Dengue virus serology early diagnosis reagent
A dengue virus and diagnostic reagent technology, applied in the field of serological detection of dengue virus, can solve the problems of expensive kits, difficult to popularize and apply at the grassroots level, and not suitable for early diagnosis of dengue virus infection, and achieve good specificity and sensitivity , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
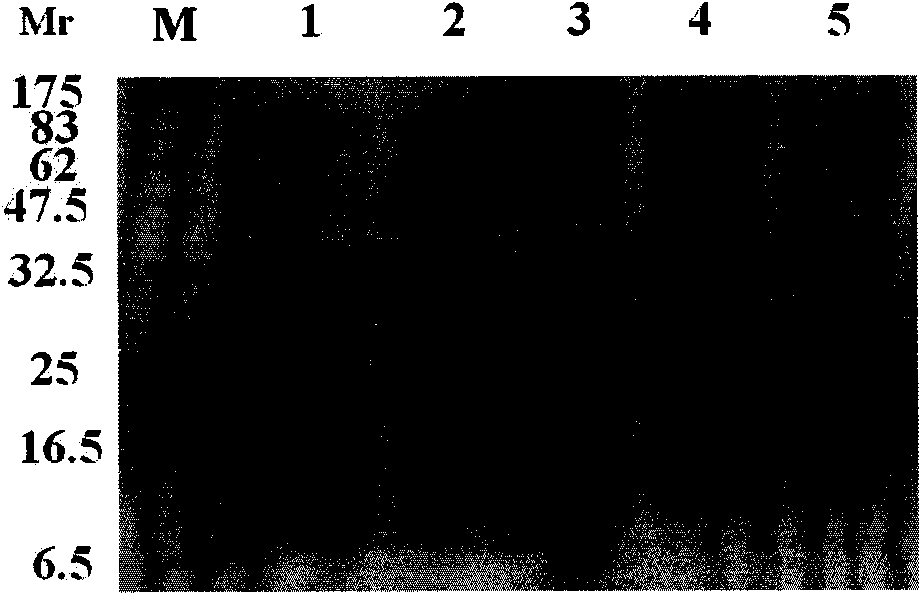
[0040] Embodiment 1 Expression of recombinant dengue virus E protein domain III and rEIII
[0041] Materials and methods
[0042] 1. Construction of DV type 1-4 EIII domain and rEIII fusion recombinant expression vector
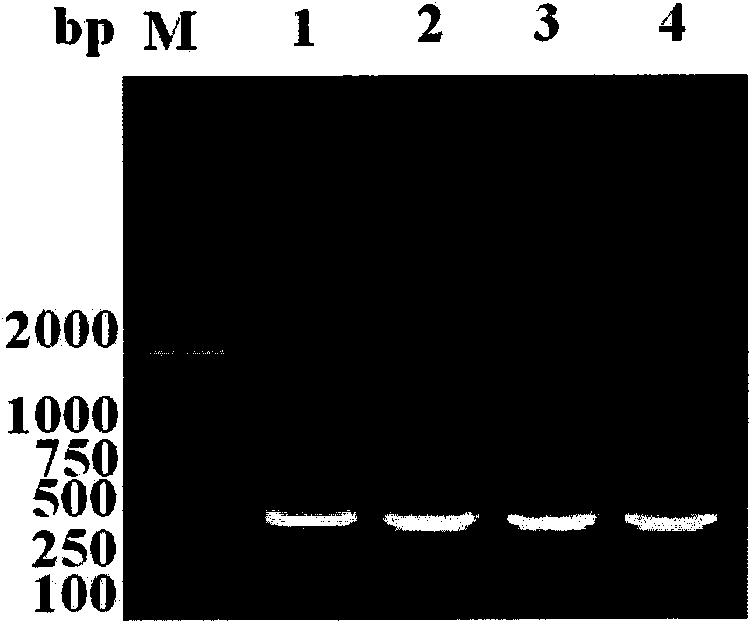
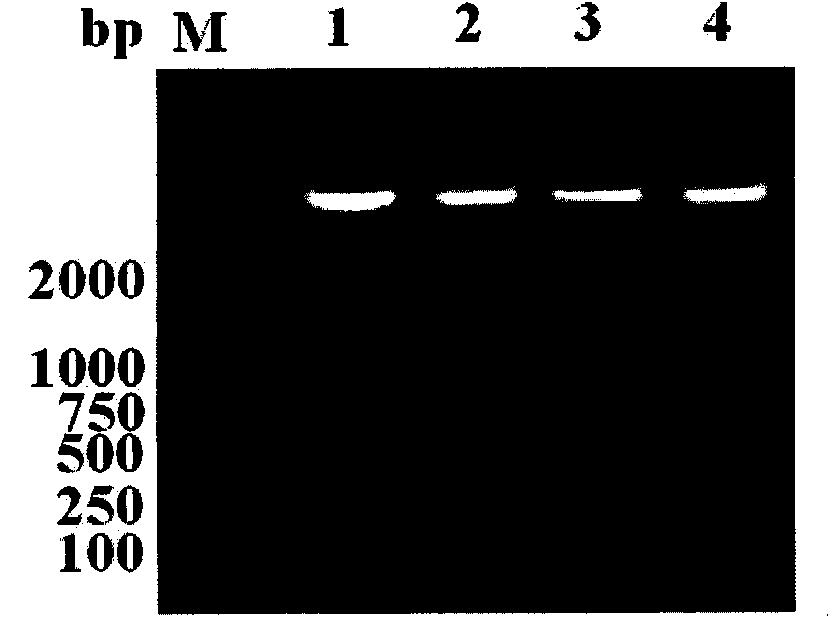
[0043] 1.1 Acquisition of DV1-4 type EIII region gene fragment and rEIII coding gene fragment
[0044] In this example, the EIII gene of DV types 1-4 was obtained by extracting dengue virus RNA and reverse transcription. Of course, those skilled in the art can artificially synthesize the EIII region gene fragments of DV1-4 types and the rEIII code by using the disclosed EIII domain sequence. Gene fragment.
[0045] 1.1.1 Acquisition of cDNA
[0046] C6 / 36 cells were cultured in 1640 growth medium (containing 30% eagle's, 10% fetal bovine serum, 1% double antibody, 1% glutamine), and cultured at 28°C, 5% CO2 until the cells grew into a monolayer, Inoculate 1-4 standard strains of dengue virus (type 1 GZ01 / 95 strain, type 2 ZS01 / 01 strain, type 3 H87 strain...
Embodiment 2
[0179] Application of embodiment 2 recombinant dengue virus EIII protein and rEIII in dengue virus fever serum detection
