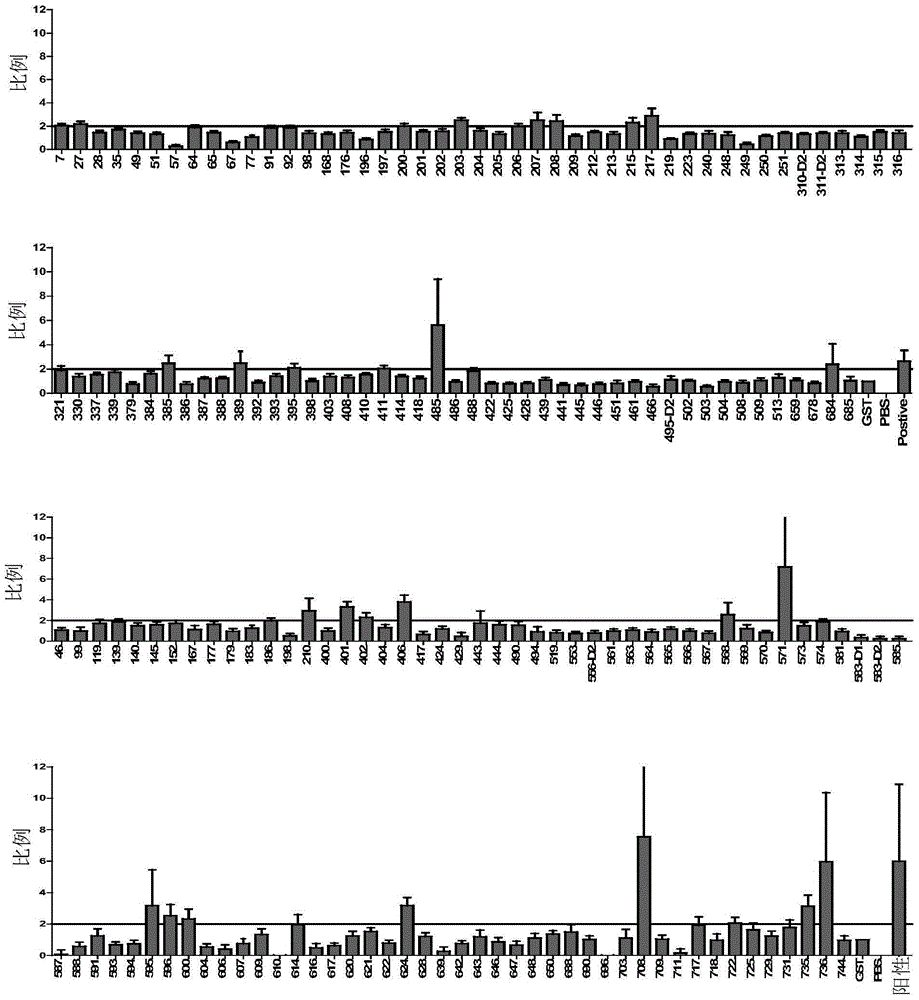
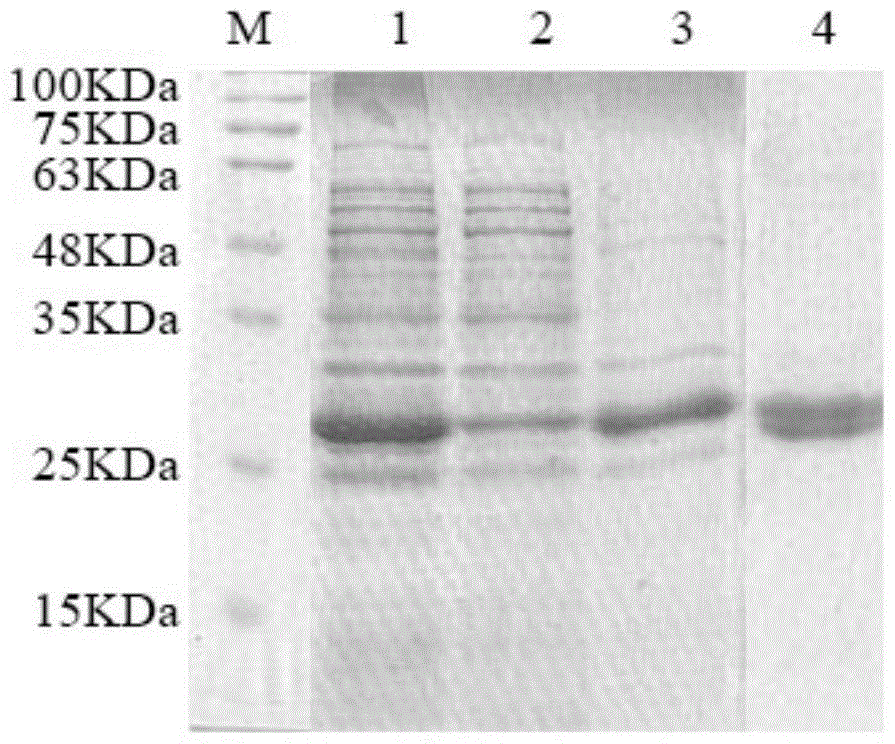
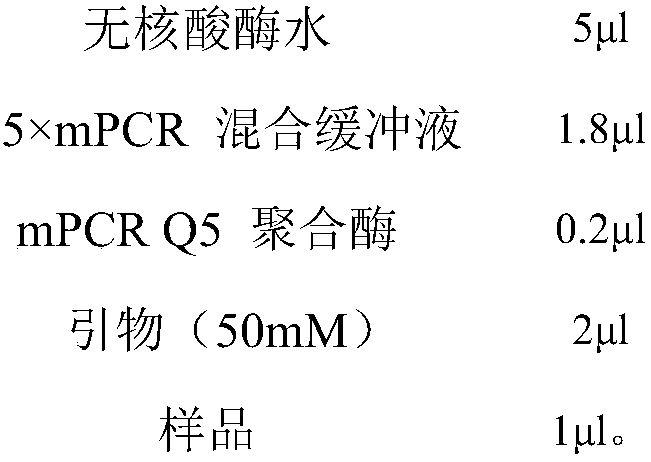
Patents
Literature
178 results about "Serological assay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Serology Assay. A serology assay enables the detection of an antibody (target) with the use of a capture protein antigen attached to the surface of a microsphere. A detection antibody that incorporates a fluorescent label is used to quantify the amount of target antibody present.
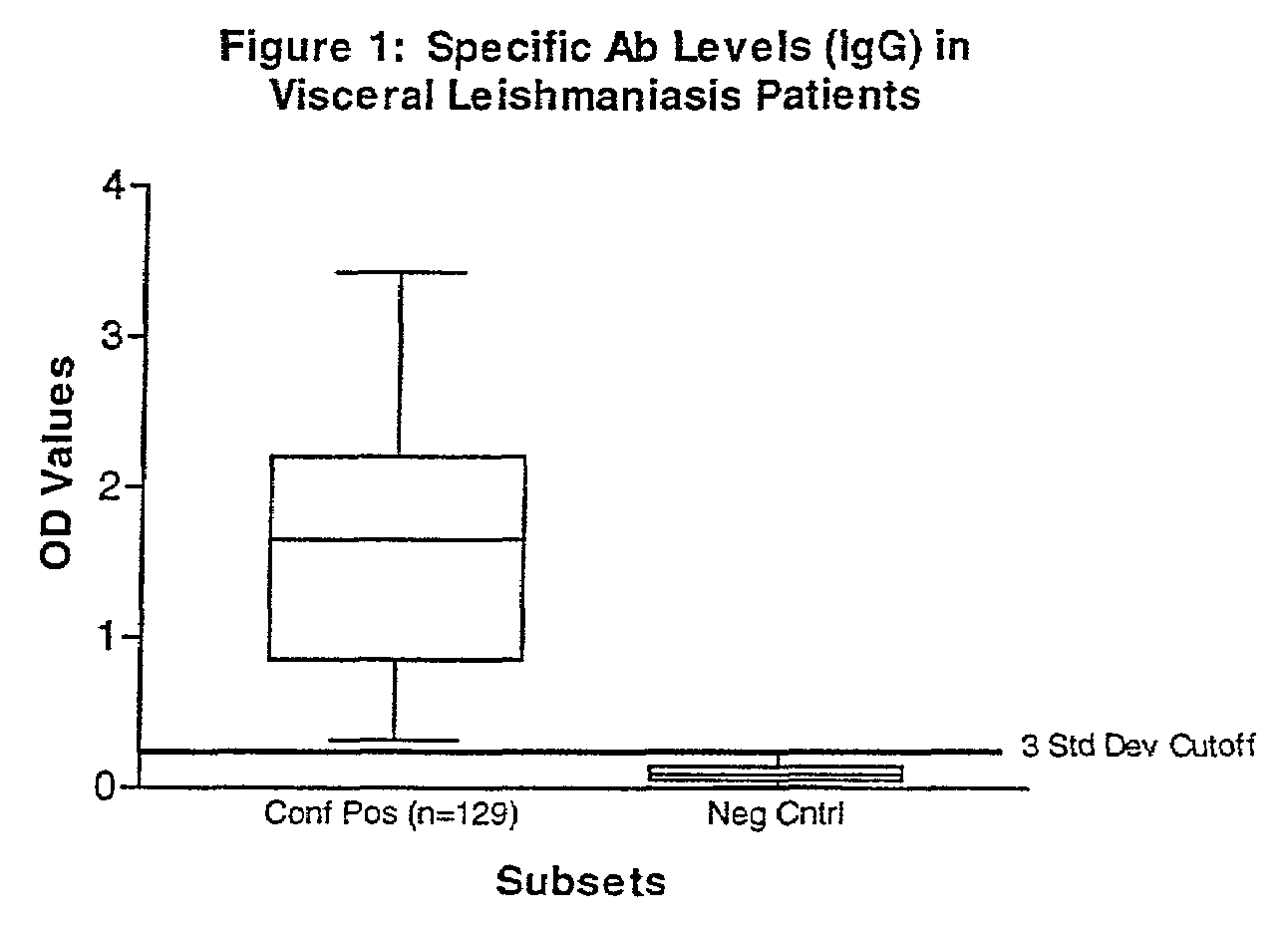
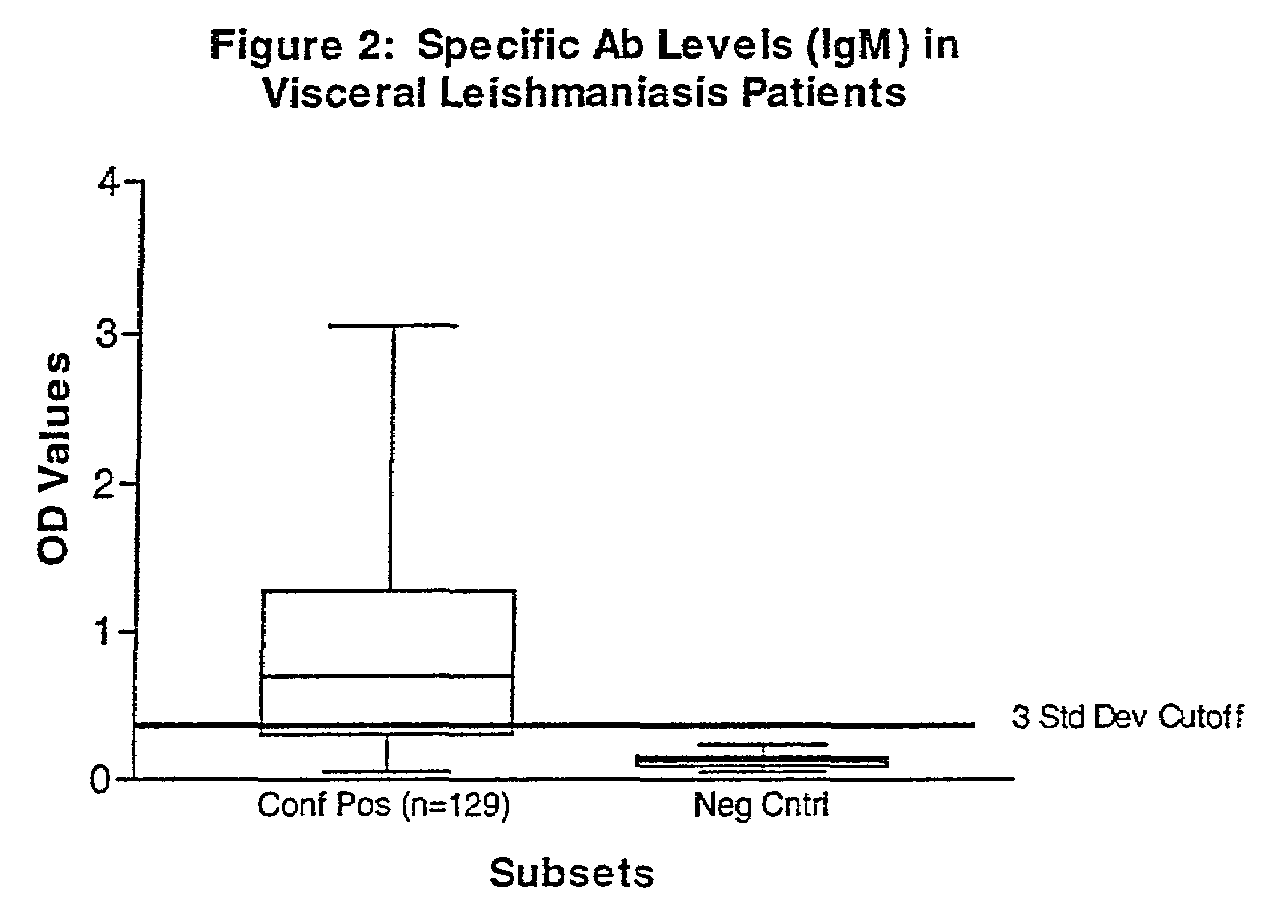
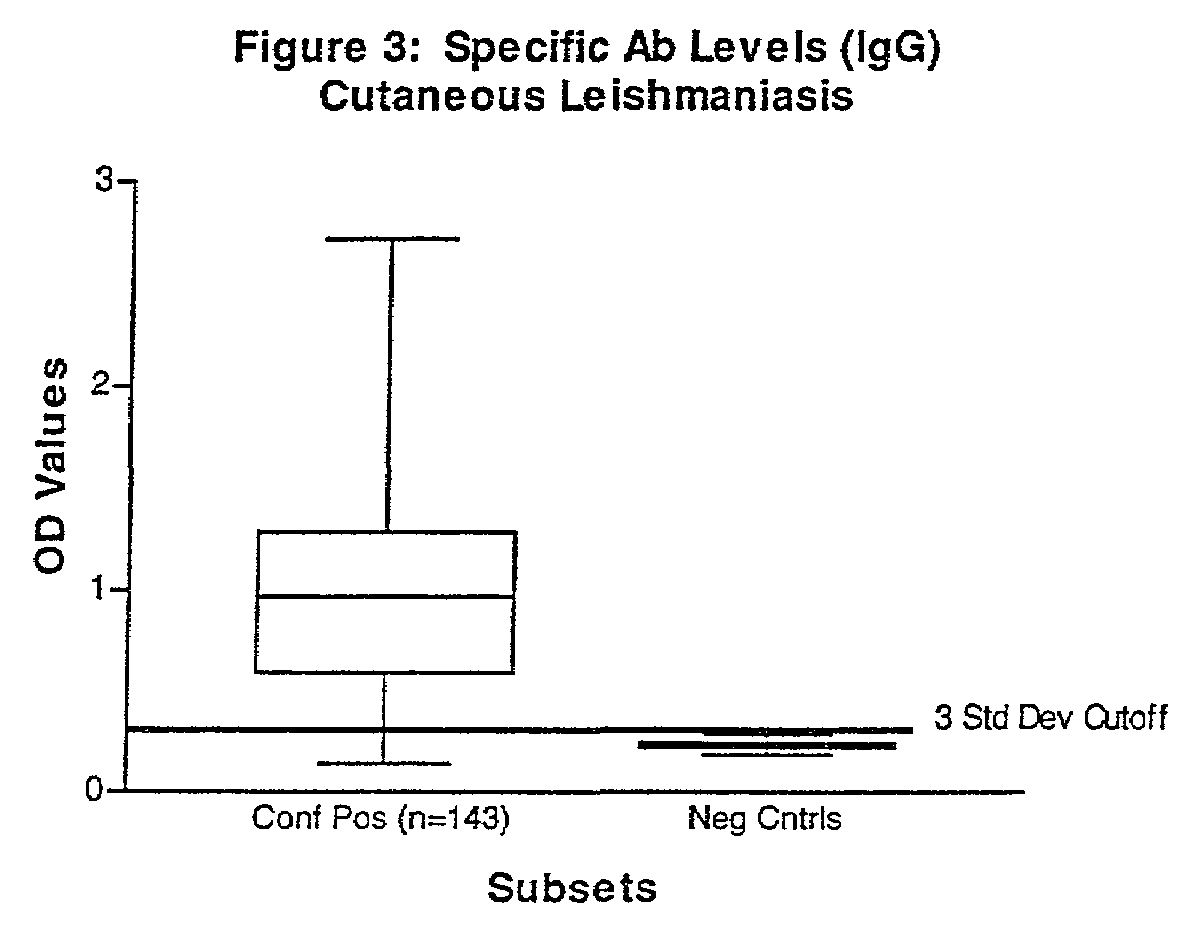
Practical serological assay for the clinical diagnosis of leishmaniasis
InactiveUS7008774B2Improve purification effectPurification is easy and lessBiocideProtozoa antigen ingredientsProtozoaAntigen capture
