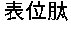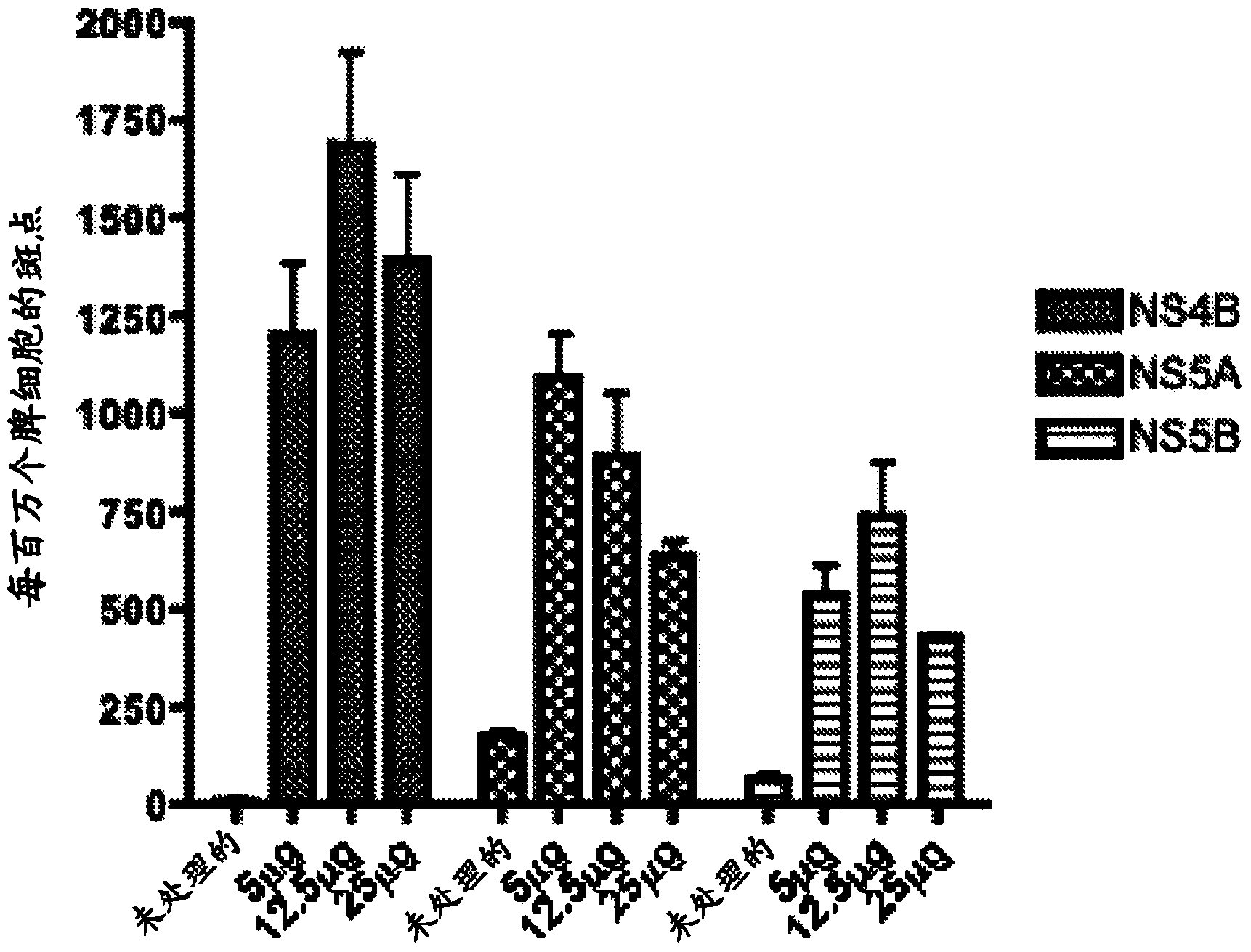Patents
Literature
38 results about "Hcv vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
There Is No Available Vaccination for HCV. So far, there is no vaccine that you or your child can take to protect against HCV. There are many strains of the virus and they mutate (change genetic characteristics) rapidly. This makes it difficult to identify a particular virus for which a vaccine could be developed.
Hcv vaccines and methods for using the same
ActiveUS20120034256A1Improved immunogenic targetOrganic active ingredientsSsRNA viruses positive-senseRecombinant vaccinesAttenuated vaccine
Owner:INOVIO PHARMA +1
Hepatitis C virus nucleic acid vaccine
ActiveUS9056090B2Reduce capacityReduce loadSsRNA viruses positive-senseViral antigen ingredientsMolecular biologyNucleic Acid Vaccines
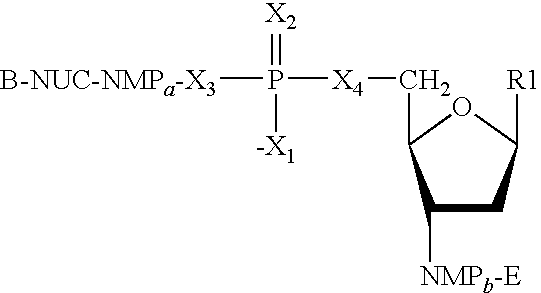
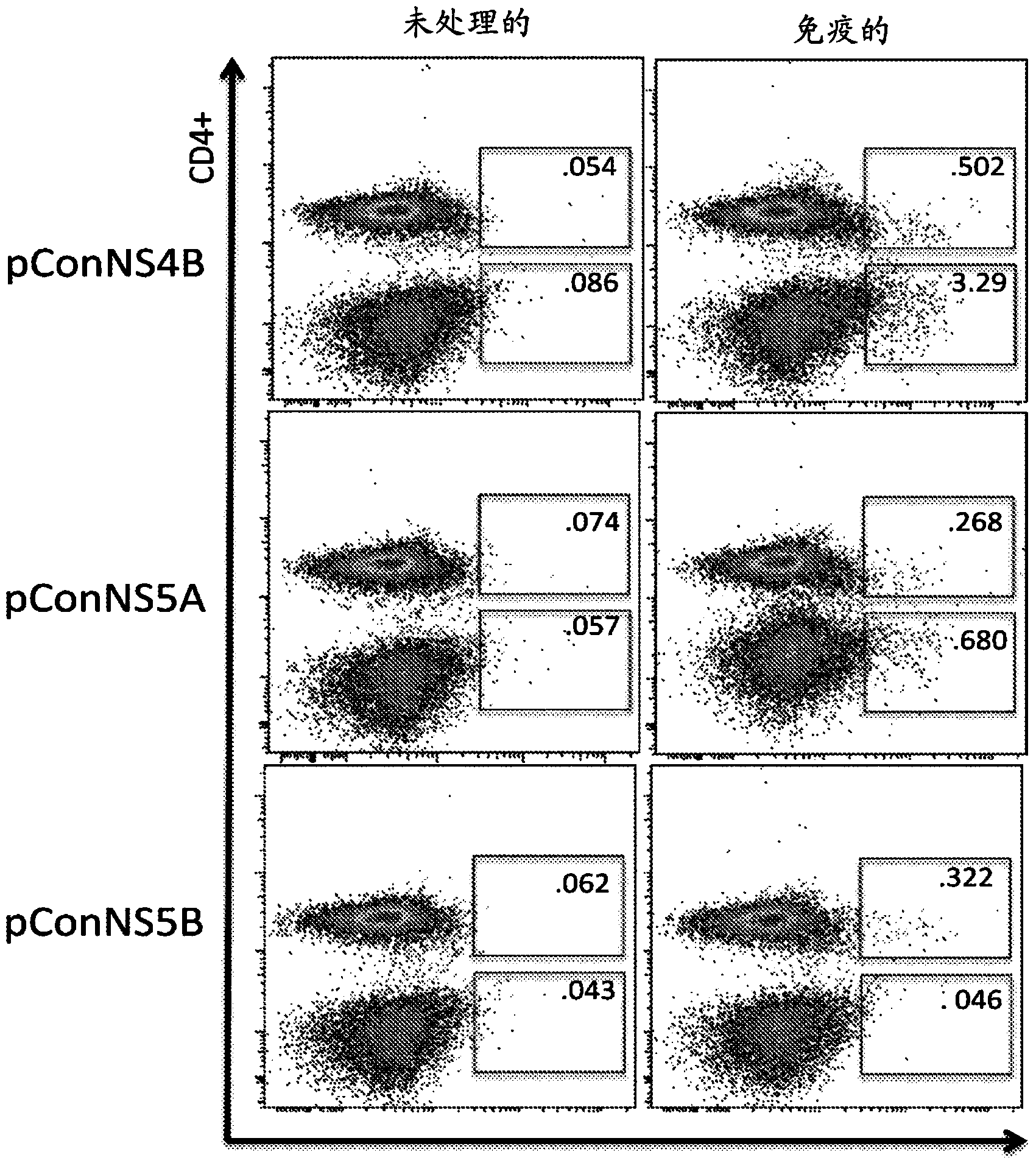
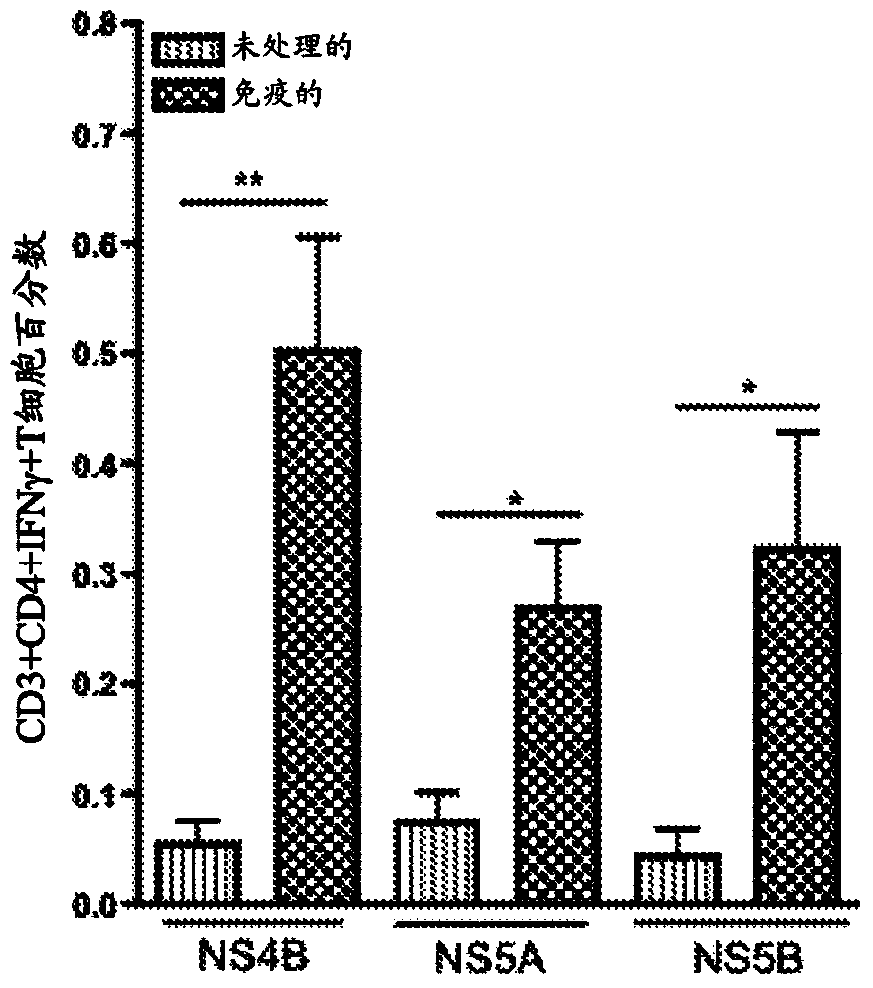
The present invention features nucleic acid constructs that can be used as a HCV nucleic acid vaccine, vaccine component, or in the production of a HCV vaccine. Described constructs include those: (1) encoding for a chimeric HCV polypeptide containing a NS3-4A region based on a first HCV strain and an NS3-NS4A-NS4B-NS5A or an NS3-NS4A-NS4B-NS5A-NS5B region based on a second strain; and (2) a chimpanzee based adenovector encoding an HCV polypeptide.