[0180] In this example, the Mac-ELISA method was established by using the dengue virus type 1-4 EIII recombinant protein and rEIII fusion protein prepared in Example 1 to explore the application of the recombinant dengue virus EIII protein and rEIII in the detection of dengue virus fever serum.
[0181] Experimental Materials
[0182] 1. Coating antibody: goat anti-human IgM-μ chain antibody (SIGMA, USA).
[0183] 2. Serum to be tested: dengue fever monitoring serum (collected from Guangdong, Guangxi, Fujian and other places), healthy human serum, epidemic hemorrhagic fever patient serum (Virus Disease Institute, Chinese Center for Disease Control and Prevention), Japanese encephalitis patient IgM positive serum (China Institute of Virology, Academy of Preventive Medicine).
[0184] 3. Enzyme-labeled antigen: HRP-labeled recombinant EIII protein and reco...
Embodiment 3
[0259] The composition of embodiment 3 dengue virus infection early diagnosis kits
[0260] According to Example 2, this example provides a detection kit for early diagnosis of dengue virus infection. The kit consists of the following parts:
[0261] 1) A microtiter plate coated with goat anti-human IgM-μ chain antibody;
[0262] 2) HRP labeled rEIII antigen;
[0263] 3) ELISA washing solution (PBST): 0.05% Tween20, 1X PBS, pH7.4;
[0264] 4) Substrates of HRP:
[0265] Solution A: 5mg tetramethylbenzidine (TMB) is dissolved in 5mL dimethyl sulfoxide (DMSO). Dissolve in acetate buffer (0.2mol / L.pH 5.8-6.0) according to 1:4;
[0266] Liquid B: 0.03% H 2 o 2
[0267] 5) ELISA reaction termination solution: 2moI / L H 2 SO 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com