Methods for the diagnosis of visceral, cutaneous and canine leishmaniasis in a subject suspected of being infected with the parasitic protozoa Leishmania is disclosed. Disclosed are antibody-capture enzyme-linked immunosorbent assays (ELISAs) for the detection of antibodies to Leishmania parasite soluble antigens and antigen-capture ELISAs for the detection of Leishmania parasite soluble antigens in host samples. Also disclosed are immunodiagnostic kits for the detection of Leishmania parasite circulating antigens or IgM and IgG antibodies in a sample from subject having visceral, cutaneous or canine leishmaniasis. In these methods and kits, detection may be done photometrically or visually. The methods and kits also allow the visualization of Leishmania amastigotes or promastigotes in a sample.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
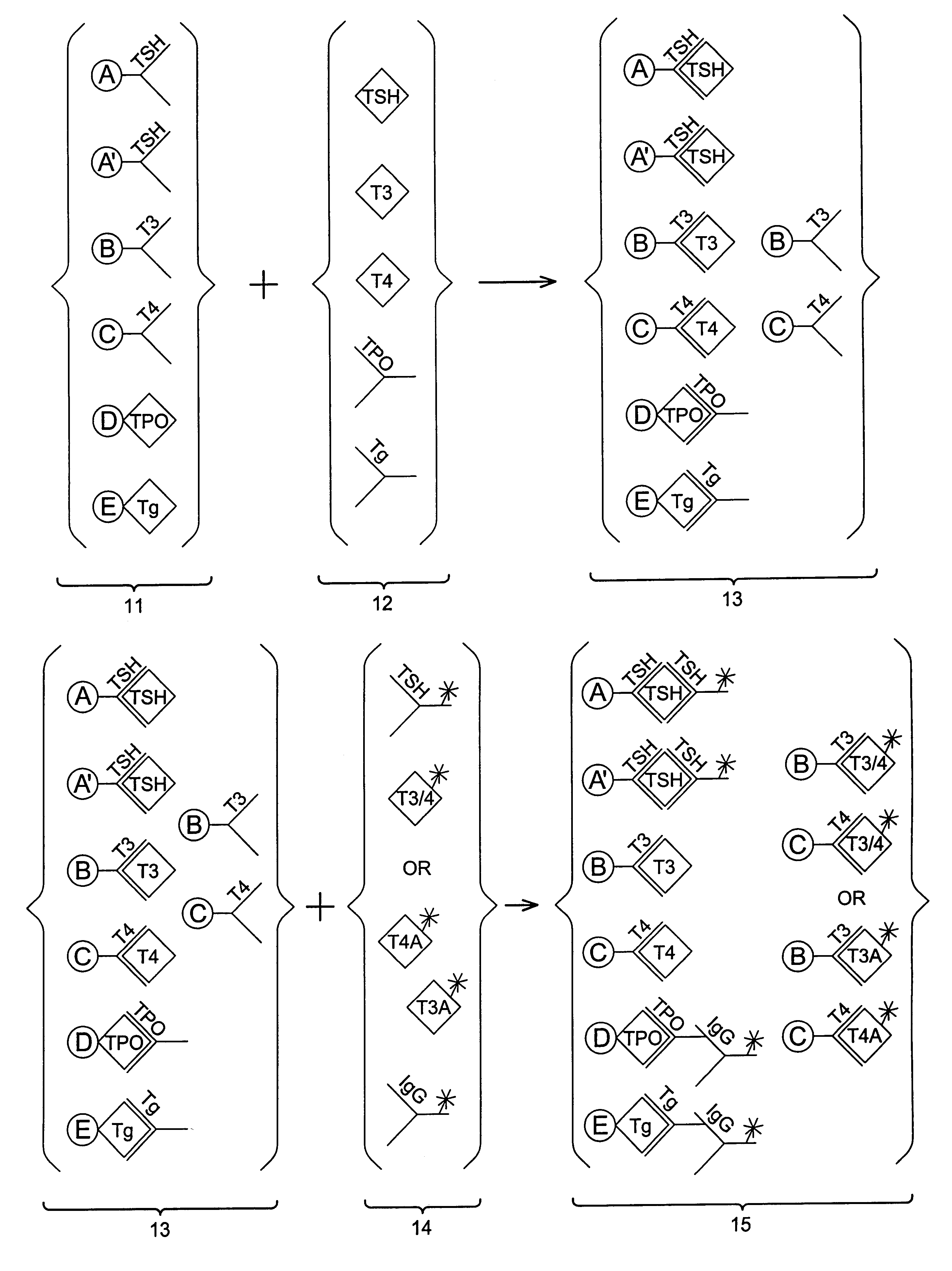
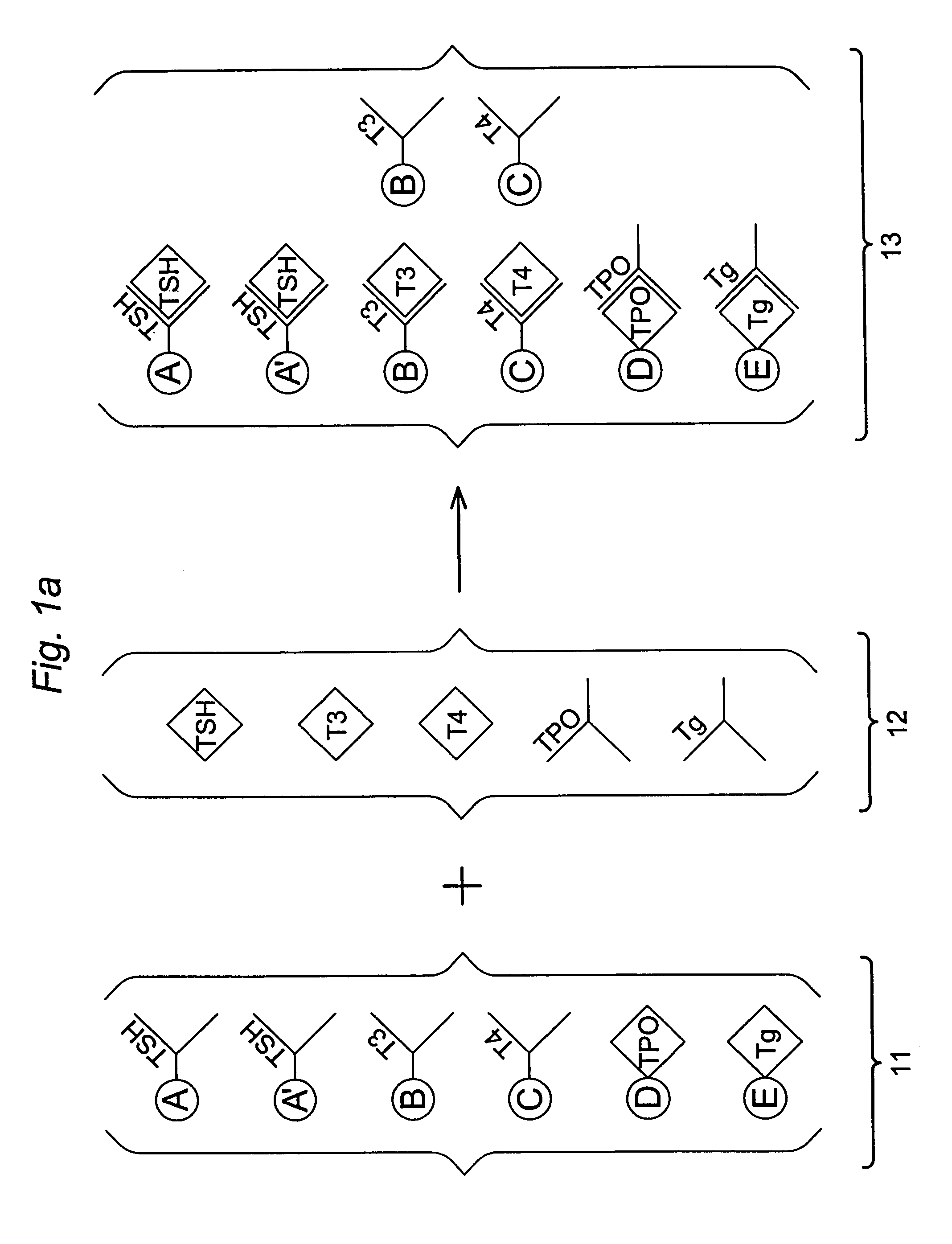
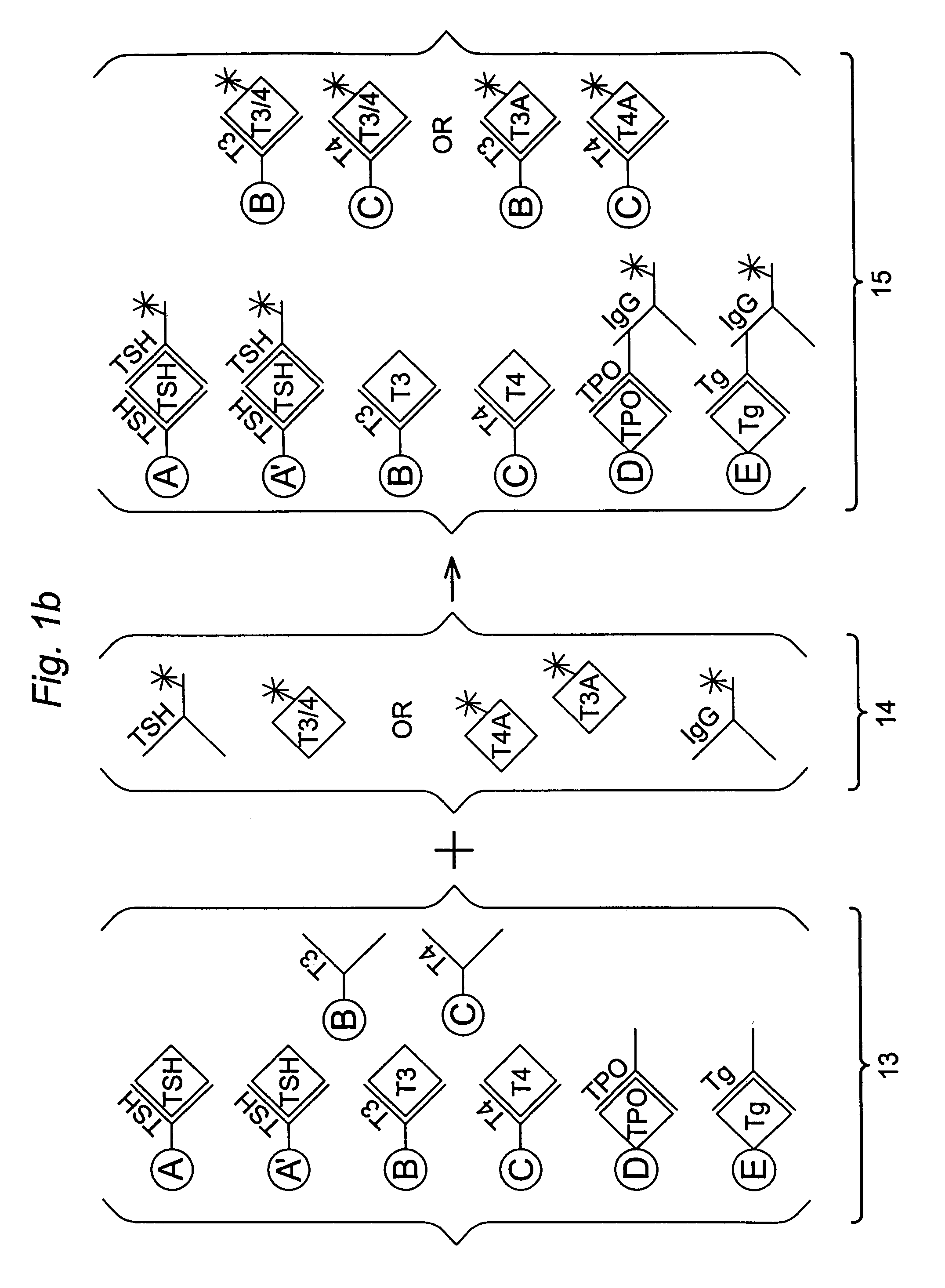
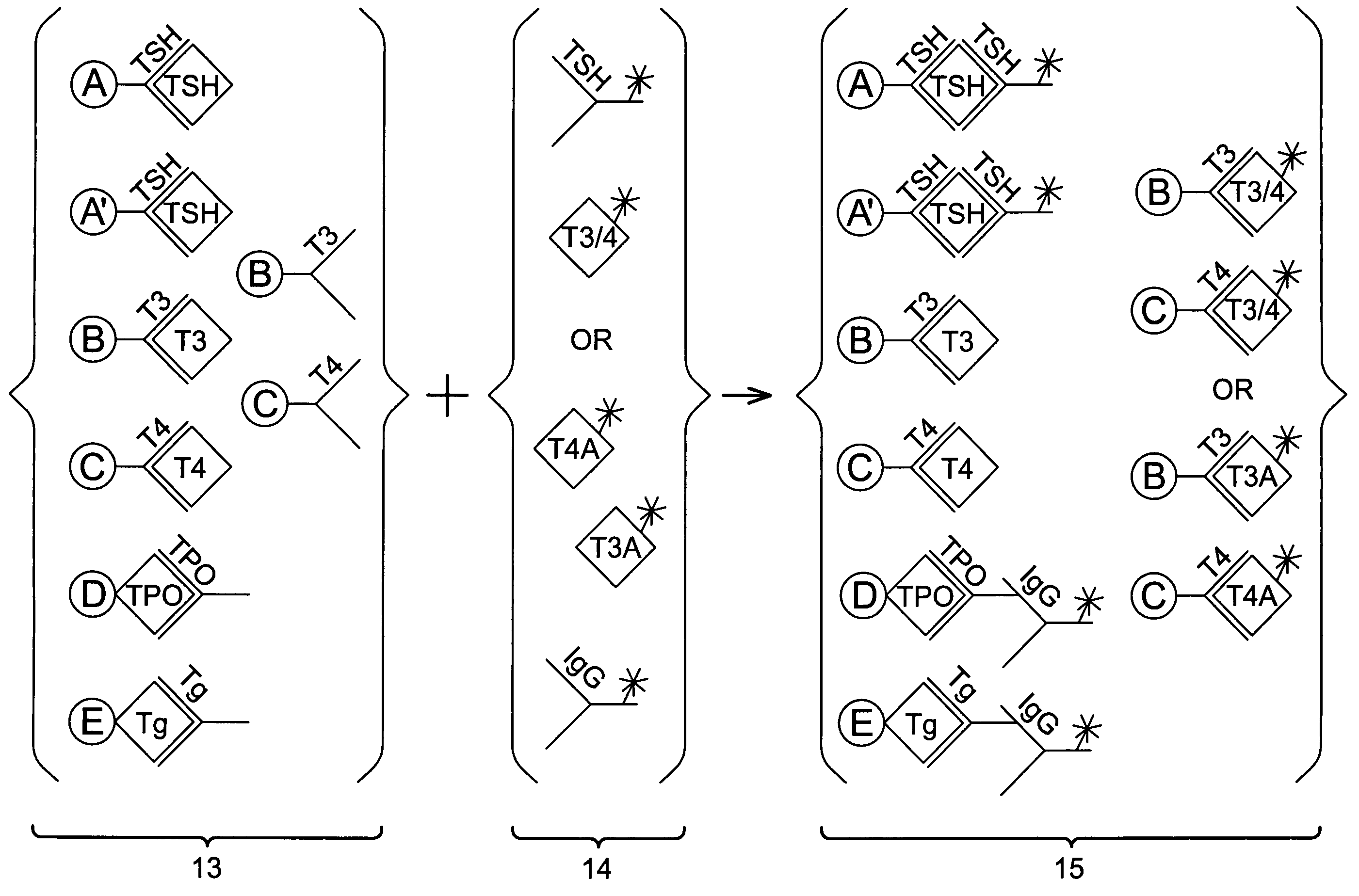
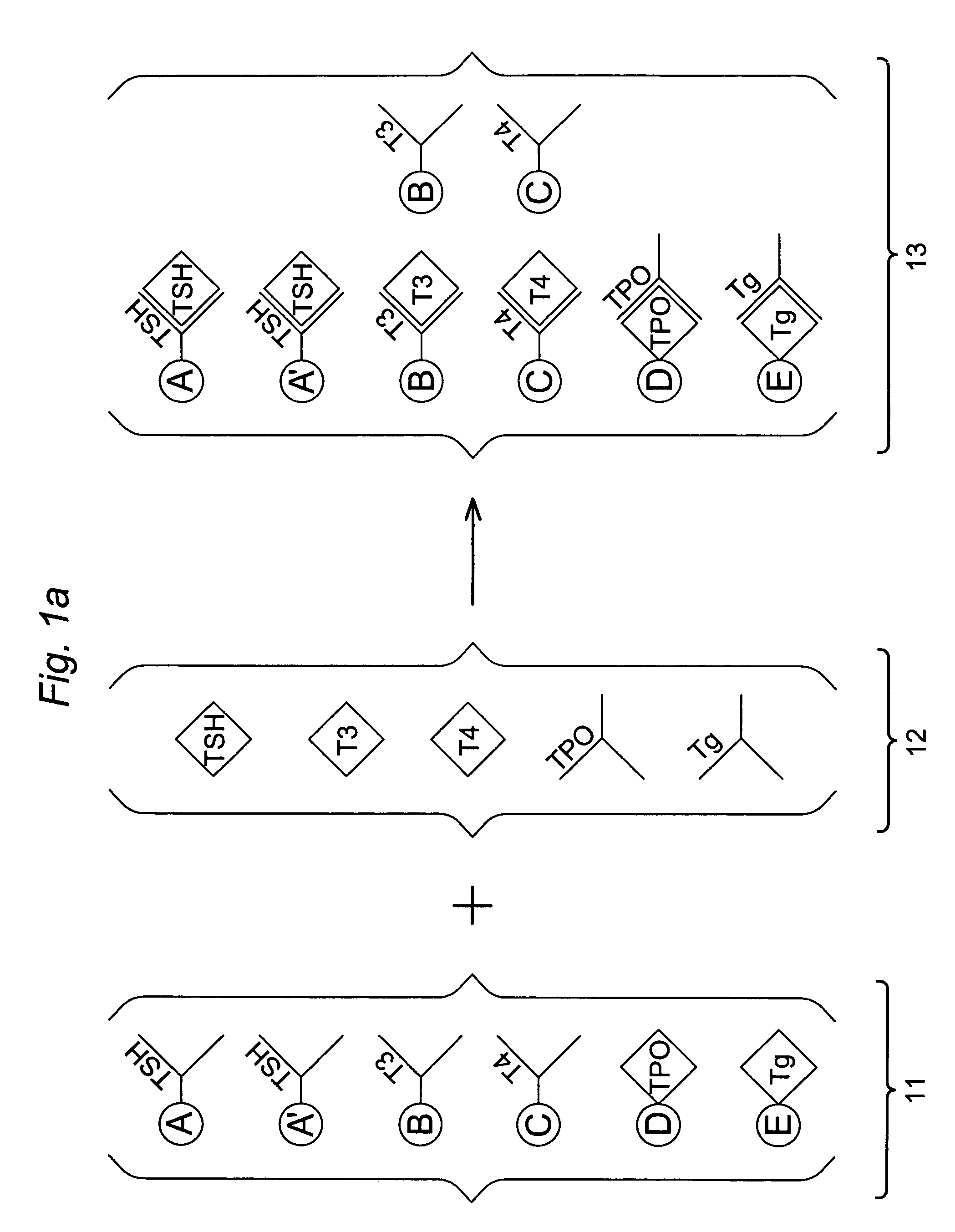