Owner:MSD ITAL
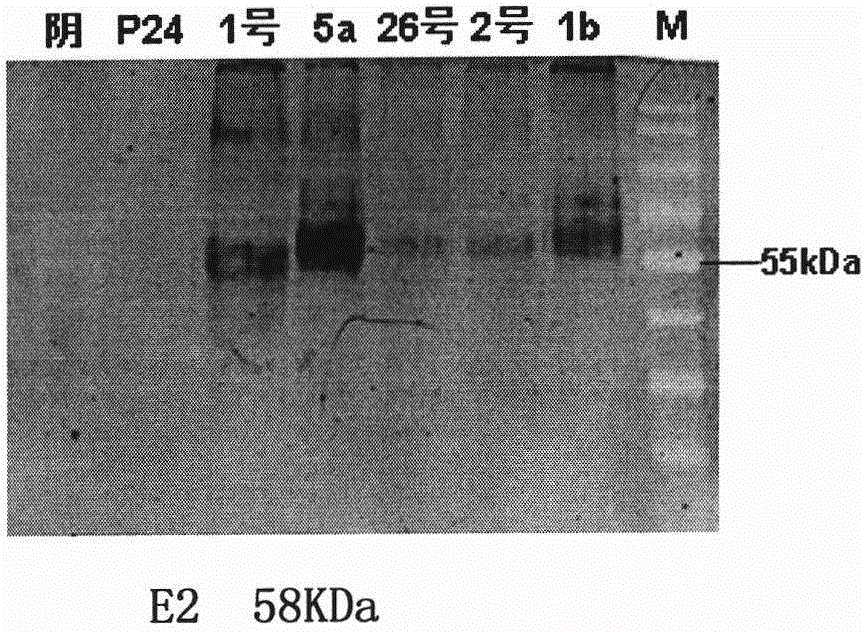
Method for expressing hepatitis C virus envelope protein E2 by mammal cell with high efficient secretion
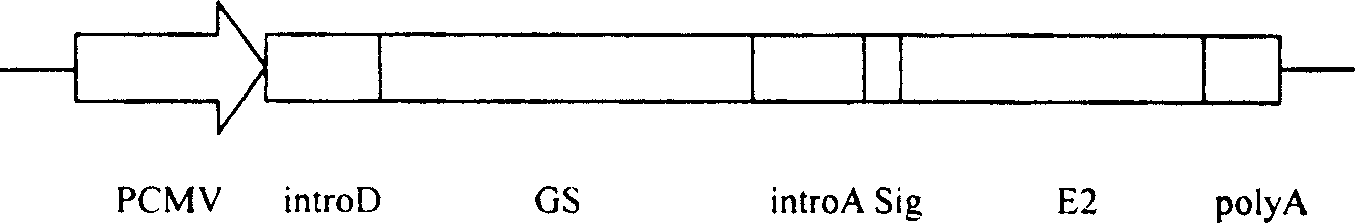
The present invention relates to biomedicine technology. HCV envelope protein E2 mediates the combination between HCV and target cell and is key protein relates to HCV infection and one kind of low expression protein hard to obtain in gene recombination process. The present invention aims at provides high efficiency secretion method for mammal to express HCV envelope protein E2. The method constitutes one new type of mammal cell expressing plasmid, which expresses target gene E2 protein in high level while expressing glutamine synthetase as the screening marker in low level. The present invention makes it possible to batch prepare recombinant HCV envelope protein E2 with the natural biological function and antigenicity of HCV envelope protein, lays the foundation for development of serological HCV infection detecting reagent and HCV vaccine, and provides HCV molecular virological research with important material.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
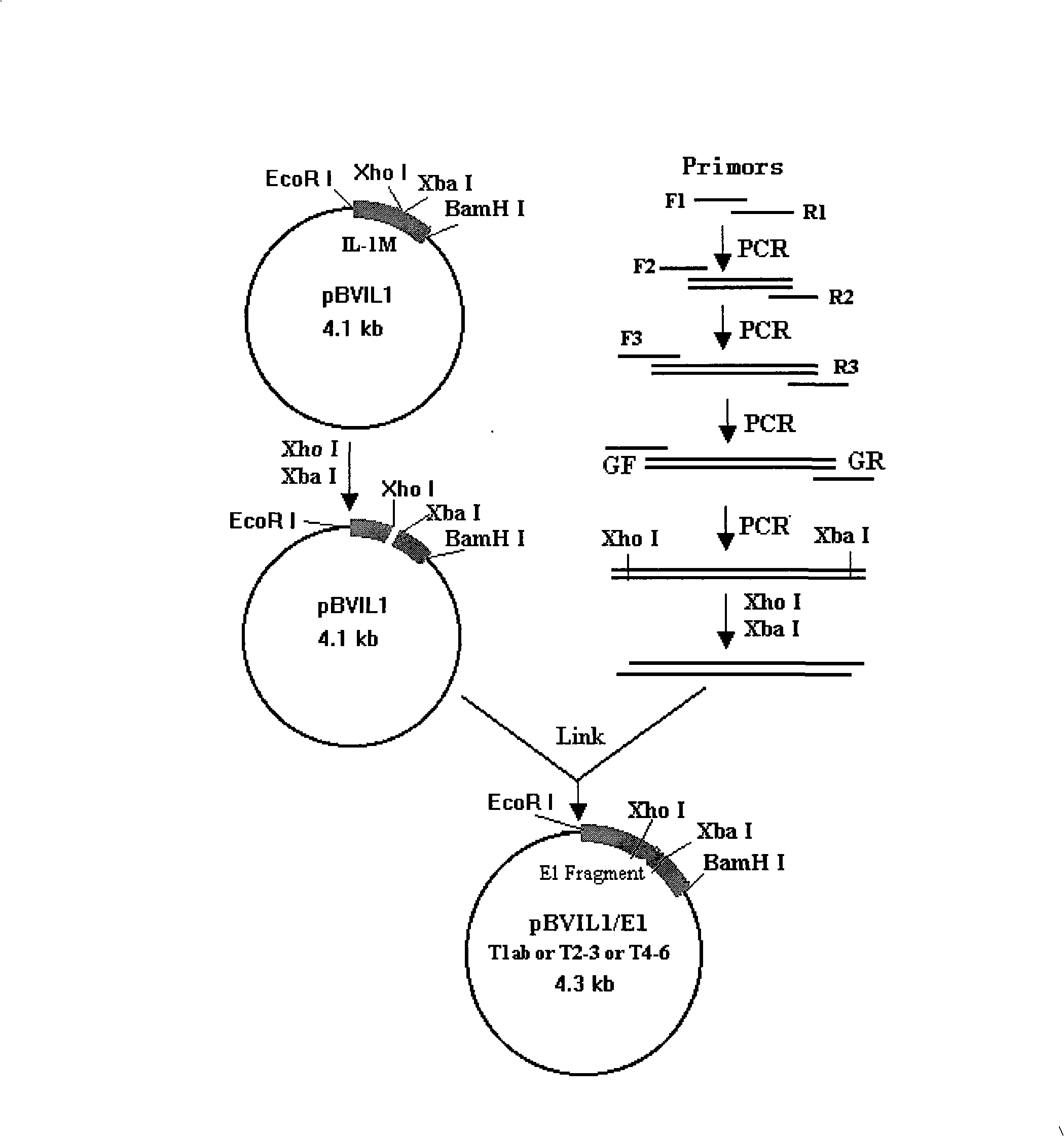
Multi-type HCV-E1 epitope complex immunogen, encoding gene and application thereof
InactiveCN101319011AOvercoming the Variation ProblemBacteriaDigestive systemEscherichia coliDiagnostic agent
The invention discloses an epitope antigen gene containing E1 in different HCV types and a prokaryotic expression plasmid pBVIL1 / E1s-PADRE of general T auxilliary cell epitope gene. Engineering bacteria obtained by transforming the plasmid into escherichia coli can efficiently express recombinant multi-type HCV / E1 epitope complex antigens through induction culture, as well as the application of the multi-type HCV / E1 epitope complex