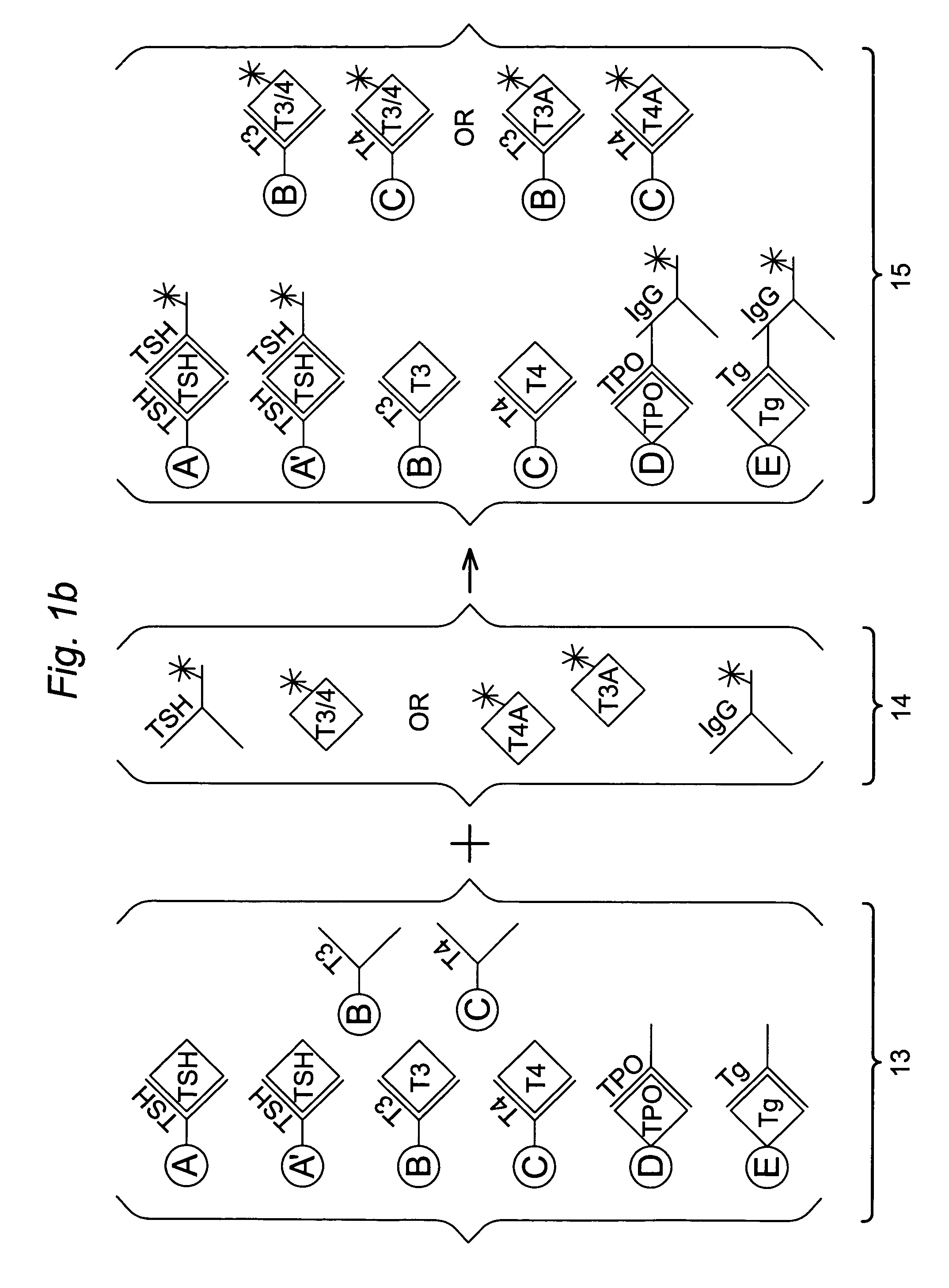
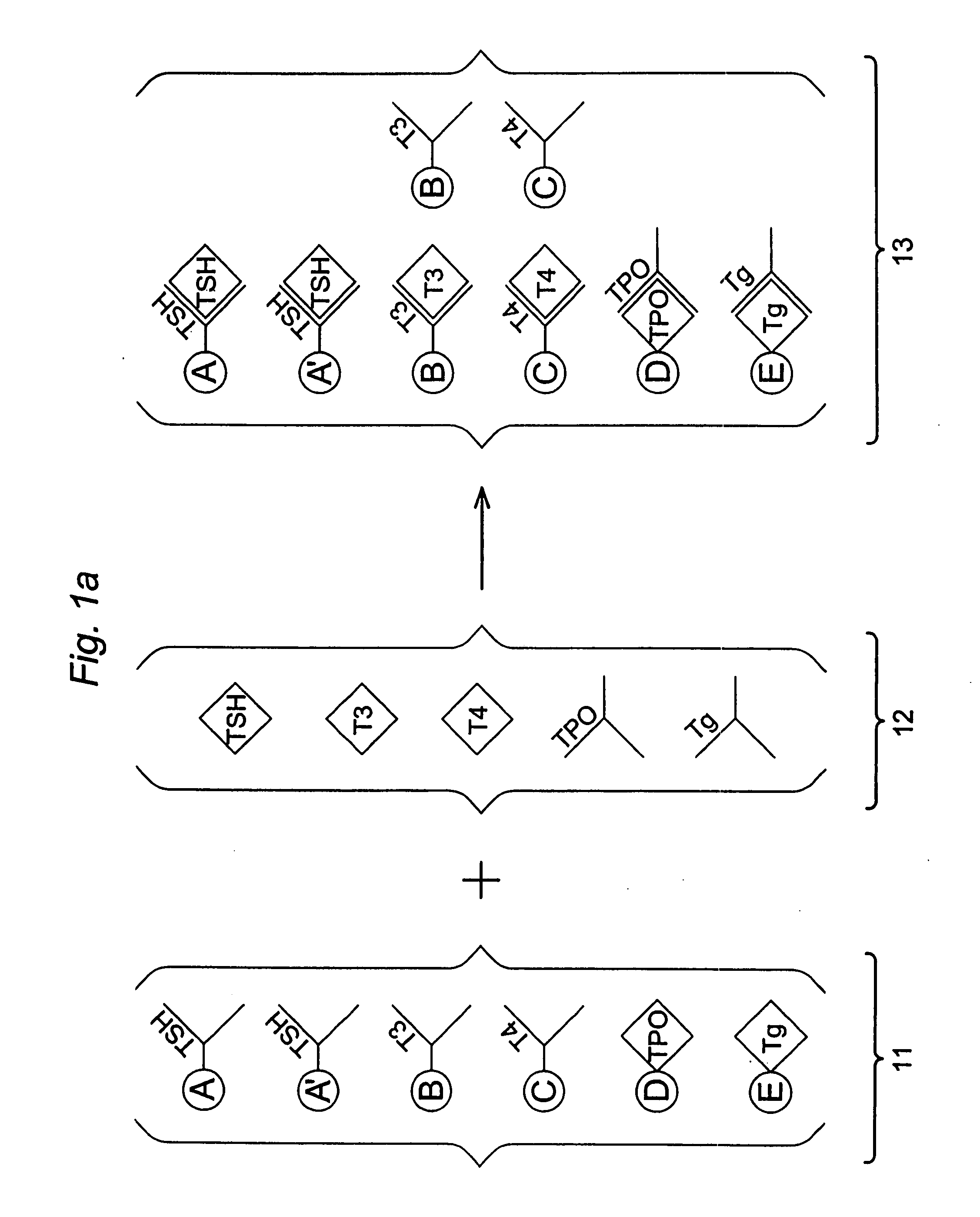
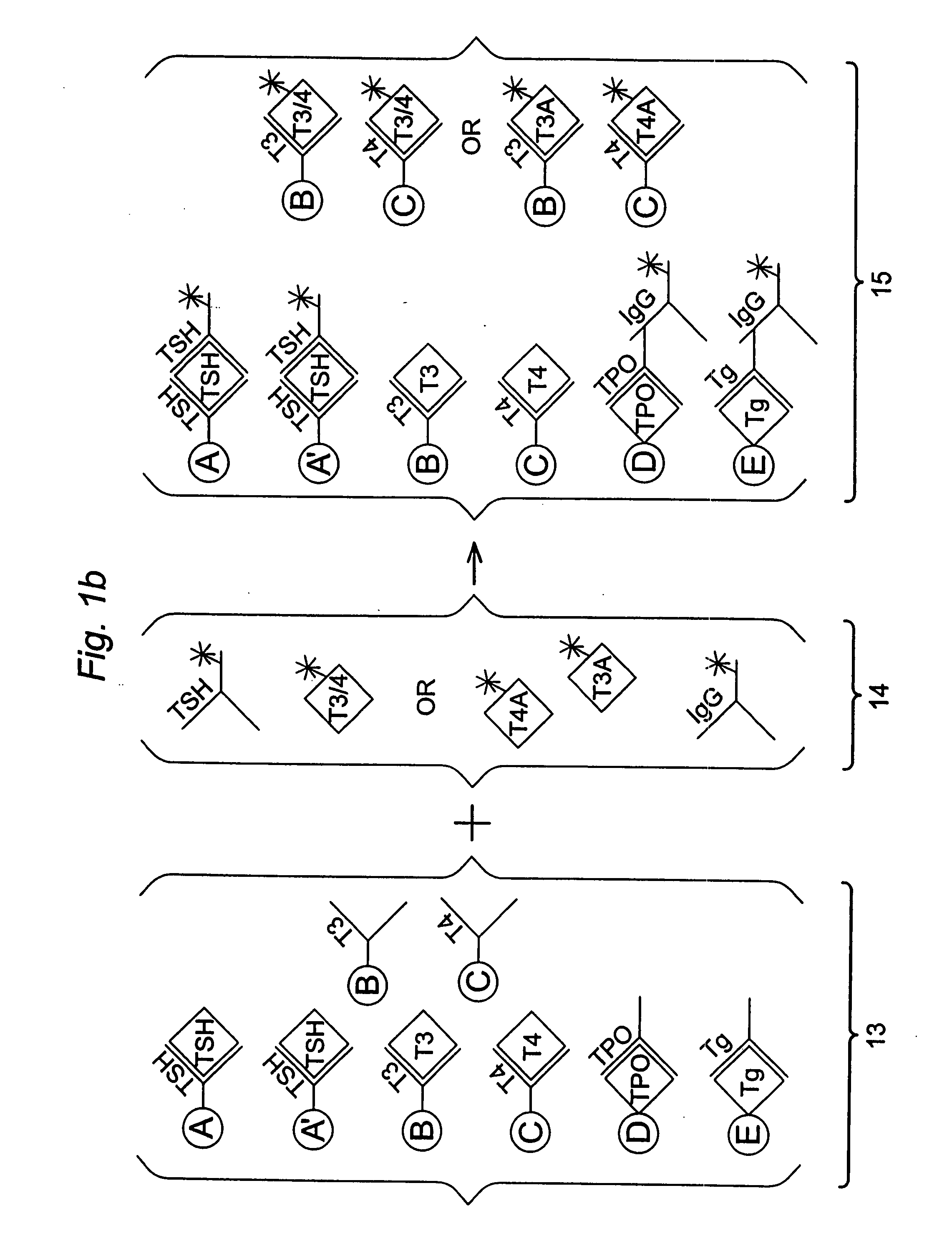
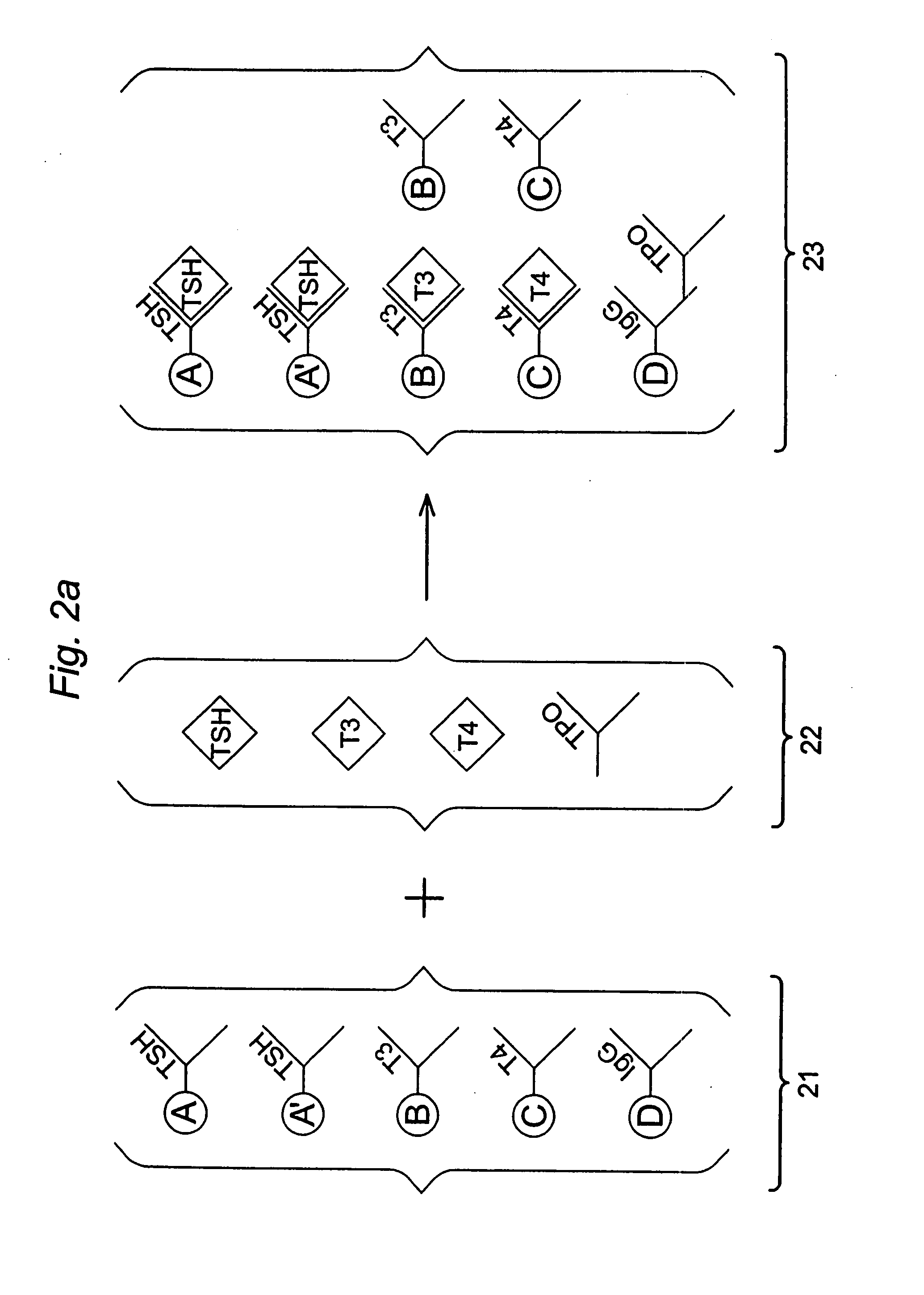
Multi-analyte diagnostic test for thyroid disorders
InactiveUS7271009B1Reduce signalingIncrease ratingsBioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseMulti analyte
Immunological assays for several biological markers for thyroid disorders in a biological sample are performed in a single test with a combination of sandwich-type, sequential competitive, and serological assays by the use of particles classified into groups that are distinguishable by flow cytometry, one group for the assay of each marker. Each group of particles is coated with a different immunological binding member, and coating densities, co-coating materials, and special buffer solutions are used to adjust for differences in the sensitivities and dynamic ranges of each of the markers in the typical sample.
Owner:BIO RAD LAB INC
Des-gamma-carboxy-pro-thrombin microplate chemiluminescence immune analysis determination reagent kit and preparing method thereof
InactiveCN101377505ASimple and fast operationFair priceChemiluminescene/bioluminescenceCelluloseThrombin activity
The invention relates to the medical field of immunoassay, more specially, the invention provides a chemiluminescent immunoassay detection kit for des-gamma-carboxyand-prothrombin (DCP) microporosity plate and a preparation method thereof, and realizes the rapid, sensitive and high-specificity serological detection of DCP with the one step chemiluminescent immunoassay method. The kit of the invention has the advantages of simplicity, convenience, rapidness, sensitivity, stability and the like, eliminates the interference of the prothrombin analogues and the serum cellulose and the analogues thereof, and has the advantage of high specificity.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Biologically safe Africa swine fever antigen multifactorial serum for ELISA diagnosis
The invention relates to a biologically safe Africa swine fever antigen multifactorial serum for ELISA (enzyme-linked immuno sorbent assay) diagnosis. A technical scheme adopted in the invention includes: adopting gene-expressed structural protein P72, K205R, P54, and A104R, conducting chemical purification, carrying out coating with a Freund's incomplete adjuvant, performing intramuscular immunization on laboratory swine in three batches, collecting swine blood after one month, separating serum, implementing serological testing, and conducting subpackaging and preservation. The serum is subpackaged into ELISA kits to undergo test according to conventional ELISA test methods.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
Monophosphoinositide proteoglycans-3 chemiluminescence immune analysis determination reagent kit and preparing method thereof
InactiveCN101377506AIncrease the effective amountReliable clinical reference valueChemiluminescene/bioluminescenceTreatment effectChemiluminescent immunoassay
The invention relates to the medical field of immunoassay, more specially, the invention provides a chemiluminescent immunoassay detection kit for phosphatidylinositol proteoglycan-3(GPC-3) and a preparation method thereof, and realizes the simultaneous serological detection of GPC-3 N terminal and C-terminal protein with the chemiluminescent immunoassay method. The kit has the advantages of simple sampling, convenient detection and accurate and specific technical method. The invention adopts a biotin-strapavidin system to coat antibodies and improve the efficiency of antibody coating and the linear range of detection as well as sensitivity, and can be conveniently used for the tracing observation of early diagnosis or treatment effect for primary carcinoma of liver.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
ELISA detection method and reagent kit for bluetongue viral antigen
InactiveCN101403759AImprove featuresIncreased sensitivityBiological testingSerum igeMonoclonal antibody
The invention discloses an ELISA detection method and a kit of blue-tongue virus antigen. The detection method uses the monoclonal antibody of anti-blue-tongue virus VP7 protein as dual-anti sandwich ELISA detection method of a coating and enzyme-marked antibody; and the kit comprises the monoclonal antibody of the anti-blue-tongue virus VP7 protein of the coating and enzyme-marked antibody. The kit and the detection method can be used for detecting the blue-tongue virus antigen, have higher specificity and sensitiveness, can be used in large-scale forserological detection, is suitable for epidemiological investigation and has wide application prospect.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
Multi-analyte diagnostic test for thyroid disorders
InactiveUS7608465B2Reduce signalingIncrease ratingsBioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseMulti analyte
Immunological assays for several biological markers for thyroid disorders in a biological sample are performed in a single test with a combination of sandwich-type, sequential competitive, and serological assays by the use of particles classified into groups that are distinguishable by flow cytometry, one group for the assay of each marker. Each group of particles is coated with a different immunological binding member, and coating densities, co-coating materials, and special buffer solutions are used to adjust for differences in the sensitivities and dynamic ranges of each of the markers in the typical sample.
Owner:BIO RAD LAB INC
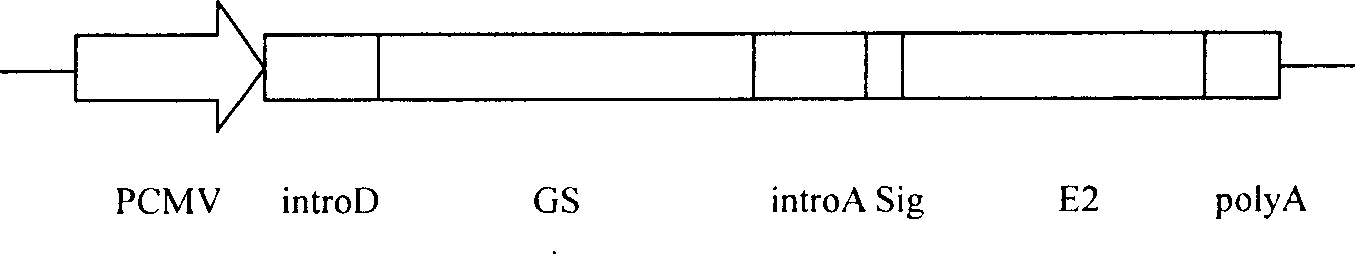
Method for expressing hepatitis C virus envelope protein E2 by mammal cell with high efficient secretion
The present invention relates to biomedicine technology. HCV envelope protein E2 mediates the combination between HCV and target cell and is key protein relates to HCV infection and one kind of low expression protein hard to obtain in gene recombination process. The present invention aims at provides high efficiency secretion method for mammal to express HCV envelope protein E2. The method constitutes one new type of mammal cell expressing plasmid, which expresses target gene E2 protein in high level while expressing glutamine synthetase as the screening marker in low level. The present invention makes it possible to batch prepare recombinant HCV envelope protein E2 with the natural biological function and antigenicity of HCV envelope protein, lays the foundation for development of serological HCV infection detecting reagent and HCV vaccine, and provides HCV molecular virological research with important material.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Enterovirus 71 type specific recombinant protein antigen and application thereof
InactiveCN102558313AIncreased sensitivityImprove featuresBacteriaMicroorganism based processesSerodiagnosesType specific
Owner:SOUTHEAST UNIV
Multi-drug resistance tuberculosis diagnosing marker set and application thereof
The invention discloses a multi-drug resistance tuberculosis diagnosing marker set and application thereof. Sequences of multi-drug resistance tuberculosis diagnosing markers correspond to SEQ:ID:NO:1 to SEQ:ID:NO:8. The multi-drug resistance tuberculosis diagnosing markers are originated from 8 antigens of tuberculosis mycobacteria and used for judging whether a tested the optimal working point of protein combinations for diagnosing multi-drug resistance tuberculosis, the specificity is 80.4%, and the sensibility is 80%, wherein the specificity and the sensibility are both higher than indexes in diagnosis of multi-drug resistance tuberculosis in the prior art. In addition, in the technology, a serological test is adopted, samples are easy to obtain, and the multi-drug resistance tuberculosis diagnosing marker set is suitable for diagnosis of various kinds of suspicious multi-drug resistance tuberculosis including multi-drug resistance tuberculosis and extrapulmonary tubereulosis.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Multi-analyte diagnostic test for thyroid disorders
InactiveUS20070178604A1Reduce signalingIncrease ratingsDisease diagnosisBiological testingDiseaseDiagnostic test
Immunological assays for several biological markers for thyroid disorders in a biological sample are performed in a single test with a combination of sandwich-type, sequential competitive, and serological assays by the use of particles classified into groups that are distinguishable by flow cytometry, one group for the assay of each marker. Each group of particles is coated with a different immunological binding member, and coating densities, co-coating materials, and special buffer solutions are used to adjust for differences in the sensitivities and dynamic ranges of each of the markers in the typical sample.
Owner:BIO RAD LAB INC
Blood group serology detection method and automatic detection system for realizing method
The invention provides an automatic blood group serology detection method and an automatic detection system for realizing the method. The method provided by the invention is convenient and effective, controllably improves the suspension effect of micro liquid and satisfies the operation requirements of blood immune reaction, so that results are more reliable and accurate; an adopted test tube luminous flux detection method is the obtained most widely-used method, can quickly judge experimental results and is high in accuracy rate, high in detection rate of weak agglutination and better in sensitivity. The detection system provided by the invention can quickly judge the experimental results through close cooperation of all parts and is high in accuracy rate, high in detection rate of weak agglutination and better in sensitivity; particularly, a bead adding device obtains a good effect and is high in degree of automation, accurate and quick in bead adding action, so that the vibration intensity of an existing oscillating mechanism is greatly reduced, the suspension effect of the micro liquid is improved, and the operation requirements of the blood immune reaction is satisfied.