recombinant protein of the expression in the preparation of HCV vaccines and diagnostic agent. Through the strategy of selecting a multi-type epitope antigen, complex immunogen containing HCV 1a, 1b, 2, 3, 4 and 6 type HCV / E1 main neutralizing epitopes is successfully constructed, which can be used as a component part of HCV vaccine immunogen so as to overcome the variation problem of HCV; meanwhile, the antigen can also be used for the antigen of HCV diagnostic agent.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA +1
Method for porcine circovirus production and pcv2 vaccines
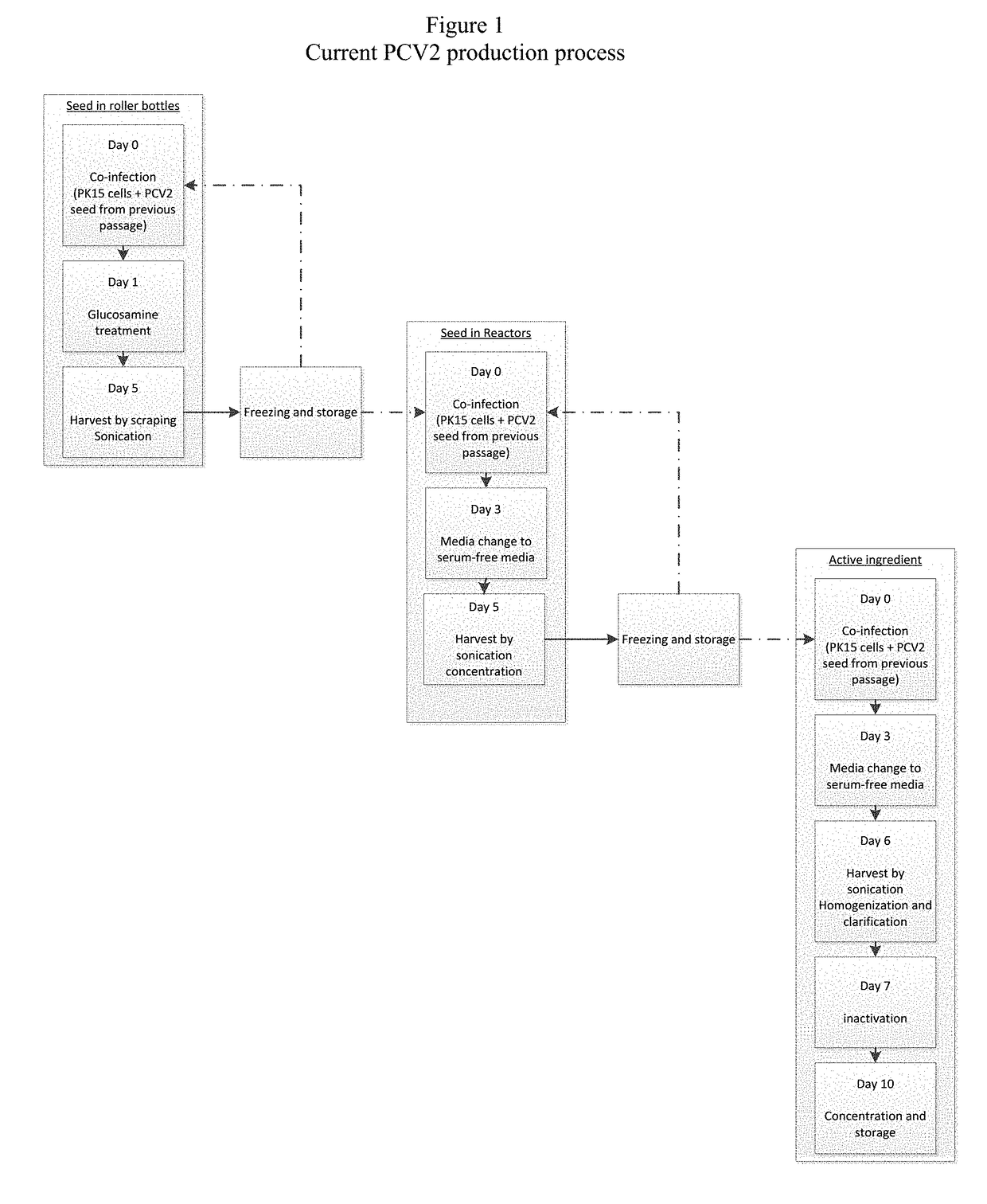
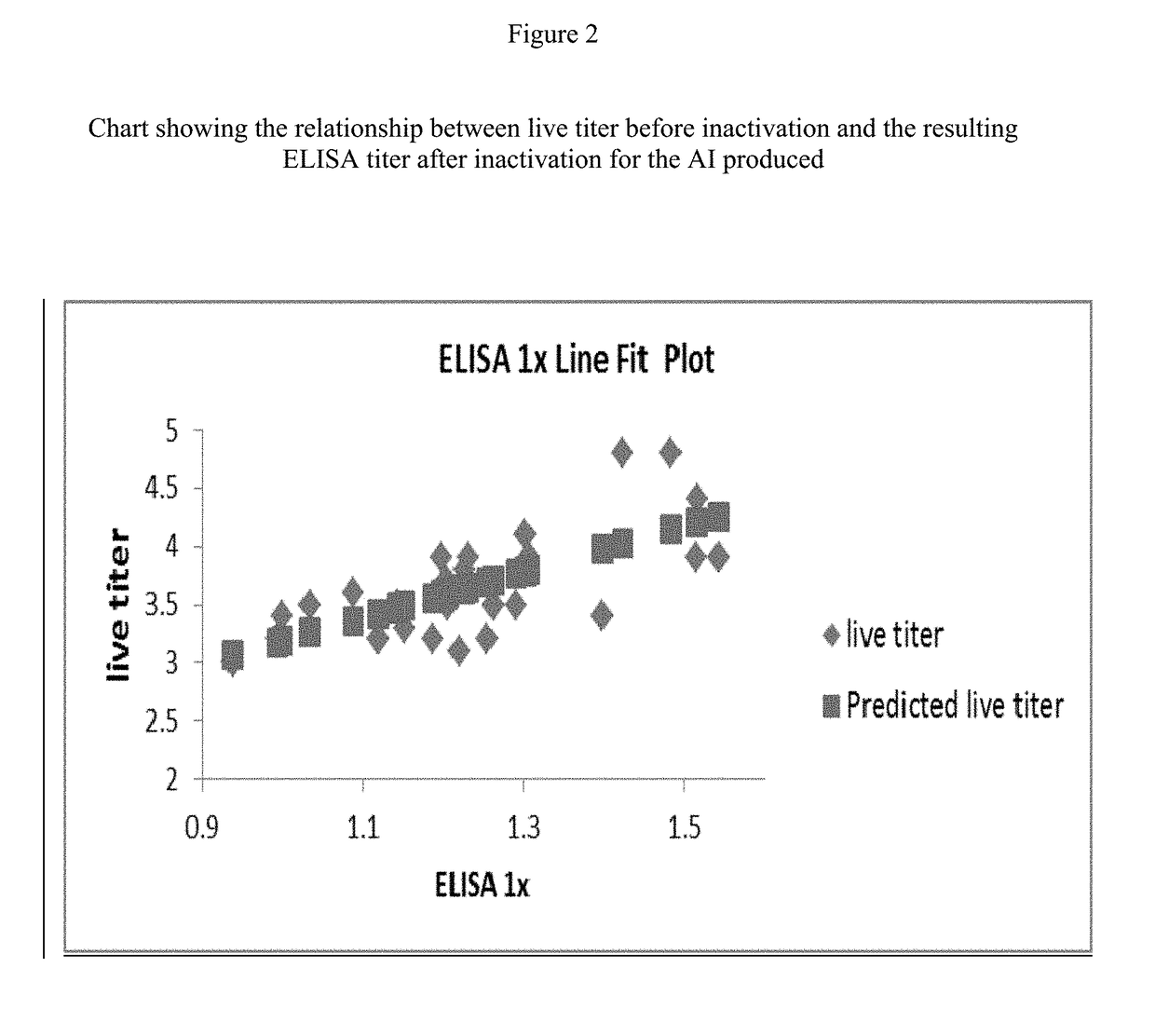
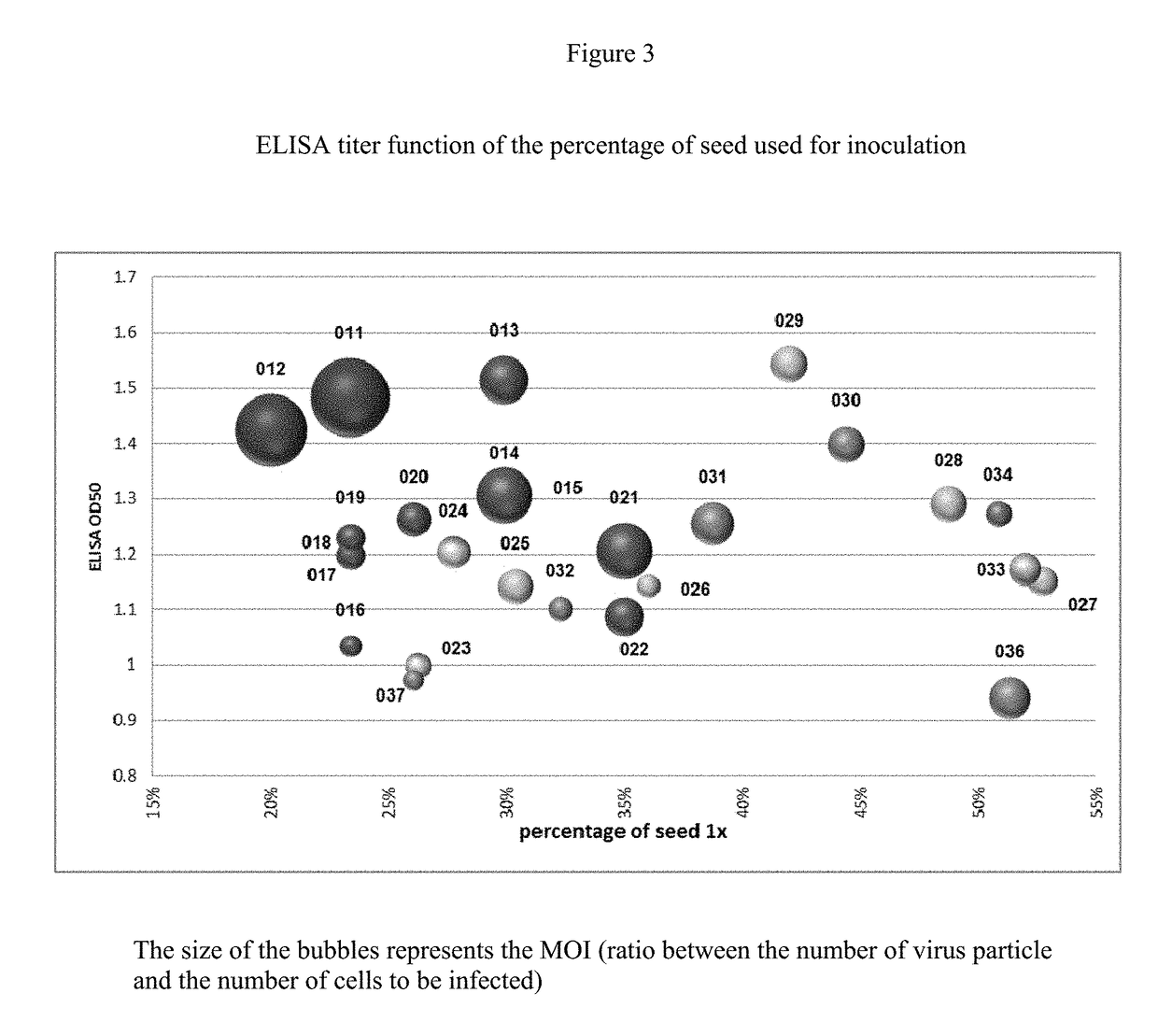
ActiveUS20160333322A1Speeding up cultivationIncrease virus yieldViral antigen ingredientsVeterinary vaccinePorcine circovirusHcv vaccine
This invention relates to a method for rapid production of PCV2 such that an optimum yield of the virus can be obtained. This invention also relates to the vaccine useful against PCV2 which includes the PCV2 propagated using such a method.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
HCV composite multi-epitope transgene plant oral vaccine
InactiveCN101070544AOptimal immune doseOptimal number of immunizationsGenetic material ingredientsDigestive systemAntigenTransgene
This invention is one kind of hepatitis C virus (HCV) compound multi-epi-position transgene edibility plant vaccine. States the HCV vaccine is the HCV compound multi-epi-position antigen gene, in the plant expresses in the host-based system to express obtains, and states the plant expresses the host is edibility. This invention provided one kind highly effective moderately-priced in view of the same type, different HCV separation not to be able to provide the overlapping protective function the effective HCV vaccine.
Owner:QINGDAO UNIV OF SCI & TECH
Hcv vaccines
InactiveUS20090130135A1Extended shelf lifeUseful and effectiveSsRNA viruses positive-senseViral antigen ingredientsEpitopeVirology
Owner:INTERCELL AG
Chimeric HCV (hepatitis C virus) vaccine taking influenza virus as carrier and preparation method thereof
ActiveCN103757032AProtection from flu virusProtection from HCVSsRNA viruses negative-senseSsRNA viruses positive-senseDNAInfluenza C Virus
The invention discloses a chimeric HCV (hepatitis C virus) vaccine taking an influenza virus as a carrier and a preparation method thereof. The invention discloses DNA (deoxyribonucleic acid) molecules as shown in SEQ ID NO.1. The chimeric HCV vaccine disclosed by the invention is a bivalent vaccine, and a foundation is laid for achieving dual or multiple purposes of the chimeric HCV vaccine taking the influenza virus as the carrier.
Owner:302 MILITARY HOSPITAL OF CHINA
Hepatitis C virus vaccine composition
InactiveCN102596244AAvoid infectionSsRNA viruses positive-senseAntibody mimetics/scaffoldsAntigenAdjuvant
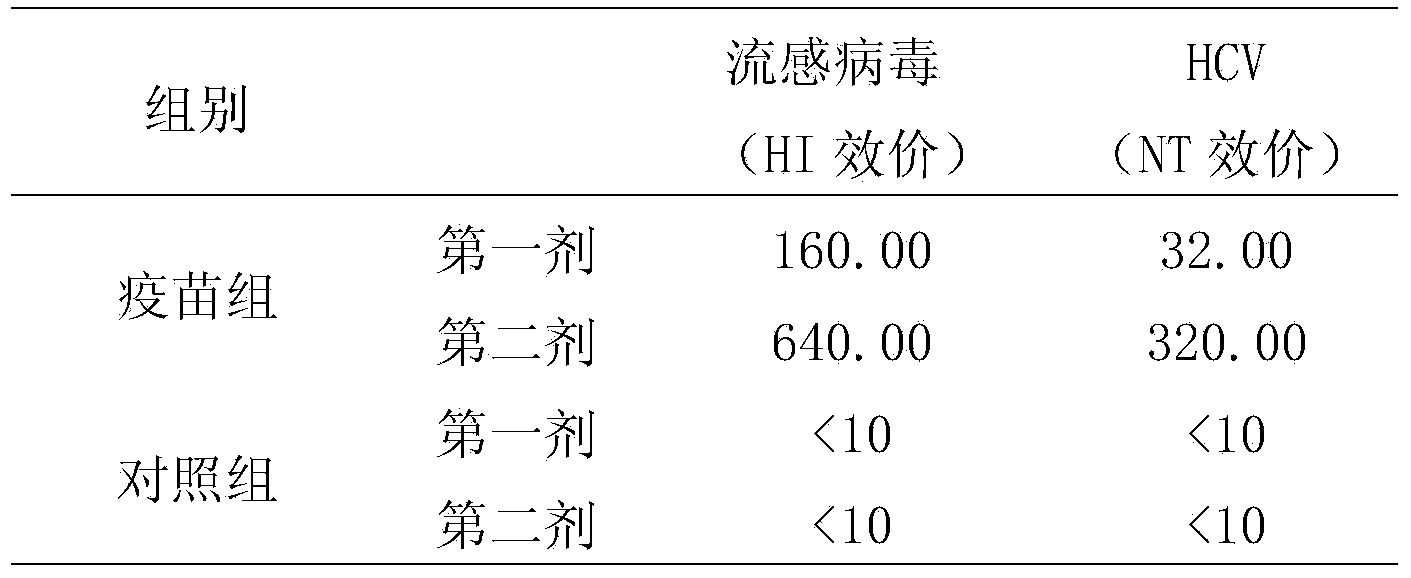
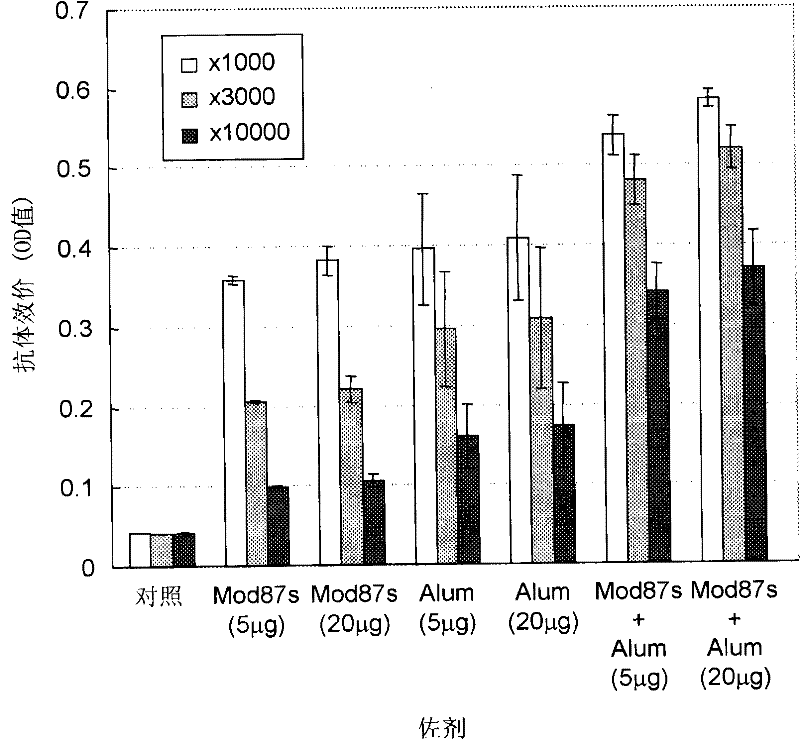
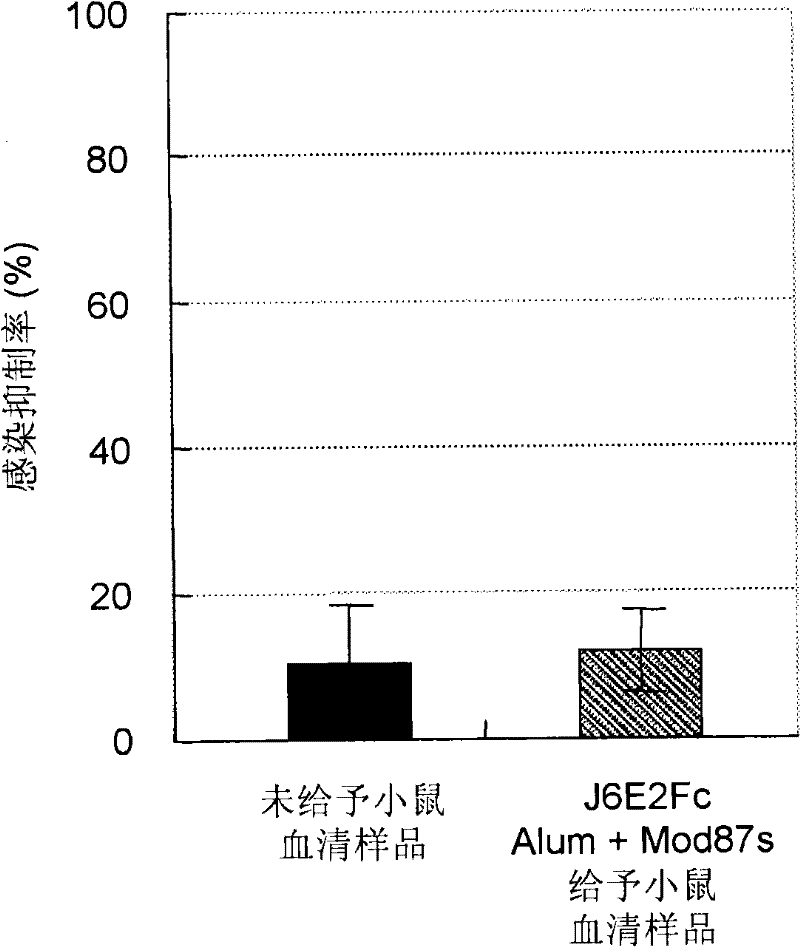
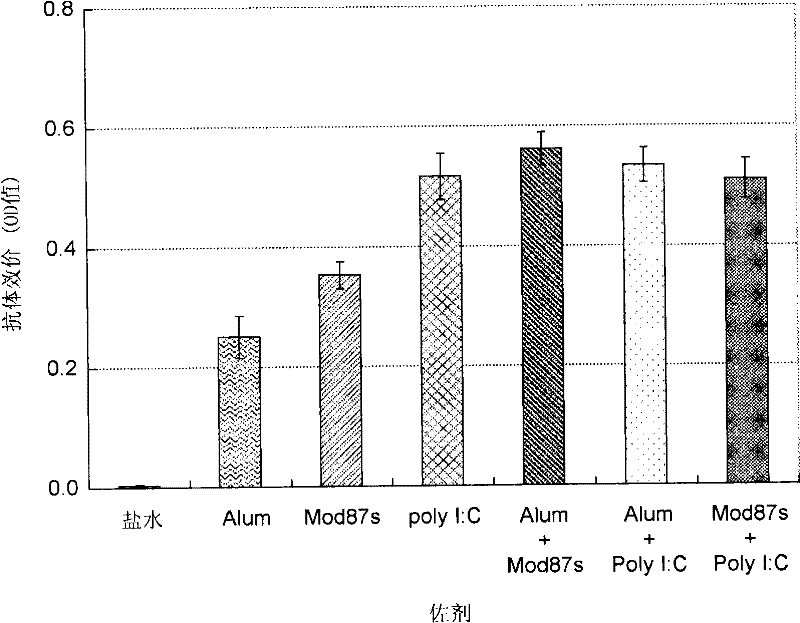
Disclosed is an effective HCV vaccine composition, which is developed as a result of the finding of an optimum combination of an HCV antigen capable of inducing an antibody having an inhibitory activity on the infection by an HCV and an adjuvant. Specifically disclosed is a hepatitis C virus vaccine composition which comprises: inactivated virus particles produced by inactivating infectious hepatitis C virus particles that are produced from hepatitis C virus genome containing sequences respectively encoding NS3 protein, NS4A protein, NS4B protein, NS5A protein and NS5B protein derived from hepatitis C virus strain JFH1; a non-methylated CpG-containing oligonucleotide represented by SEQ ID NO:5 shown in the Sequence Listing; and aluminum hydroxide.