Owner:JIANGSU KELAISIKE BIOLOGICAL TECH
Orrhology detection method and use of substrate metal protease MMP11
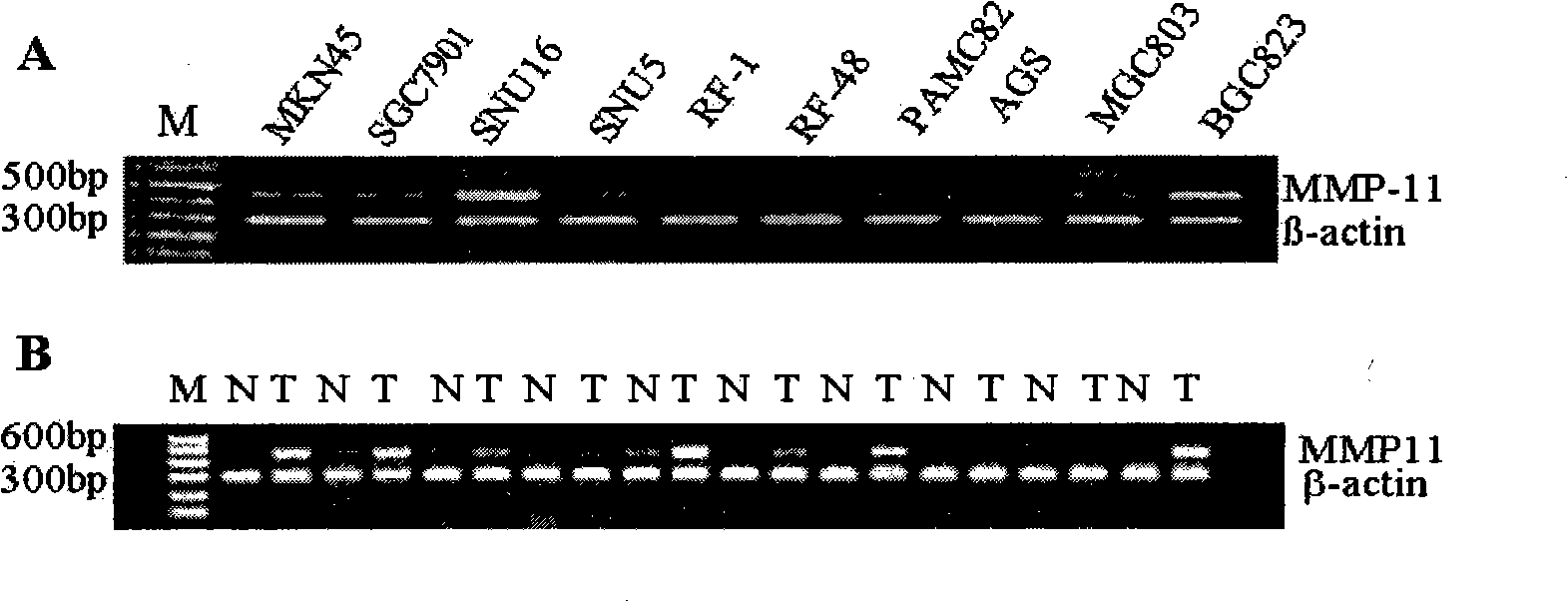
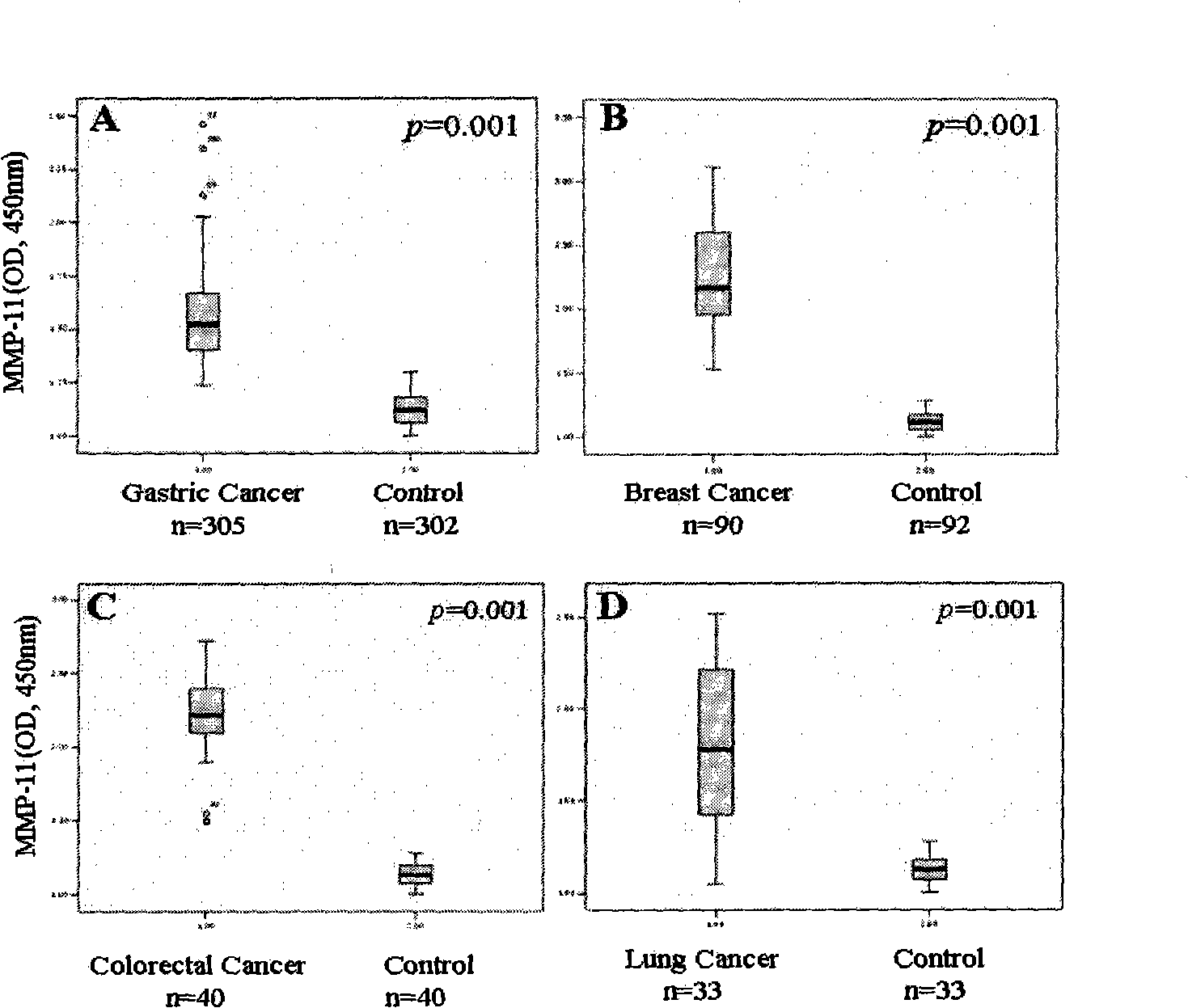
The invention discloses a method for serologically detecting Matrix metalloproteinase 11 (MMP11) and the application thereof. The gene chip technology and the bioinformatics method are adopted to obtain stomach cancer gene expression spectra, and by taking the MMP11 gene for example, the expression characteristics of the gene in the tumor cell lines and tissues are verified at the levels of mRNA and protein. At the same time, a detection kit for the MMP11serum is established by utilizing the technique of the enzyme linked immuno sorbent assay (ELISA), and the expression level of the MMP11 protein in the serum of stomach cancer patients is detected. The level of the MMP11 protein in the serum of stomach cancer patients is obviously higher than that of a non-cancer comparison group (p is lower than 0.001), and the same tendency is also found in the breast cancer, the colon cancer / rectum cancer and the lung cancer. In the serum detection to the stomach cancer patients, the sensitivity of the MMP11 is higher than that of a tumor molecule marker such as CEA, CA199, CA72.4, CA242, etc., and MMP11 and CA199 have good correlation (p is equal to 0.017). Through the analysis of the clinic pathological data, the level of the serum MMP11 has the obvious correlation (p is equal to 0.009) with the cancerometastasis state of the stomach cancer patients. The MMP11 is possible to become a novel tumor serum marker for the diagnosis and the prognostic judgement of the tumors.
Owner:BEIJING CANCER HOSPITAL PEKING UNIV CANCER HOSPITAL
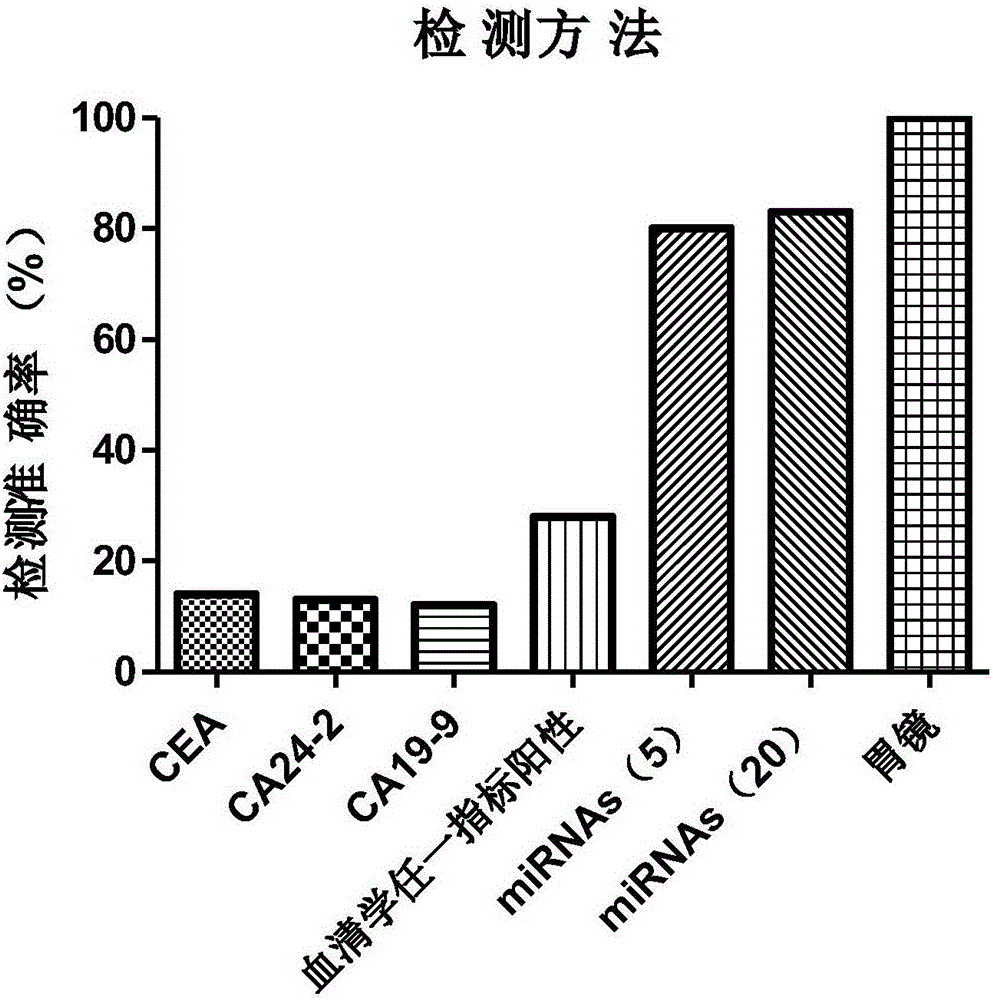
Stomach cancer serological detection and identification kit and method
ActiveCN106755377AExclude false positive resultsAvoid pressure lossMicrobiological testing/measurementAntigenCarbohydrate antigen
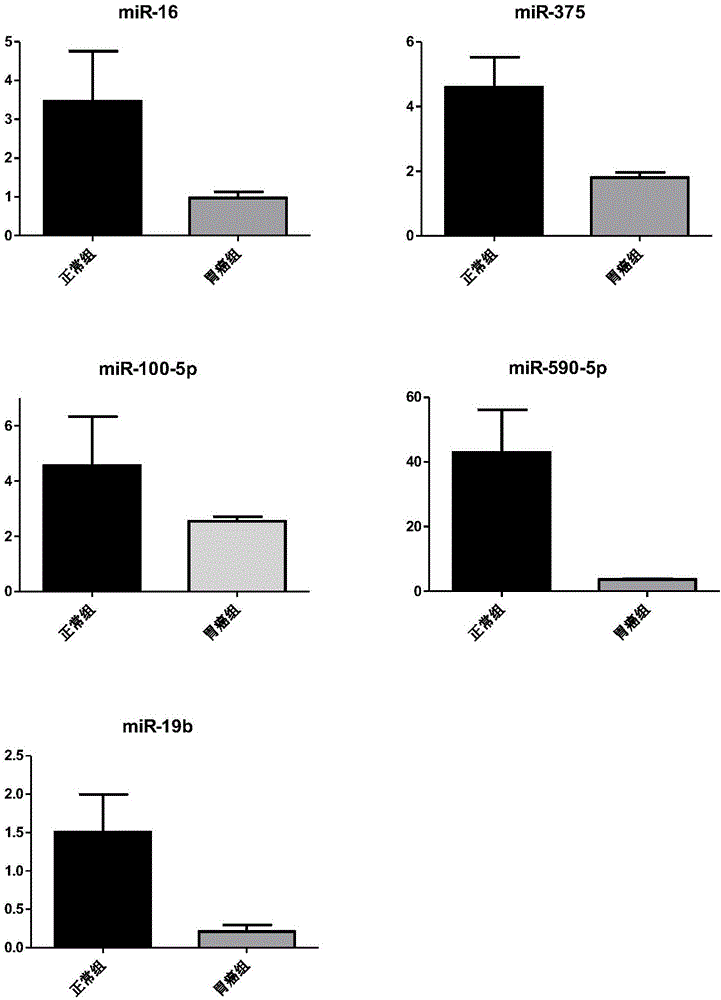
The invention discloses a stomach cancer serological detection and identification kit and a method. The stomach cancer serological detection and identification kit is prepared from a cel-miR-39-5p fragment, a primer, an exosome isolating reagent, an exosome miRNA (Micro Ribonucleic Acid) extracting reagent, a reverse transcription reagent and a real-time fluorescent quantitative PCR (Polymerase Chain Reaction) reagent, wherein the primer comprises a cel-miR-39-5p internal reference primer, an hsa-miR-375 primer, an hsa-miR-590-5p primer, an hsa-miR-19b-3p primer, an hsa-miR-100-5p primer and an hsa-miR-16 primer. When any results of detecting stomach cancer CA (Carbohydrate Antigen)19-9, CA24-2 and CEA (Carcino Embryonie Antigen) of a patient by using serum are positive, and the stomach cancer serological detection and identification kit provided by the invention is then used for carrying out aided detection, false positive results of serological detection can be effectively removed, so that huge mental stress and property loss brought to the patient can be avoided; meanwhile, aiming at the situation that when the results of detecting the stomach cancer CA19-9, CA24-2 and CEA of the patient by using the sera are negative, and the stomach cancer serological detection and identification kit provided by the invention is then used for carrying out the aided detection,, the false negative results of the serological detection can be effectively found, so that the life of the patient can be rescued in time.
Owner:ZHEJIANG PROVINCIAL HOSPITAL OF TRADITIONAL CHINESE MEDICINE
LAMP-based (loop-mediated isothermal amplification-based) visual fluorogenic and chromogenic genetic testing method for microorganisms
InactiveCN102586438AMeet the requirements for rapid detection of pathogenic microorganismsThe detection process is fastMicrobiological testing/measurementFluorescence/phosphorescenceMicroorganismFluorescence
The invention discloses an LAMP-based visual fluorogenic and chromogenic genetic testing method for microorganisms, which relates to a genetic testing method for microorganisms. The testing method includes the following steps: (1) buffered peptone water (BPW) is used for culturing a sample to be tested according to a national standard method for 4 hours; (2) a water boiling method is used for extracting DNA (deoxyribonucleic acid) from the sample to be tested; (3) the DNA is added into an LAMP reaction system, and the temperature of 65 DEG C is kept for 1 hour; (4) by means of comparison with a control group, the result of the sample to be tested is observed with naked eyes, and qualitative analysis is carried out on the sample. Compared with the conventional culture method, the genetic testing method has the advantages that: testing is rapid, only taking 5 hours, and plus the extraction of the sample DNA, testing takes less than 6 hours in total; the specificity is good, and the sensitivity is high; the steps are simple, and the repeatability is high; and a plurality of samples can be tested at the same time. The genetic testing method can qualitatively test microorganisms, and can take the place of the conventional culture method and the serological testing method which are used up to now.