Owner:TORAY IND INC +2
HCV vaccines and methods for using the same
ActiveUS8829174B2Improved immunogenic targetsOrganic active ingredientsSsRNA viruses positive-senseRecombinant vaccinesAttenuated vaccine
Improved anti-HCV immunogens and nucleic acid molecules that encode them are disclosed. Immunogens disclosed include those having consensus HCV genotype 1 a / 1 b NS3 and NS4A. Pharmaceutical composition, recombinant vaccines comprising and live attenuated vaccines are disclosed as well methods of inducing an immune response in an individual against HCV are disclosed.
Owner:INOVIO PHARMA +1
Hcv vaccines
InactiveUS20110300170A1Increase the number ofPrecise processingSsRNA viruses positive-senseViral antigen ingredientsEpitopeHepatitis
Disclosed are methods and compositions for inducing immune responses against Hepatatis C virus (HCV). The compositions comprise one or more epitope from a hotspot epitope. In certain embodiments, an HCV vaccine comprising at least two epitopes, each from a different hotspot epitope, is provided.
Owner:INTERCELL AG
Hcv vaccines
InactiveUS20090155294A1Extended shelf lifeUseful and effectiveSsRNA viruses positive-senseViral antigen ingredientsEpitopeHepatitis
Owner:BUSCHLE MICHAEL +5
DNA (Deoxyribonucleic acid) segment and applications thereof in constructing HCV (hepatitis C virus) whole-genome expression animal model
The invention discloses a DNA (deoxyribonucleic acid) segment and applications thereof in constructing an HCV (hepatitis C virus) whole-genome expression animal model. The nucleotide sequence of the DNA segment comprises the following sequentially series connected sequences: an operator gene sequence of bacteria tetracycline drug-resistance operon, an immediate early promoter gene sequence of human macrophage, a gene sequence of HCV whole genome, and a gene sequence of ribozyme shearing the HCV whole genome activity on DNA and / or RNA (ribonucleic acid) level. The DNA segment can be used for constructing a tetracycline or tetracycline derivative-induced HCV whole genome-expressed transgenic animal model. The immunity of the animal model is normal, thereby being capable of producing specific anti-virus immunoreaction aiming at the expression of the HCV, and further being effectively applied to the research on the immunopathogenesis damage mechanism of drug evaluation and virus infection of the HCV vaccine.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
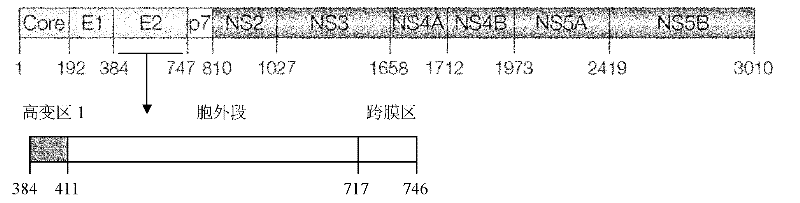
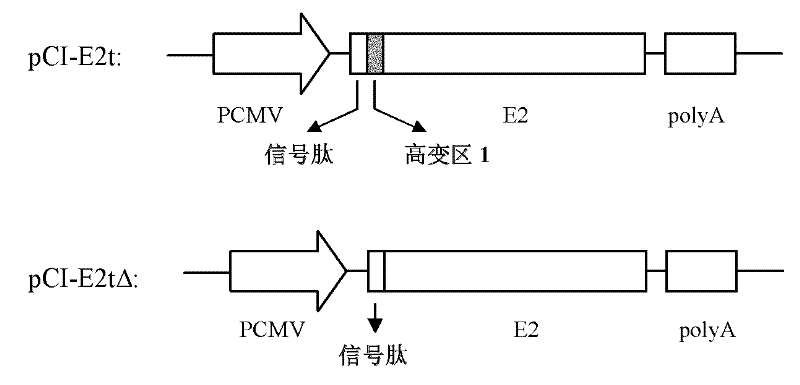
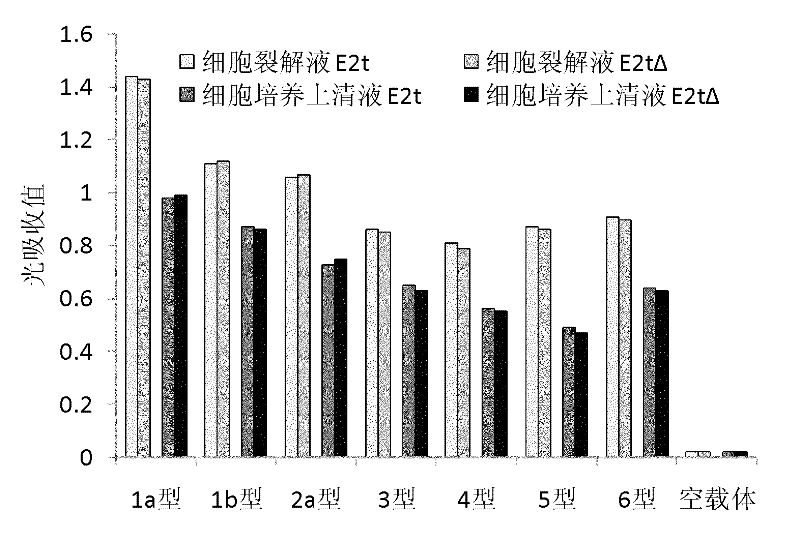
HCV envelope protein E2 with deleting hypervariable region 1 and use thereof
InactiveCN102206256AGood inhibitory effectImmunogenicity suppressionMicrobiological testing/measurementVirus peptidesGenotypeNeutralizing antibody
The invention relates to the technical field of biological engineering. At present, there is no effective vaccine for preventing hepatitis C virus (HCV) infection. The amino terminal of HCV envelope protein E2 has 27 amino acid residues with highest variability, known as hypervariable region 1(HVR1). The invention provides an HCV envelope protein E2 without HVR1, also provides an application of the HCV envelope protein E2 without HVR1 on HCV vaccines and HCV infection immunity diagnostic reagents. Animal immunization