Owner:WUHAN UNIV
Detection kit for distinguishing cow pathogenic mycobacteria infection from non-pathogenic mycobacteria infection and method thereof
ActiveCN101533018AStrong characteristicIncreased sensitivityHybrid peptidesMaterial analysisBCG immunizationMycobacterium Infections
The invention belongs to the field of immunodetection and relates to a detection kit for distinguishing cow pathogenic mycobacteria infection from non-pathogenic mycobacteria infection and a method thereof. The detection reagent comprises combined fusion protein rE6-M63-H70 used as a specific stimulation origin, the combined fusion protein can effectively stimulate sensitized peripheral blood lymphocyte cultured in vitro to release Gamma-interferon (IFN-Gamma) at a high level. The cow IFN-Gamma release test established by using the detection reagent rE6-M63-H70 combined fusion protein as the stimulation origin overcomes the insufficiencies of serology detection method and the IFN-Gamma release test with PPD as the stimulation origin, thus enjoying very high sensitivity and specificity and distinguishing cow pathogenic mycobacteria ( such as mycobacterium bovis) infection from non-pathogenic mycobacteria (such as mycobacterium avium or non-pathogenic mycobacteria) infection and even distinguishing the cow pathogenic mycobacteria infection from BGG immunity; therefore, the detection kit and the method of the invention can be effectively used to detect the clinical cow pathogenic mycobacteria infection.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
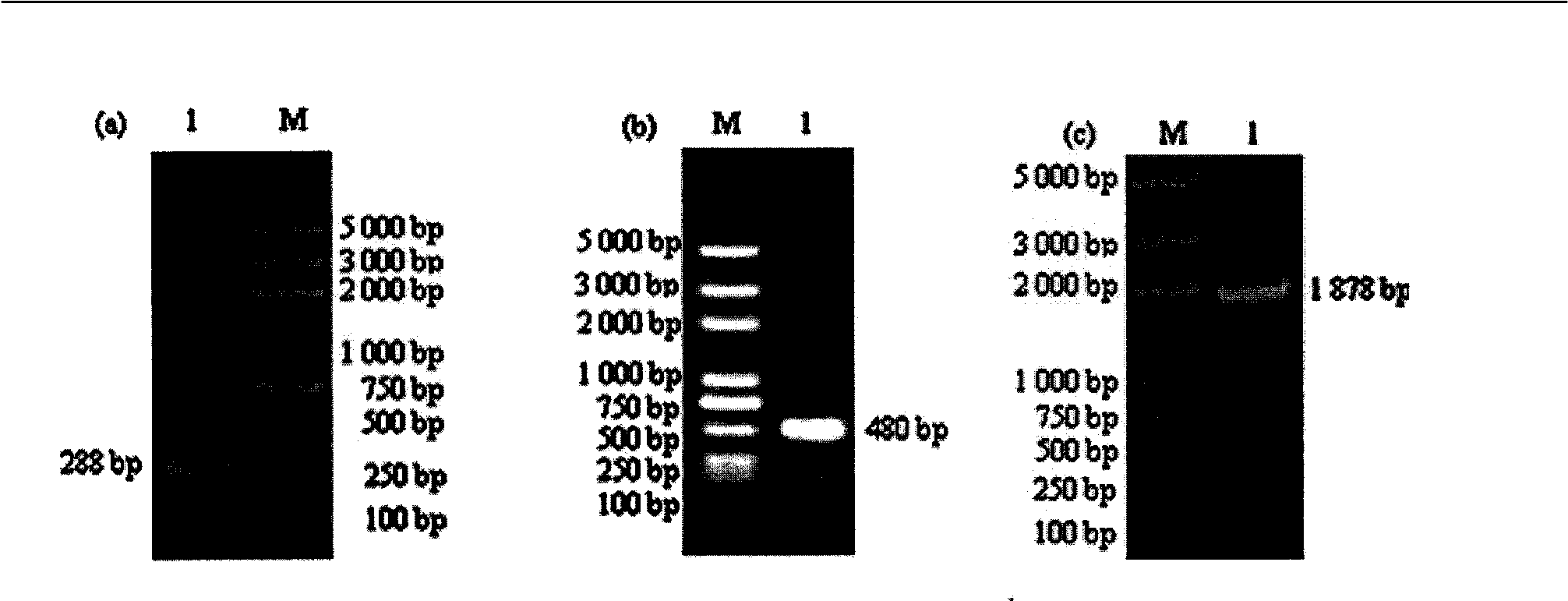
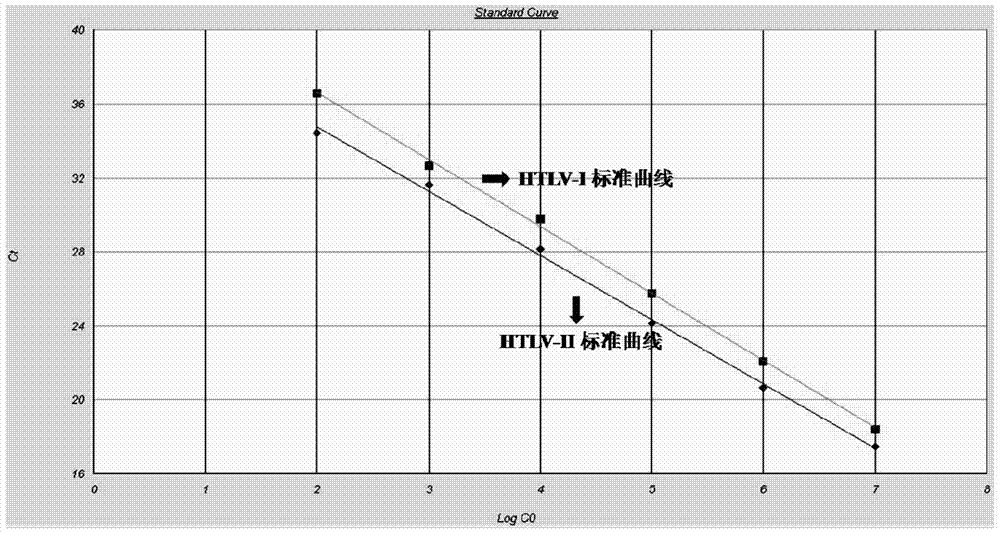
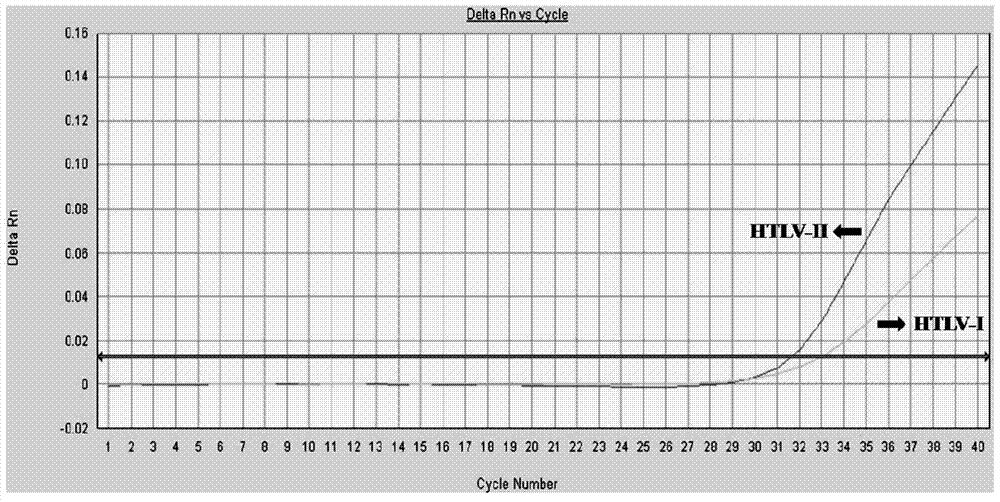
Primer and method for detecting HTLV (human T-lymphotropic virus)-I and HTLV-II proviruses in same pipe
ActiveCN103898239AHelp monitorLimit transmissionMicrobiological testing/measurementDNA/RNA fragmentationCarrying capacityRetroviral provirus
The invention provides a primer and a method for detecting HTLV (human T-lymphotropic virus)-I and HTLV-II proviruses. The primer comprises a specific primer and a probe for detecting an HTLV-I provirus and a specific primer and a probe for detecting an HTLV-II provirus, the two primers and the two probes are added in the same PCR (polymerase chain reaction) pipe according to a reasonable concentration ratio, and a sample is detected by means of optimized Real-time PCR reaction conditions. The detection method can be used for detecting whether HTLV infection exists before serological changes, thereby greatly shortening the window phase; meanwhile, compared with a conventional serological detection method, the detection method has the advantages of short detection period, high specificity, high accuracy, high sensitivity, little dependence on the conditions, low pollution risk and the like. The detection result is beneficial to monitor the change of the carrying capacity of the HTLV provirus of a patient and limit the propagation of the HTLV virus.
Owner:SHANGHAI ADICON CLINICAL LAB LNC
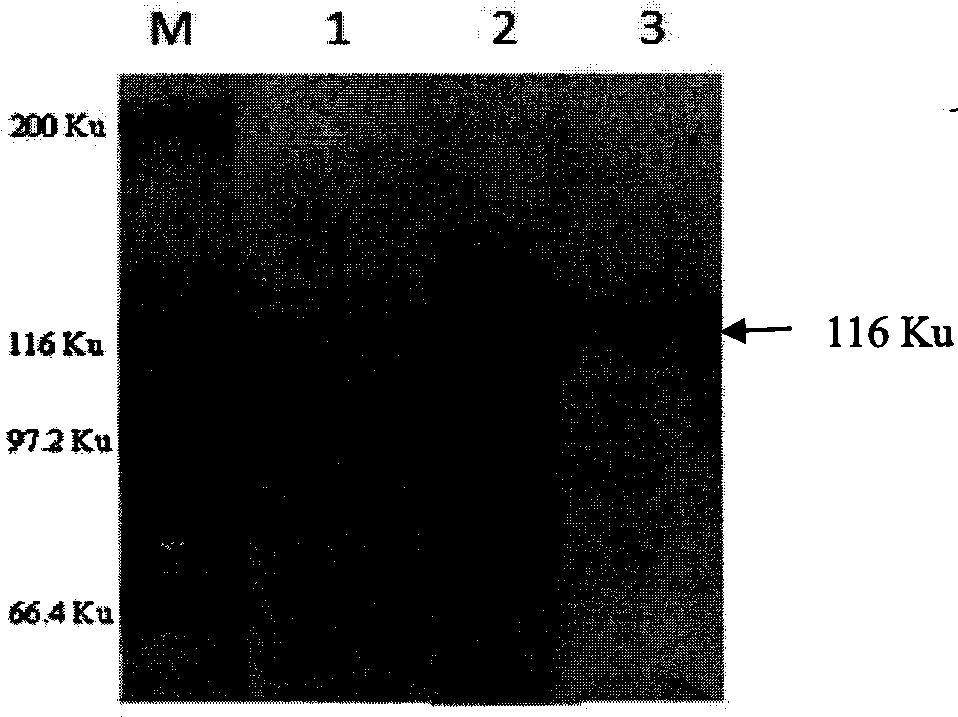
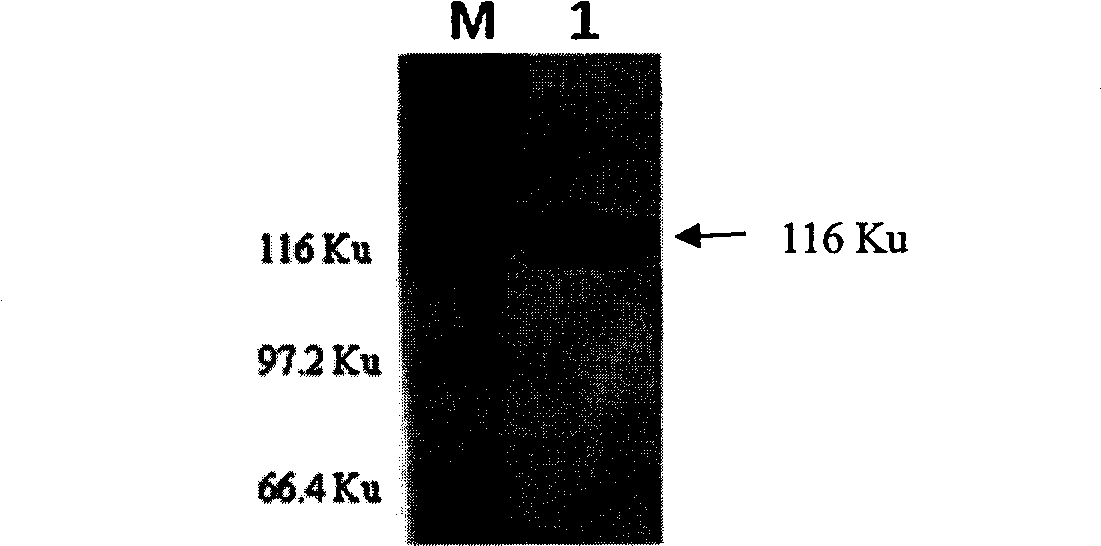
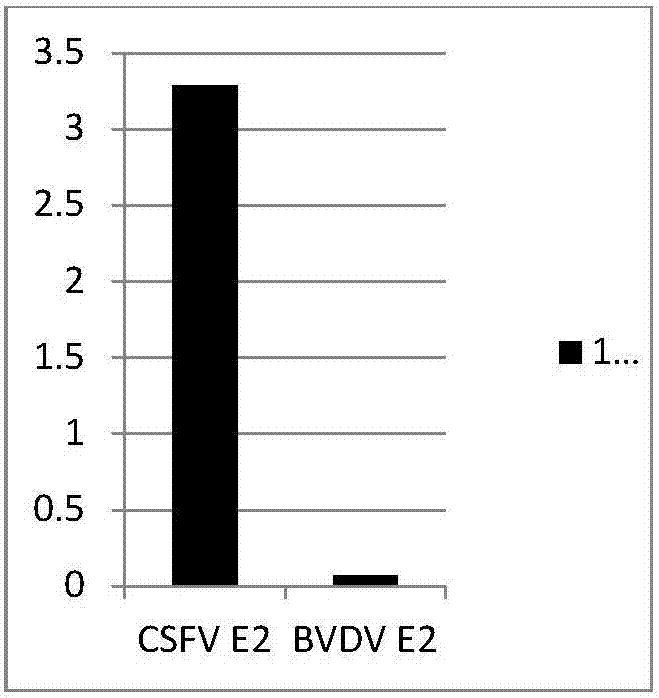
Cell strain of monoclonal antibodies of E2 protein resisting hog cholera virus and application thereof
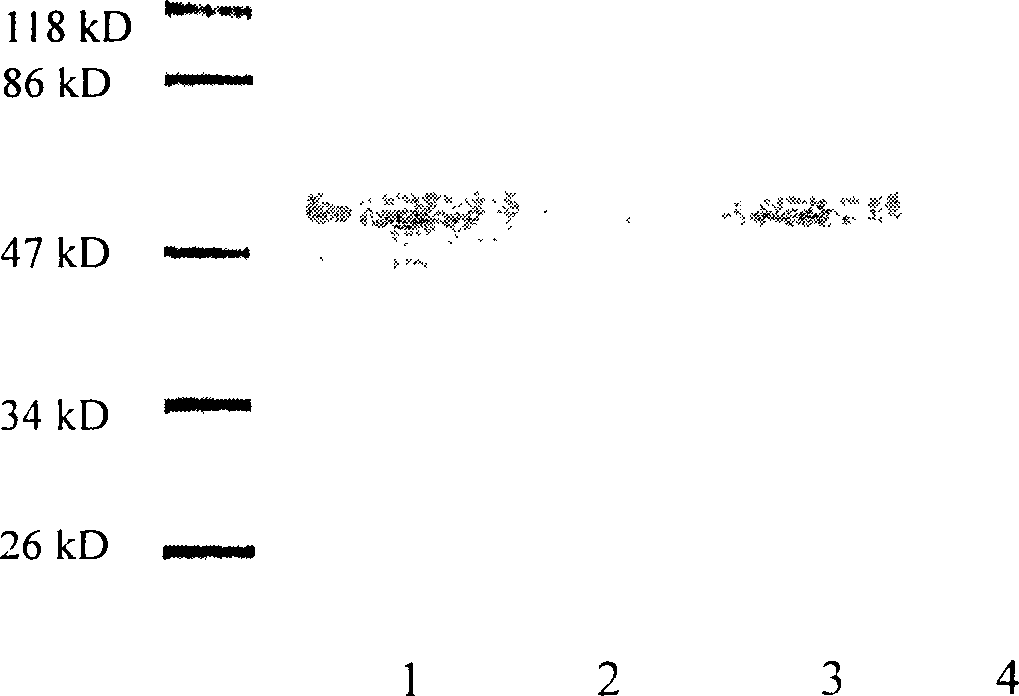
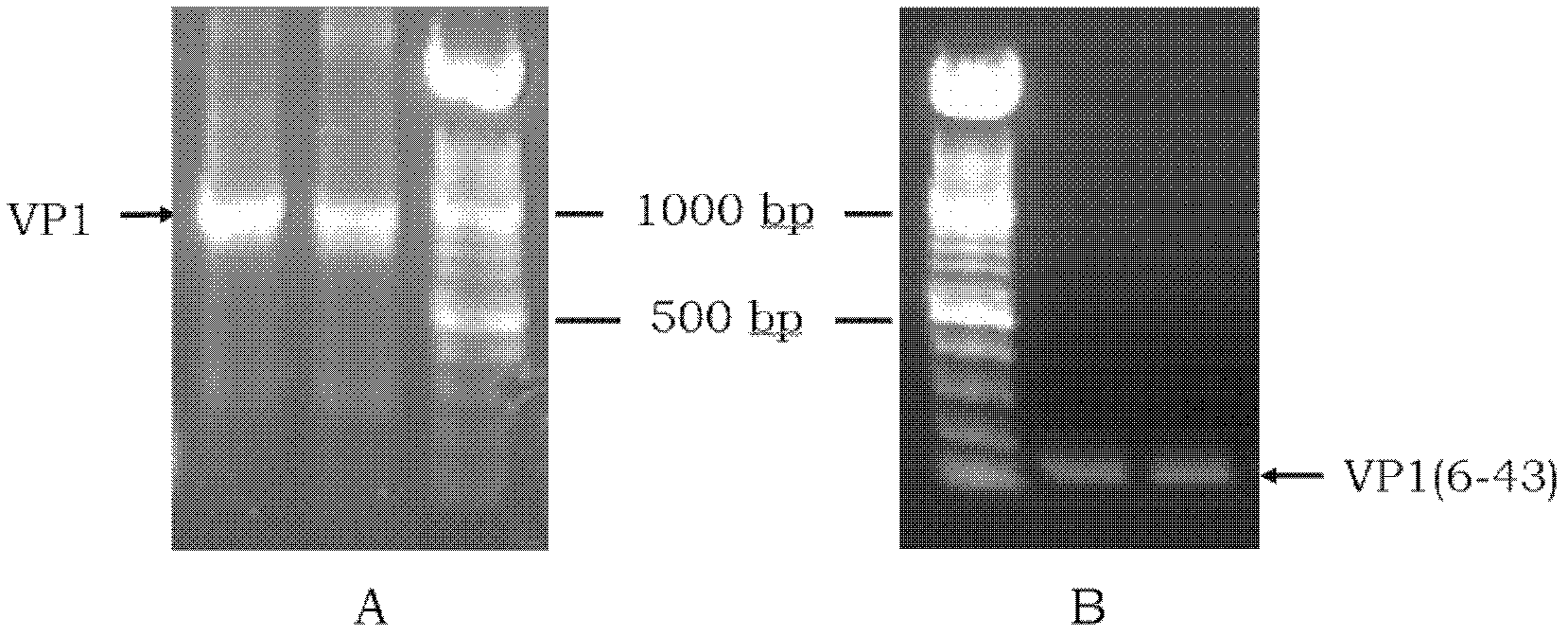
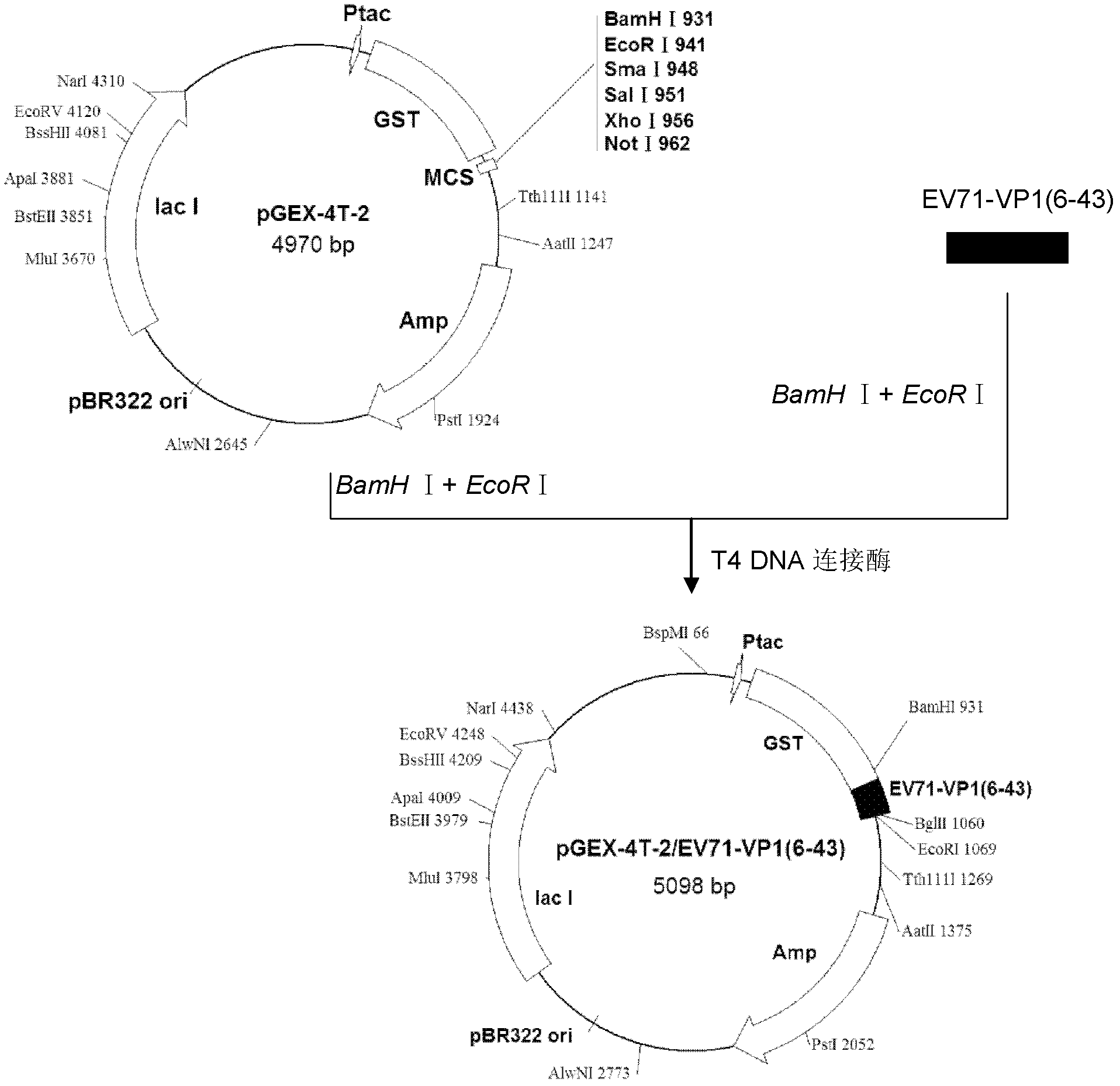
ActiveCN107058239AMicroorganism based processesImmunoglobulins against virusesElisa kitSerological assay
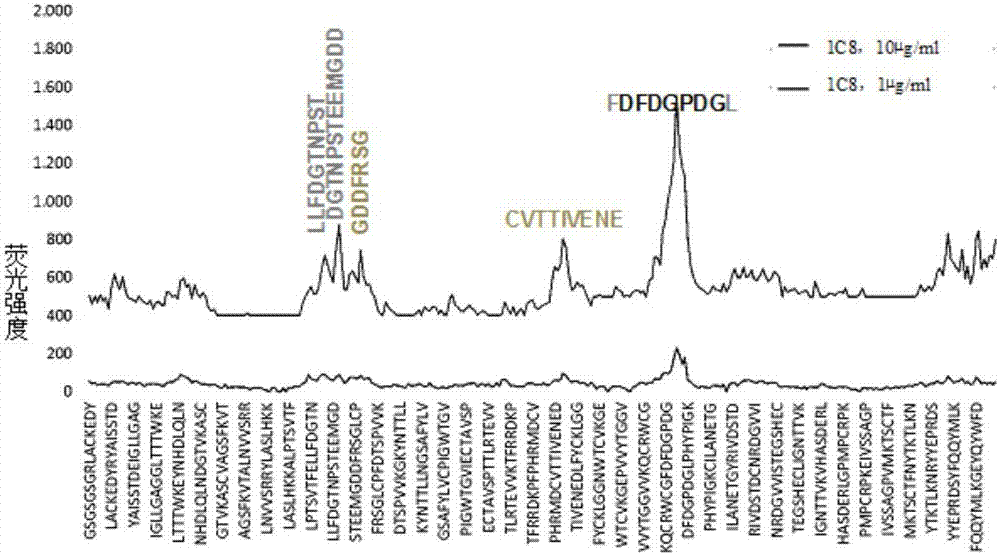
The invention relates to a cell strain of monoclonal antibodies of E2 protein resisting hog cholera virus and an application thereof. A hybridoma cell strain stably secreting the protein E2 resisting the hog cholera virus is obtained by screening, the monoclonal antibodies CSFV-1C8 secreted by the cell strain can generate specific reaction with the E2 protein of CSFV, and does not react with E2 protein of bovine viral diarrhoea / mucosal disease virus; and the monoclonal antibodies can recognize the E2 protein of the hog cholera virus specifically, and the epitope of recognition is FDFDGPDGL. The monoclonal antibodies and the epitope of the E2 protein of the hog cholera virus recognized by the monoclonal antibodies can be prepared into a reagent for detecting the CSFV, and thus laying foundation for establishing a serological detection method of antibodies of the hog cholera virus. A competitive ELISA kit for detecting the antibodies for the hog cholera and established by the invention has the advantages of specificity, sensitivity, simplicity and convenience in operation and the like and is suitable for large-scale screening of the antibodies for the hog cholera.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Dengue virus serology early diagnosis reagent
InactiveCN101962411AImprove featuresHigh sensitivityMicroorganism based processesFermentationSerodiagnosesSerological assay