tests have discovered that HVR1 in 1-6 genotype HCV envelope protein E2 has substantial inhibition effect against immunogenicity of conservative neutralizing epitope in the envelope protein E2, the effectiveness of inducting broad spectrum neutralizing antibody by the E2 protein is significantly enhanced by deleting HVR1, the HCV envelope protein E2 with deleting HVR1 possibly can be used as an effective target antigen of HCV vaccines. The invention has proved that the HCV envelope protein E2 with deleting HVR1 has strong reaction with E2 antibody in serums of HCV infections and can be used as an immunity diagnostic antigen of HCV infections.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
HCV vaccines and methods for using the same
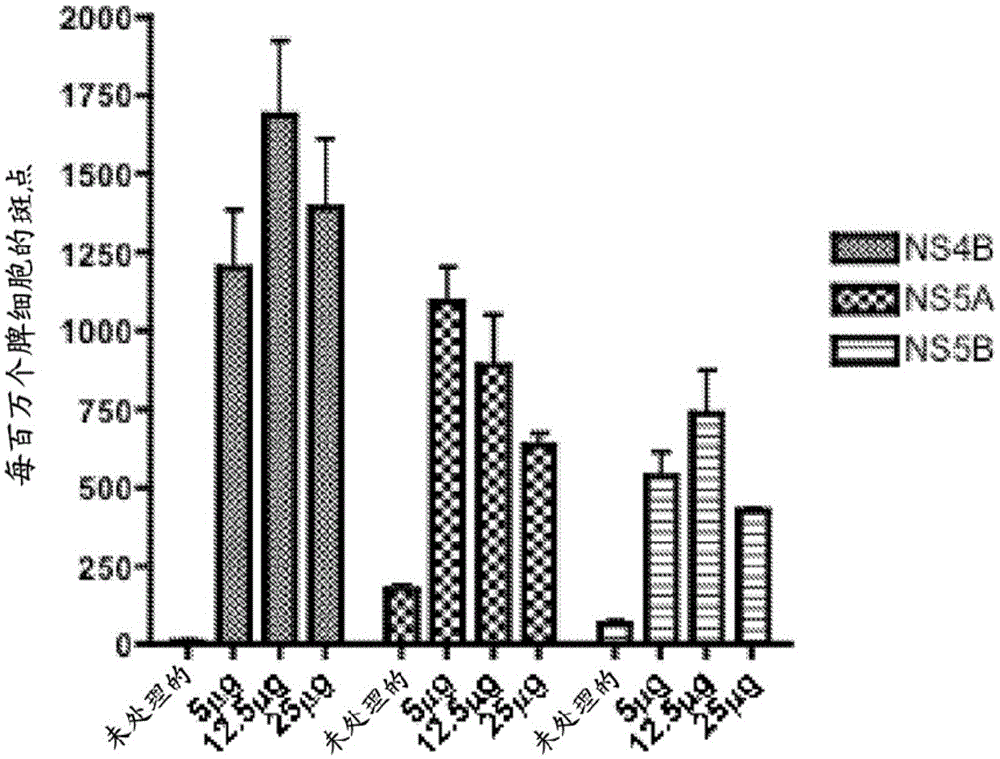
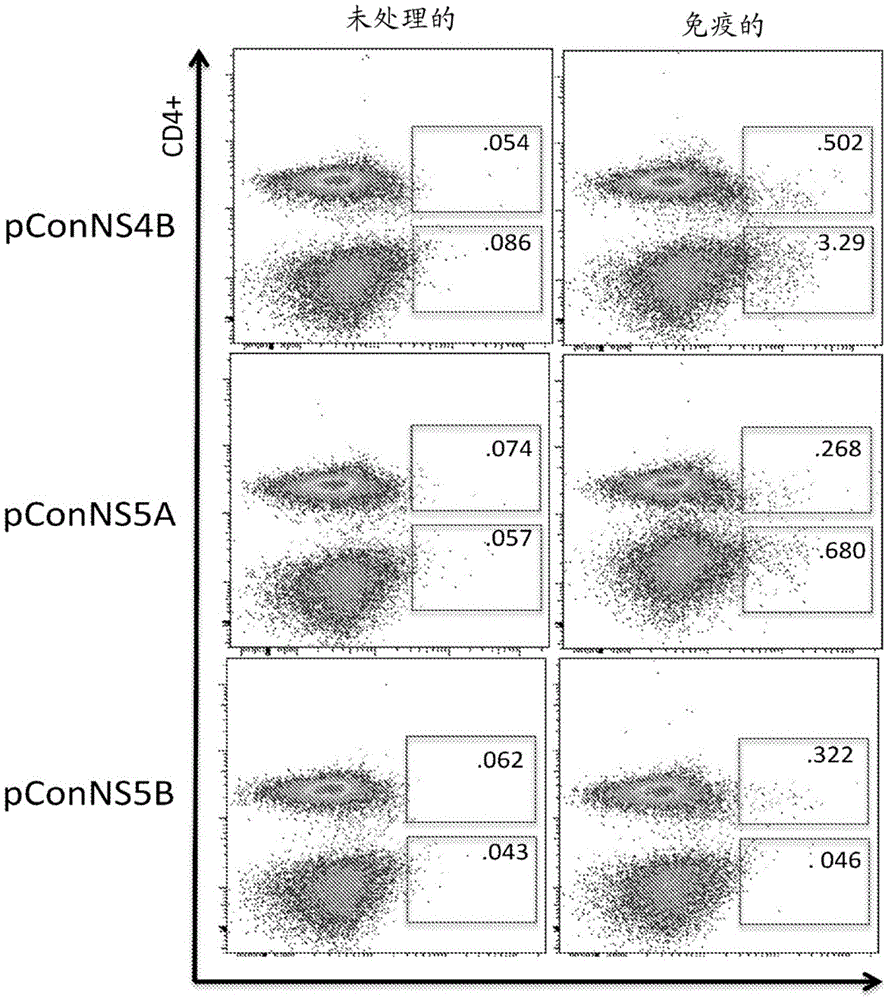
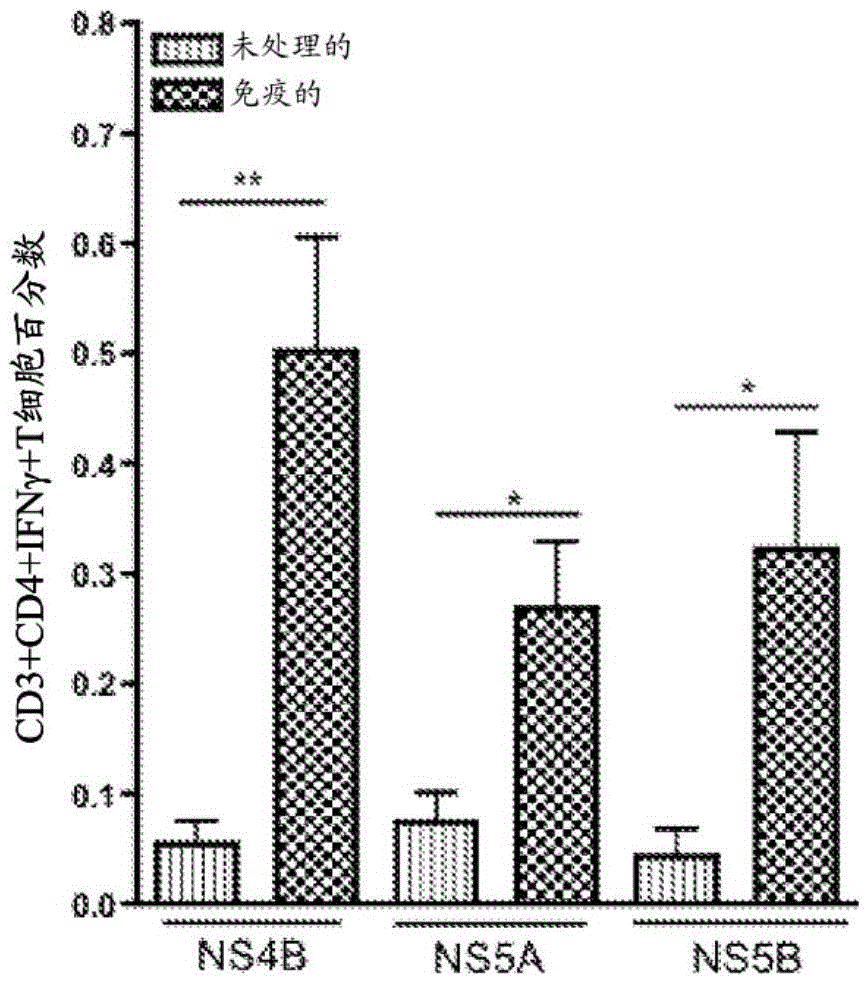
Improved anti-HCV immunogens and nucleic acid molecules that encode them are disclosed. Immunogens disclosed include those having consensus HCV genotype 1a, including for example, NS4B, NS5A and NS5B. Pharmaceutical composition, recombinant vaccines comprising and live attenuated vaccines are disclosed as well methods of inducing an immune response in an individual against HCV are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Method for porcine circovirus production and pcv2 vaccines
ActiveUS20180201906A1Speeding up cultivationIncrease virus yieldViral antigen ingredientsVeterinary vaccineCircovirusTGE VACCINE
This invention relates to a method for rapid production of PCV2 such that an optimum yield of the virus can be obtained. This invention also relates to the vaccine useful against PCV2 which includes the PCV2 propagated using such a method.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