The invention provides a dengue virus serology early diagnosis reagent, in particular, EIII structural domains of dengue viruses 1, 2, 3 and 4 are serially connected to obtain a recombinant fusion protein rEIII used for serodiagnosis of the dengue virus infection. In addition, the invention also provides an early diagnosis agent kit suitable for the dengue virus infection. The invention provides a new idea for the serodiagnosis of the dengue virus infection. The dengue virus infection can be effectively diagnosed by using the diagnosis reagent, especially, the Mac-ELISA (Enzyme Linked Immunosorbent Assay) diagnosis agent kit can be effectively used for the early serodiagnosis of the dengue virus infection. The diagnosis reagent has the advantages of better specificity and sensitivity and simple operation, and is suitable for being widely popularized and used.
Owner:中国疾病预防控制中心病毒病预防控制所
Universal and differential serologic assay for swine influenza virus
InactiveUS7892729B1Peptide/protein ingredientsMicrobiological testing/measurementSerological assayAntibody
A universal and differential assay kit for the detection of antibodies to swine influenza virus (SIV) in a biological sample comprising SIV non-structural 1 (NS1) protein and SIV nucleoprotein (NP); a universal and differential assay method for detecting antibodies to SIV in a biological sample comprising assaying the biological sample for the presence of an antibody to SIV NS1 protein and an antibody to SIV NP; primers; and fusion proteins.
Owner:IOWA STATE UNIV RES FOUND
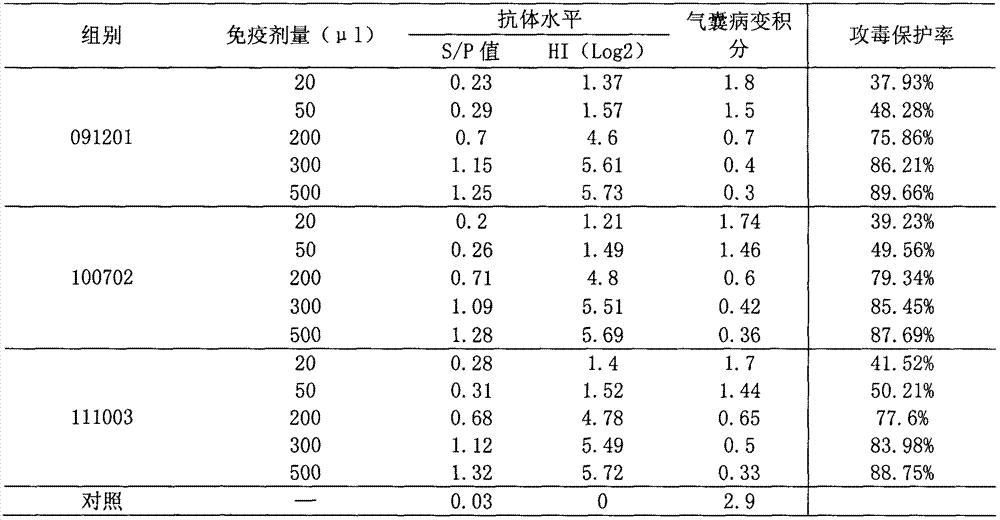
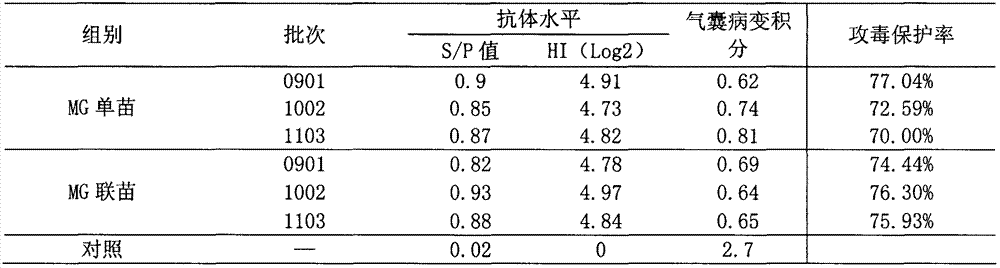
Efficacy test method of mycoplasma gallisepticum inactivated vaccine and application thereof
The invention relates to the technical field of animal biological products and discloses an efficacy test method of mycoplasma gallisepticum inactivated vaccine. Simultaneously, the efficacy test method disclosed by the invention can also be applied to quality control of the vaccine, in particular comprising the determination of immune toxicity attack protection rate and vaccine quality standard, measurement of immunization deadline and clinical monitoring. Experiments prove that the animal toxicity attack test is replaced by a serology detection method (comprising ELISA (Enzyme-Linked Immuno Sorbent Assay) and HI (Hemagglutination Inbition)); the efficacy test method is simple, convenient, rapid, accurate in result and good in repeatability and specificity; due to the establishment of the efficacy test method, subjectivity for checking airbag pathological change integrals after attacking toxicity is reduced; toxicity dispersion is avoided; a basis for establishing a rational immune program is also provided; and the efficacy test method has general popularization significance.
Owner:兆丰华生物科技(南京)有限公司
Hybridoma cell strain secreting monoclonal antibody against banana bunchy top virus and application of monoclonal antibody thereof
ActiveCN103911351AAccurate detectionSensitive detectionImmunoglobulins against virusesTissue cultureAntibody typesBALB/c
The invention discloses a hybridoma cell strain secreting a monoclonal antibody against banana bunchy top virus, and an application of the monoclonal antibody thereof. A BALB / c mouse is immunized with purified banana bunchy top virus (BBTV) virions as antigens, and a hybridoma cell strain 22E3 which can be subcultured stably and secretes a monoclonal antibody against BBTV is obtained through cell fusion, screening, and cloning, and the accession number is CGMCC No. 8780. The 22E3 monoclonal antibody has an ascetic indirect ELISA titer of up to 10<-7>, and the antibody type and subtype are IgG1, kappa chain. Specific reaction can be carried out between the monoclonal antibody and BBTV. A dot-ELISA method for detecting BBTV in bananas established with the 22F3 monoclonal antibody can detect the virus even when a diseased leaf is diluted by 1:320 times (w / v, g / mL). The acquisition of the hybridoma cell secreting monoclonal antibody against BBTV and its monoclonal antibody, and the establishment of a related serological detection method provide technical and material support for the diagnosis, prediction and scientific prevention and control of the viral disease.
Owner:ZHEJIANG UNIV
Polypeptide-ELISA kit for detecting novel coronavirus S protein unique antibody
PendingCN112213497AStrong specificityHigh purityBiological testingImmunoassaysElisa kitHorse radish peroxidase
The invention discloses a polypeptide enzyme-linked immunosorbent assay kit for detecting a novel coronavirus surface spinous process protein S unique specific antibody. The kit comprises an elisa plate coated with SARS-CoV2 surface spinous process protein S unique specificity dominant linear B cell antigen polypeptide, a sample diluent, negative control serum, positive control serum, a horse radish peroxidase labeled antibody, a concentrated washing solution, an enzyme substrate solution and a stop solution. The kit disclosed by the invention is high in specificity and good in repeatability,can be simply and conveniently used for detecting the novel coronavirus unique antibody, reduces false positive, can be used for large-scale serological detection and epidemiological investigation, and evaluates the infection condition of the novel coronavirus.
Owner:ZHEJIANG MEDICAL COLLEGE
Chemical luminescence immune assay determination reagent kit for rubella virus IgM antibody and preparation method thereof
InactiveCN101368960ASensitive highStrong specificityChemiluminescene/bioluminescenceIgm antibodyEpidemiologic survey
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Indirect ELISA kit for detecting porcine transfusion transmitted virus 2 (TTV2) antibody
InactiveCN103235121AFast detection methodThe detection method is simpleMaterial analysisTransfusion transmitted virusPorcine serum
The invention relates to an indirect ELISA kit for detecting a porcine transfusion transmitted virus 2 (TTV2) antibody and belongs to the field of biotechnology. The invention comprises antigen recombinant protein preparation, indirect ELISA establishing, and use of determination standard and clinical serological test. Through pcoldI prokaryotic expression vectors, a gene engineering bacterium pcoldI-ORF1 for expression of a porcine TTV2ORF1 truncated protein is constructed and the expressed antigen recombinant protein is purified and is used as an antigen so that an indirect ELISA detection method is established. The indirect ELISA detection method is used for detecting a TTV2 antibody level of porcine serum, has good repeatability and high singularity, can be used for porcine TTV2 serology investigation and is a fast and simple serological test method for prevention, treatment and prevalence state control of porcine transfusion transmitted diseases. The indirect ELISA detection method utilizes the porcine TTV2ORF1 recombinant protein to detect the porcine TTV2 antibody first in China.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
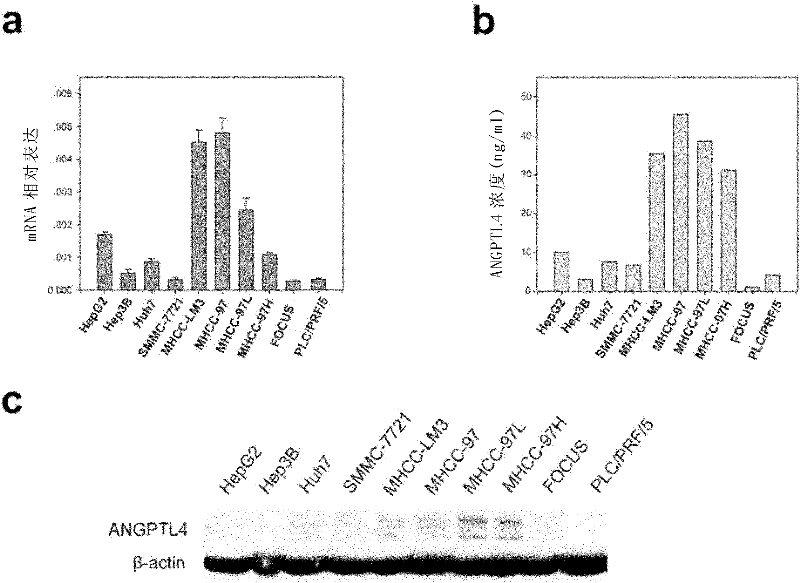
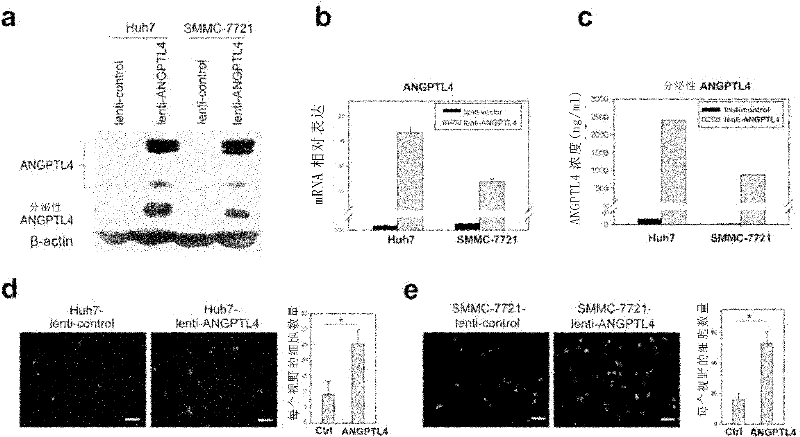
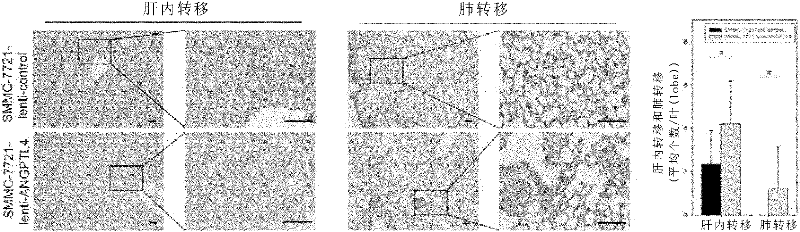
ANGPTL4 as a marker of liver cancer metastasis by serological detection and application thereof
The invention relates to ANGPTL4 as a marker of liver cancer metastasis by serological detection and an application thereof. Specifically, the invention provides an application of angiopoietin-like protein 4(ANGPTL4 protein) in (a) the preparation of a diagnostic reagent or kit for the detection of liver cancer metastasis and (b) the preparation of a diagnostic reagent or kit for serological detection of liver cancer. The invention also provides a related detection kit. The invention also provides a method for inhibiting hepatoma cell metastasis by the use of an antagonist of the ANGPTL 4 protein.
Owner:SHANGHAI INST OF ONCOLOGY
Screening and application of active tuberculosis diagnosis molecules
ActiveCN106589082AHigh sensitivityStrong specificityAntibody mimetics/scaffoldsImmunoglobulins against bacteriaHigh-Throughput Screening MethodsSerological assay
The invention provides screening and application of active tuberculosis diagnosis molecules. In particular, the invention discloses high throughput screening of mycobacterium tuberculosis's important antigens and application thereof in active tuberculosis diagnosis. Based on a high throughput functional protein screening technology of GST fusion expression, 92 positive antigens recognizable by tuberculosis patients' serum are screened out, wherein 14 antigens present strongly positive reaction. Active tuberculosis serologic detection shows that the positive antigens have high sensitivity and specificity in detection of active tuberculosis. Specifically, TBGP1, TBGP2, TBGP3, TBGP4, TBGP5 and TBGP6 6 proteins form an antigen combination for tuberculosis serologic detection, and laboratory verification shows that the combination has high sensitivity and specificity in detection of mycobacterium tuberculosis, and has application value in tuberculosis diagnosis and monitoring.
Owner:TONGJI UNIV
Targeted database creation kit for plurality of pathogens
ActiveCN108517350ARapid differential diagnosisNarrow down the scope of identificationMicrobiological testing/measurementDNA/RNA fragmentationProtozoaSerological assay
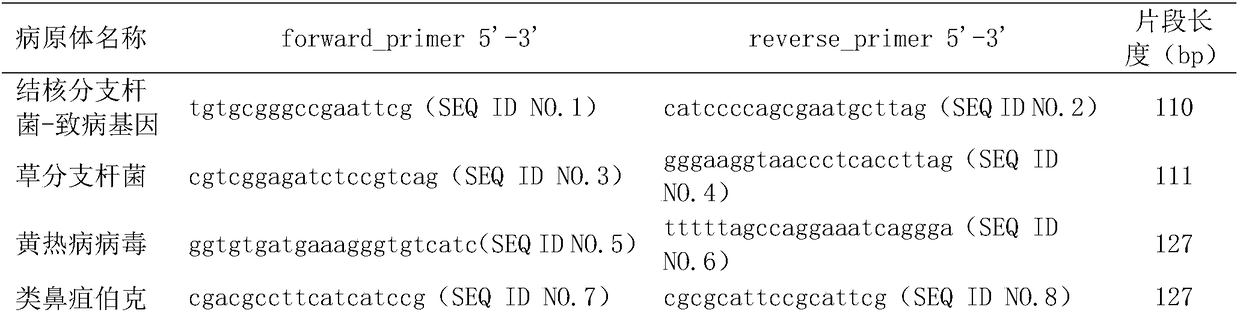
The invention relates to a targeted database creation kit for 43 pathogens. The pathogens comprise bacteria, viruses, fungi and protozoa. Targeted detection primer sequences of the pathogens are shownas SEQ ID NO. 1 to 86. According to the kit, targeted database creation can be performed on pathogen nucleic acids in an early infection stage for rapid diagnosis of the pathogens after next-generation sequencing, and directions are provided for subsequent pathogen isolation and identification, serologic detection, clinical diagnosis and symptomatic treatment.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
Kit for detecting Golgi protein 73 by magnetic particle chemiluminescence method and preparation method thereof
PendingCN113533734ARelieve painShort reaction timeChemiluminescene/bioluminescenceBiological testingStreptavidinGolgi protein
The invention discloses a kit for detecting Golgi protein 73 by a magnetic particle chemiluminescence method and a preparation method thereof, and belongs to the technical field of immunoassay. The kit comprises a streptavidin-coated magnetic particle suspension solution, a biotinylation labeled GP73 antibody solution, an alkaline phosphatase labeled Golgi protein 73 antibody solution, a calibrator, a washing solution and a substrate solution. The problems that pathological specimens need to be obtained in histological detection, the GP73 state of a patient who does not obtain cancer tissue pathology cannot be judged, and repeated detection is difficult are solved. Serological detection has the characteristics of convenience, repeatable measurement, quantification, objectivity, real-time detection and the like. The method has the advantages that the reaction time is short, radioactive contamination is avoided, the experimental operation process is simplified, the binding between the biotin and the streptavidin has extremely high affinity, and the reaction has high specificity. Therefore, non-specific interference is not increased while the sensitivity is improved.
Owner:TAIZHOU ZECEN BIOTECH CO LTD
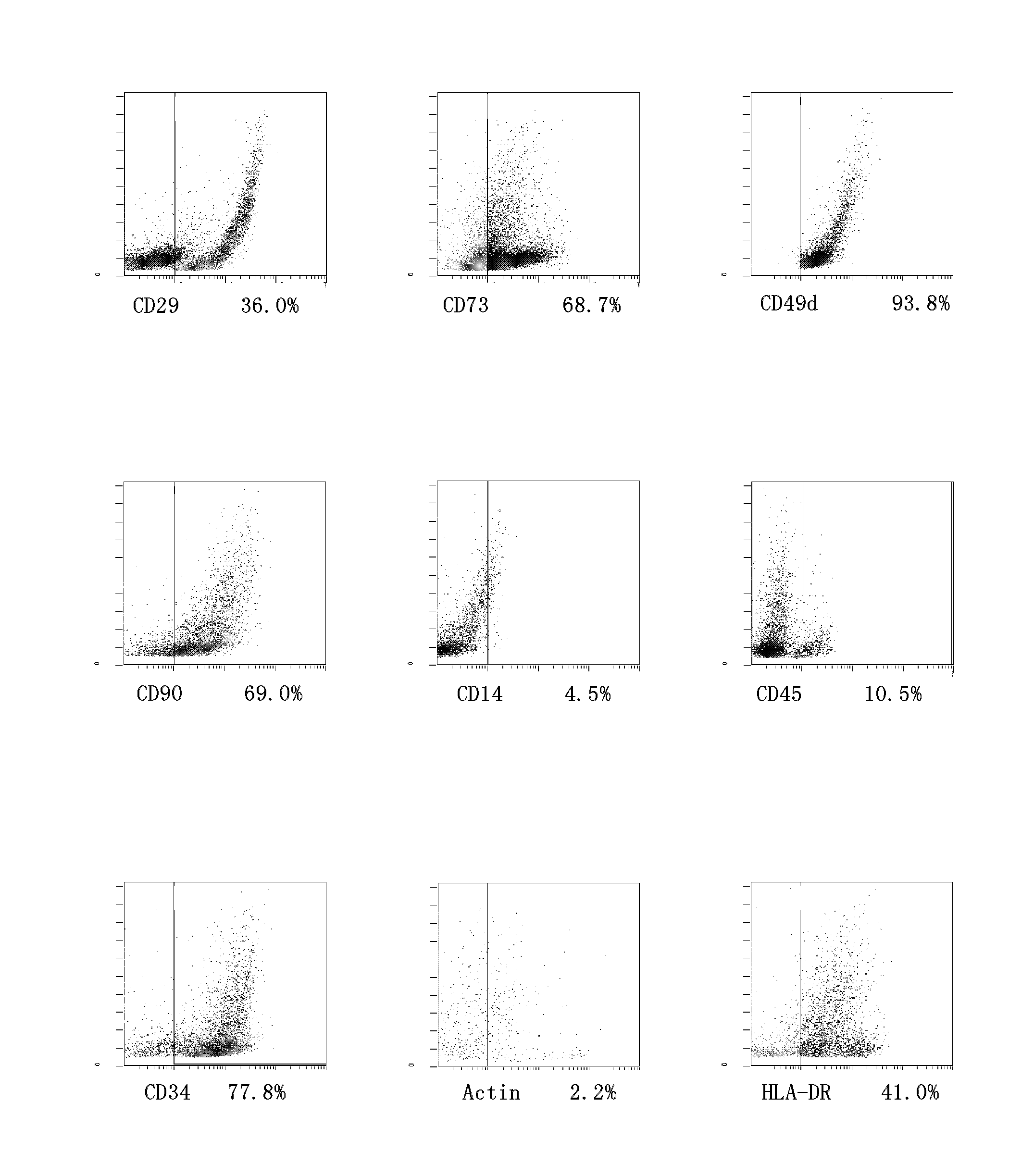
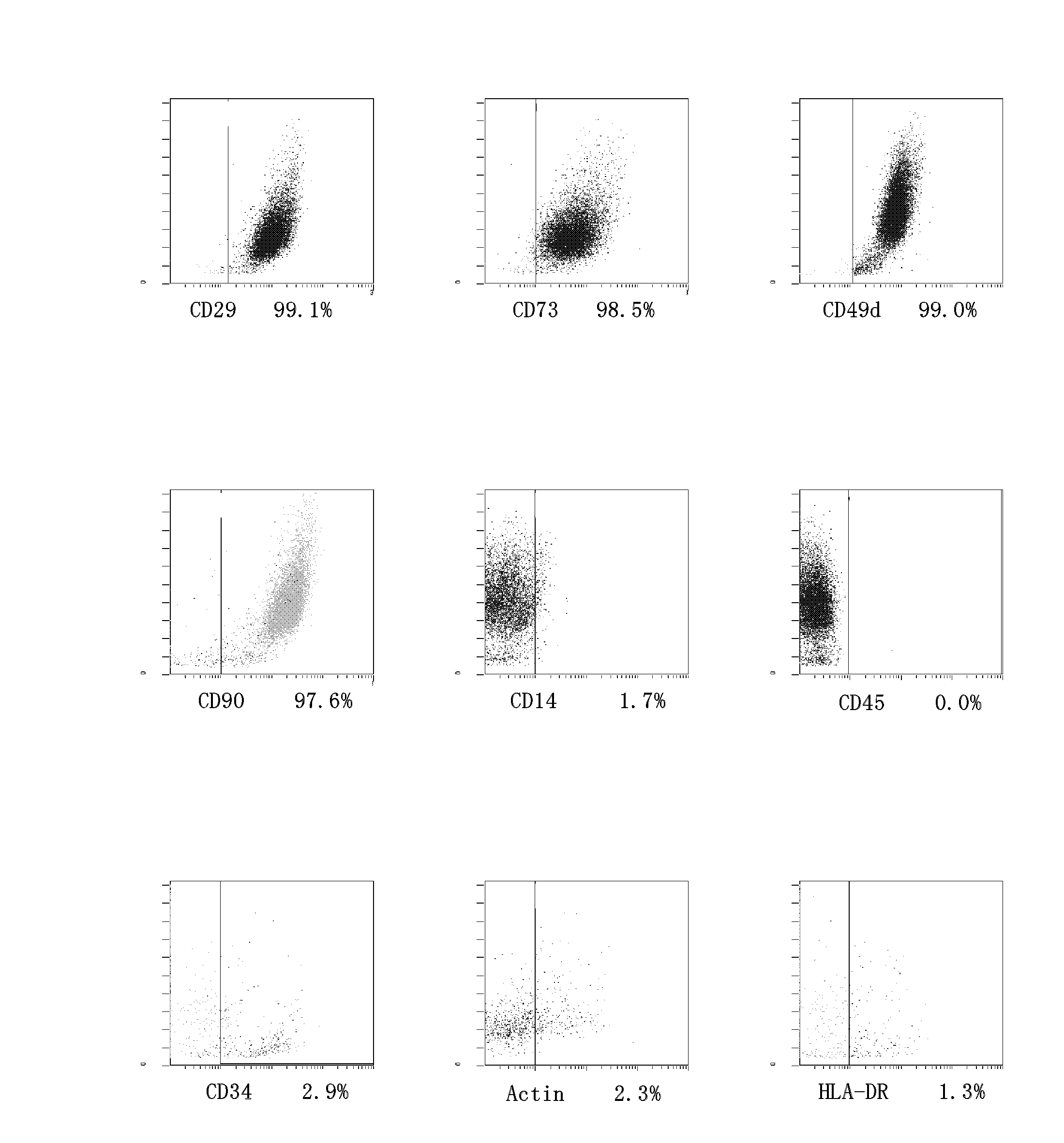
Evaluation system of anti-aging effect of autologous adipose-derived stem cell
ActiveCN102486475AGenetic material ingredientsMammal material medical ingredientsPhysiologyLow-density lipoprotein
The invention provides an evaluation system of anti-aging effect of autologous adipose-derived stem cell. The system comprises that serology detection is carried out on an individual re-transfused with adipose-derived stem cells a period after a re-transfusion; and the serology detection comprises one or more indexes selected from the group of vitamin D, high density lipoprotein, low density lipoprotein, hormothyrin, testosterone and estradiol. The evaluation system of the invention can objectively evaluate anti-aging effect of adipose derived stem cell and has advantages of accuracy, rapidness and thrift.
Owner:CELLULAR BIOMEDICINE GRP SHANGHAI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com