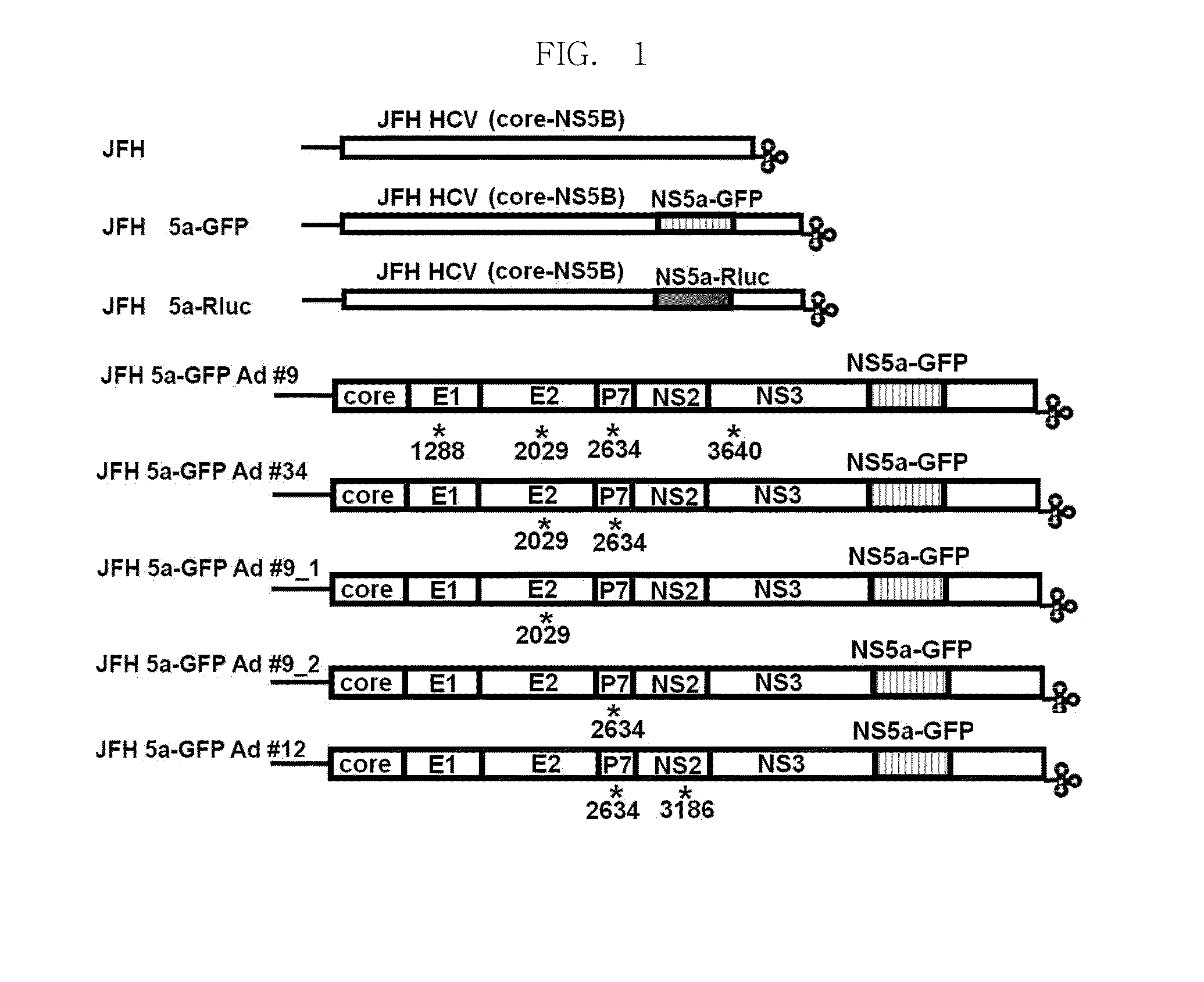
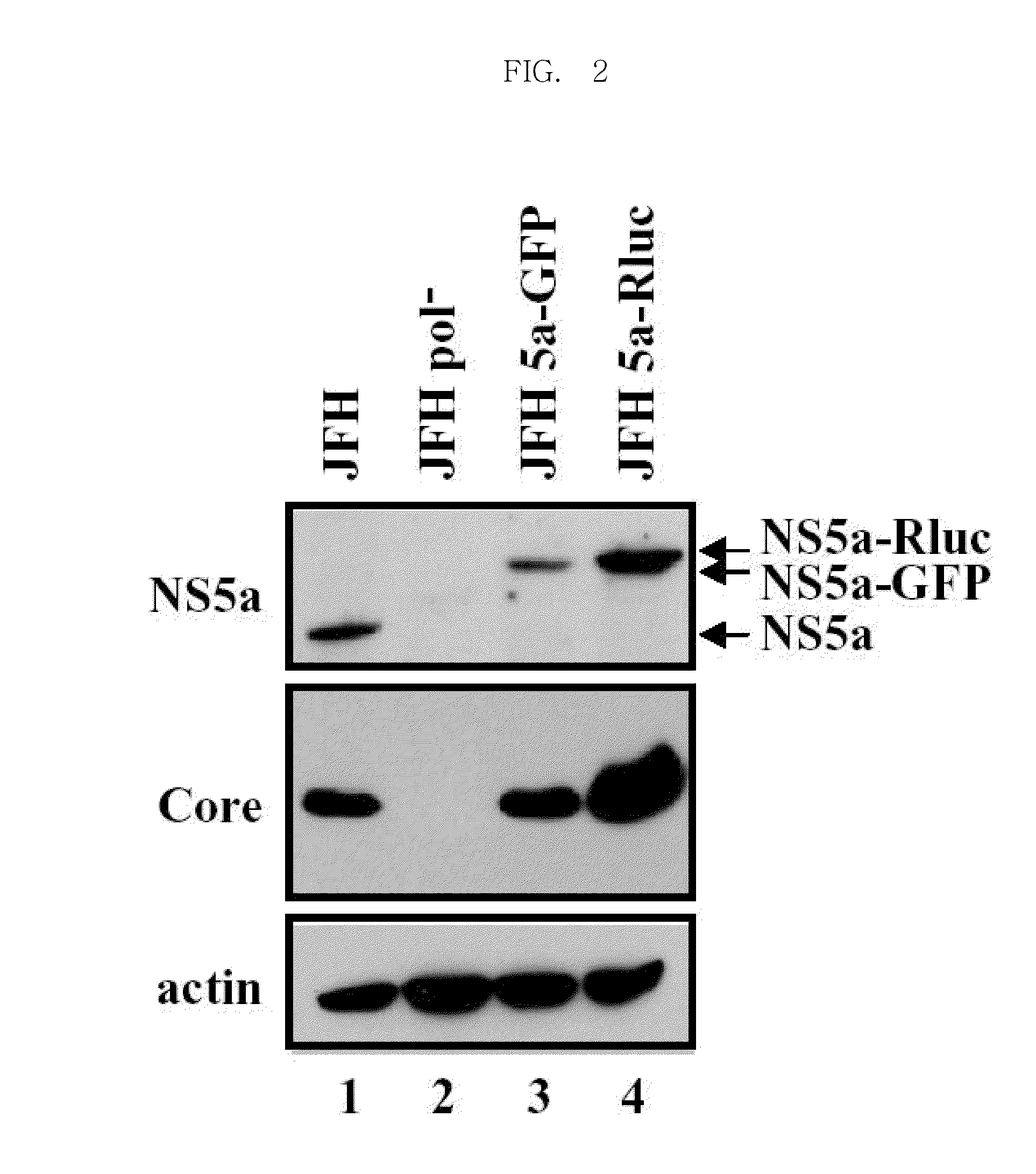
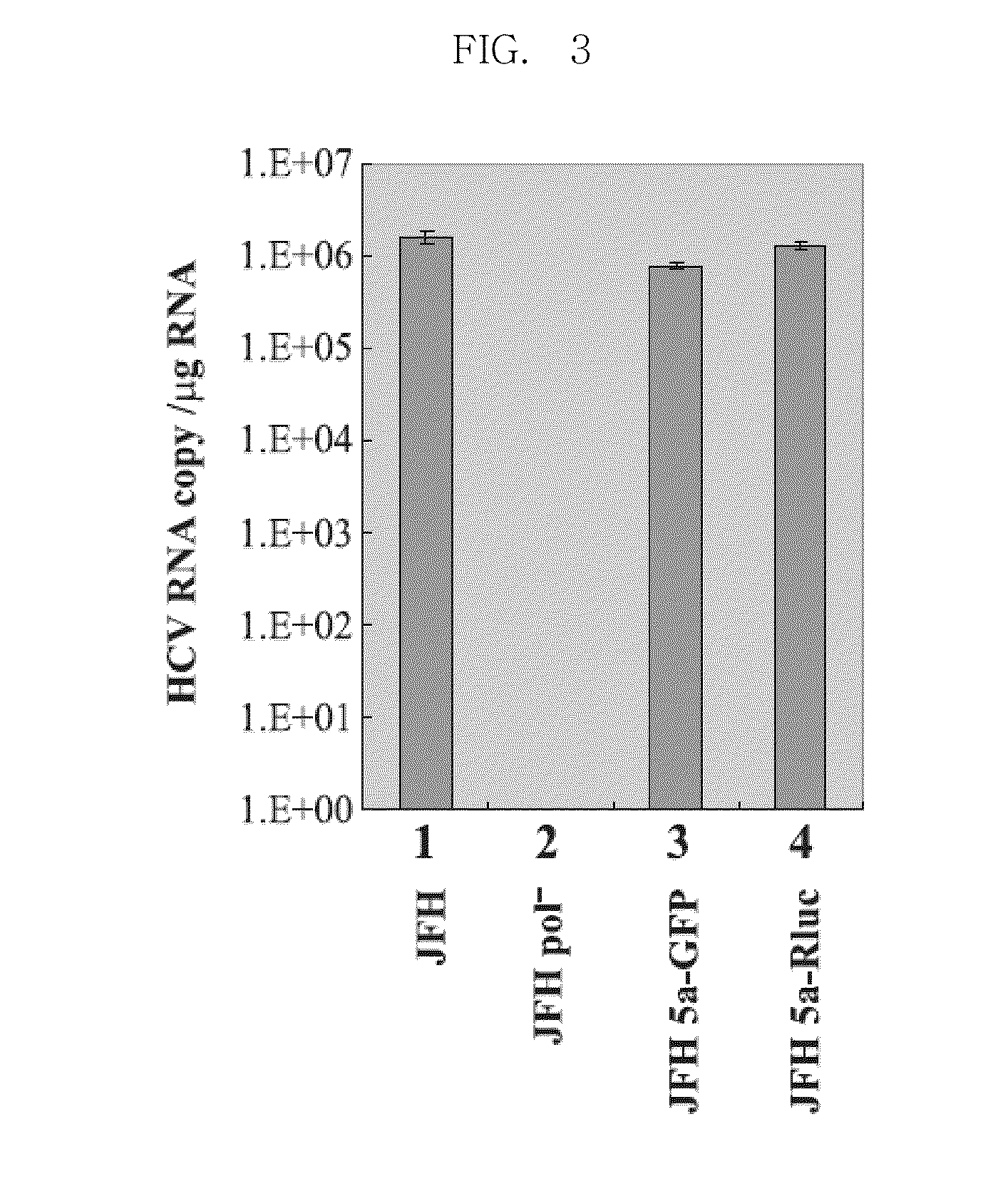
Efficiently replicable heptitis c virus mutant, a heptitis c virus mutant comprising reporter gene, a method of preparing of hcv vaccine using the same and a method of screening anti hcv composition using the same
The present invention relates an efficiently replicating a modified hepatitis virus (HCV) mutant, and a modified HCV further comprising reporter gene, a method of preparing HCV vaccine using the same, and a method of screening anti-HCV material using the same. The present invention is to overcome the defect that the conventional HCV cell culture systems are unable to produce a sufficient amount of virus, thereby causing it difficult to efficiently induce or measure HCV infection. Because the present invention can allow production of HCV in a large amount an efficiently observing HCV infection in a living cell, it can make it possible to achieve many studies that were previously highly challenging, including studies on infection routes, and assembly and release of HCV. In addition, the present invention contributes to studies for searching anti-HCV agents being inhibiting all stages of the HCV life cycle, not being limited to HCV replication.
Owner:POSTECH ACAD IND FOUND +1
Assay to detect HCV receptor binding
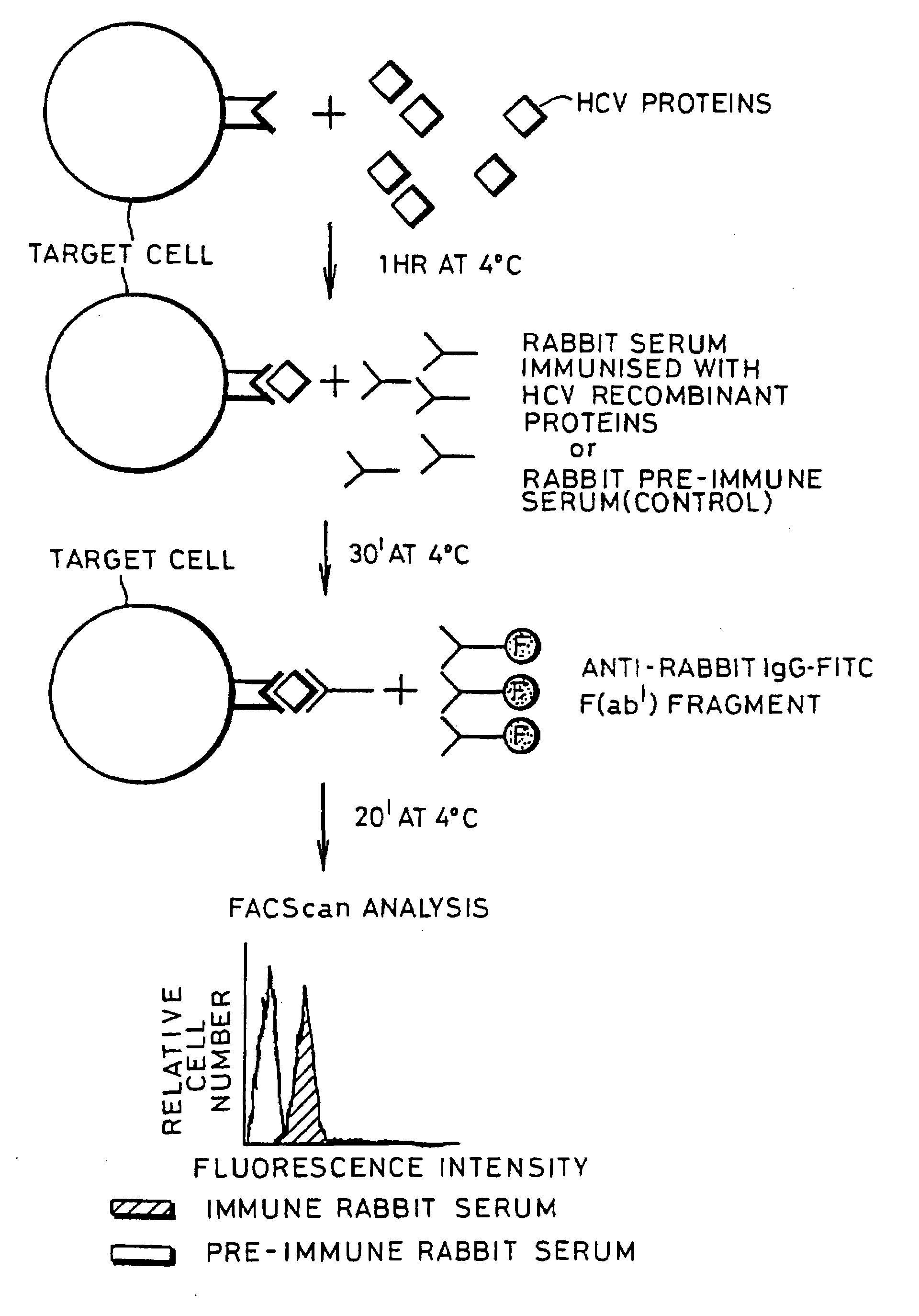
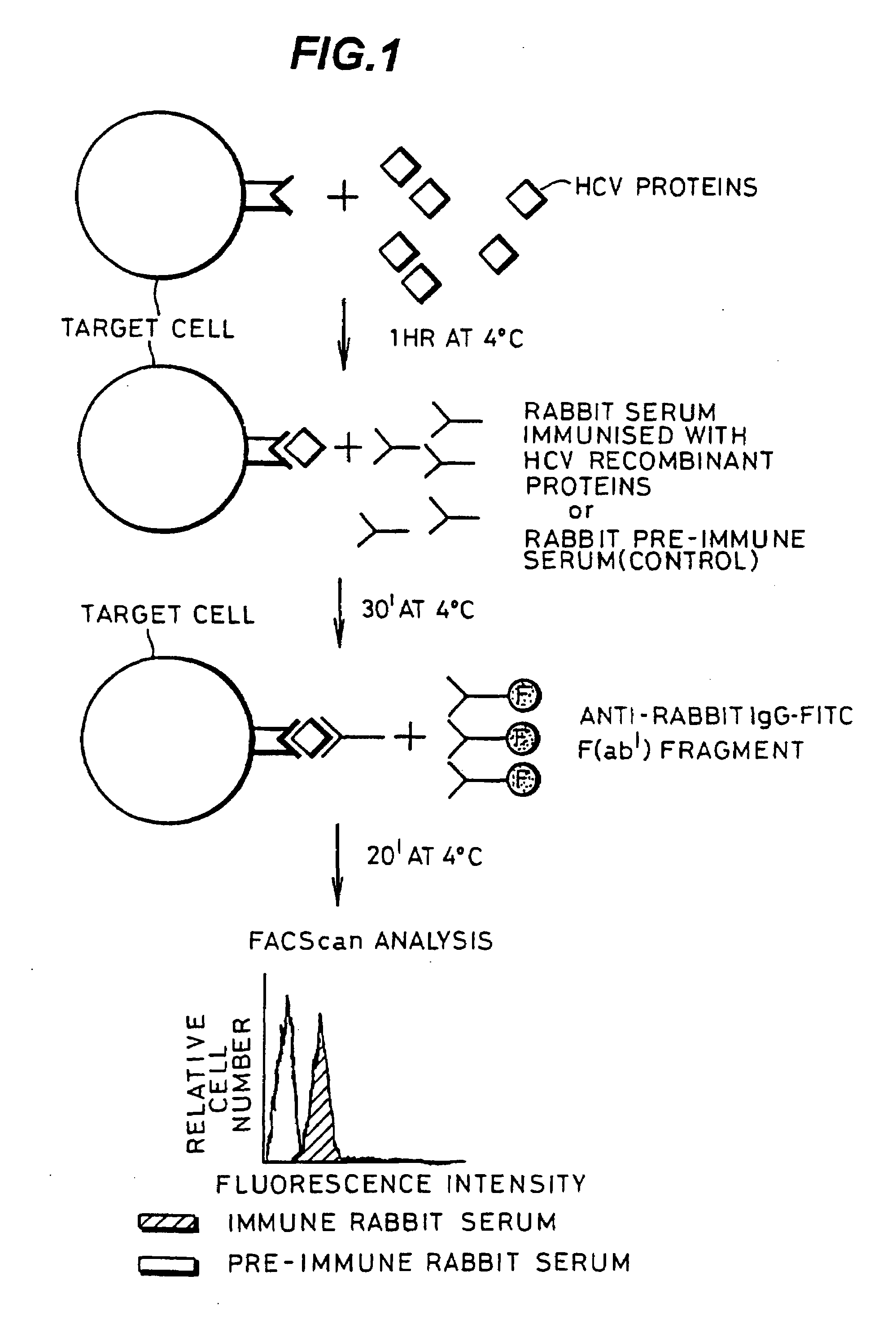
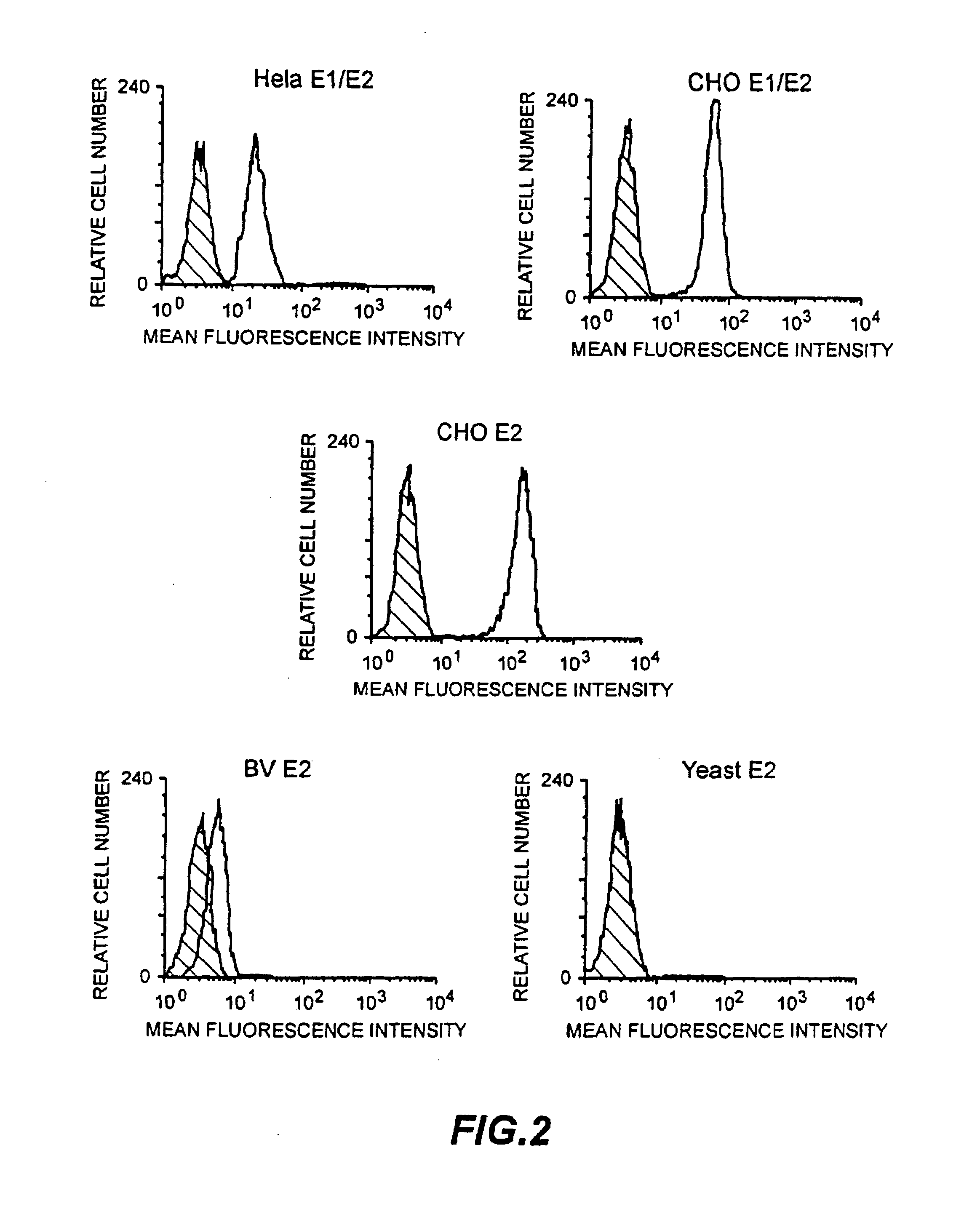
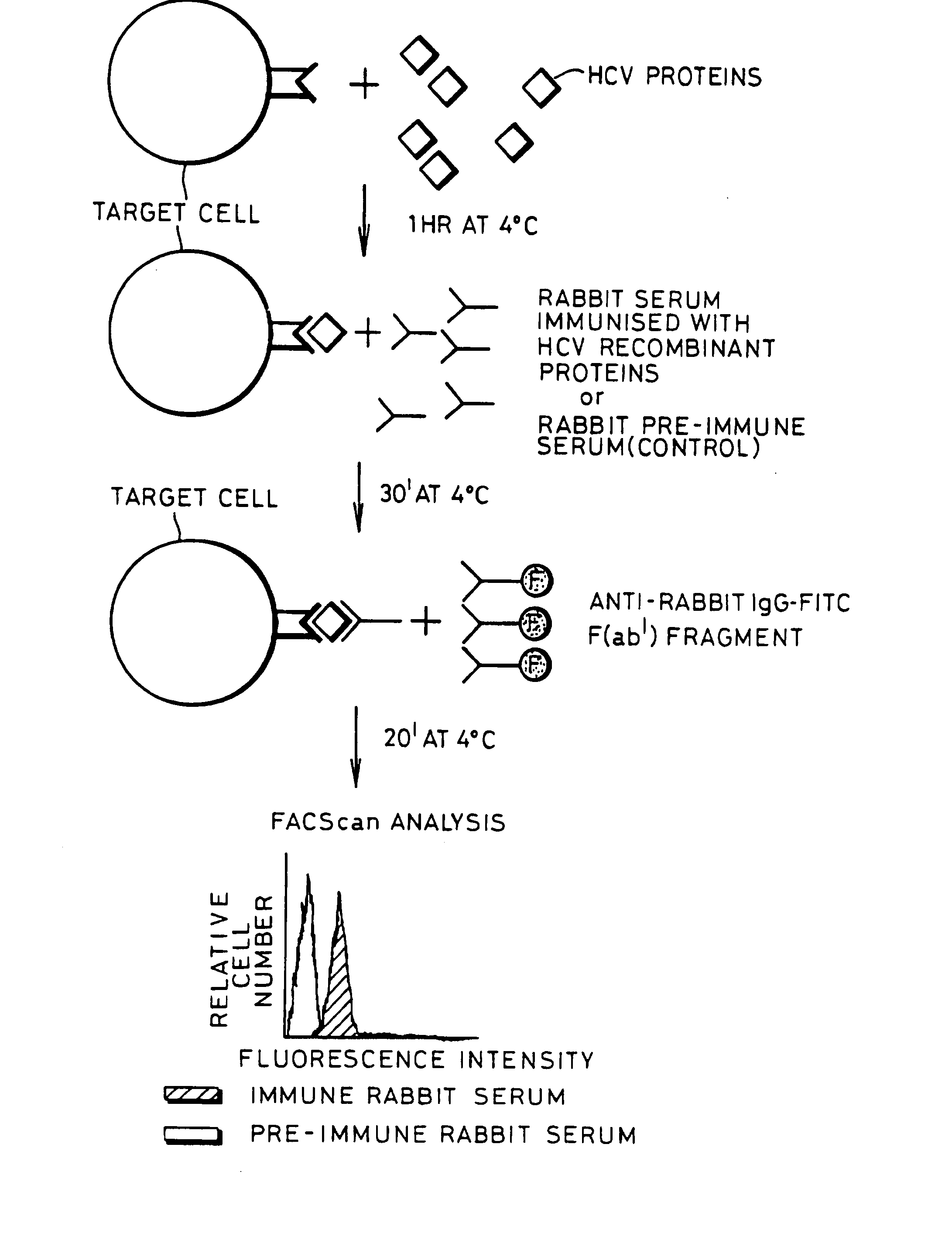
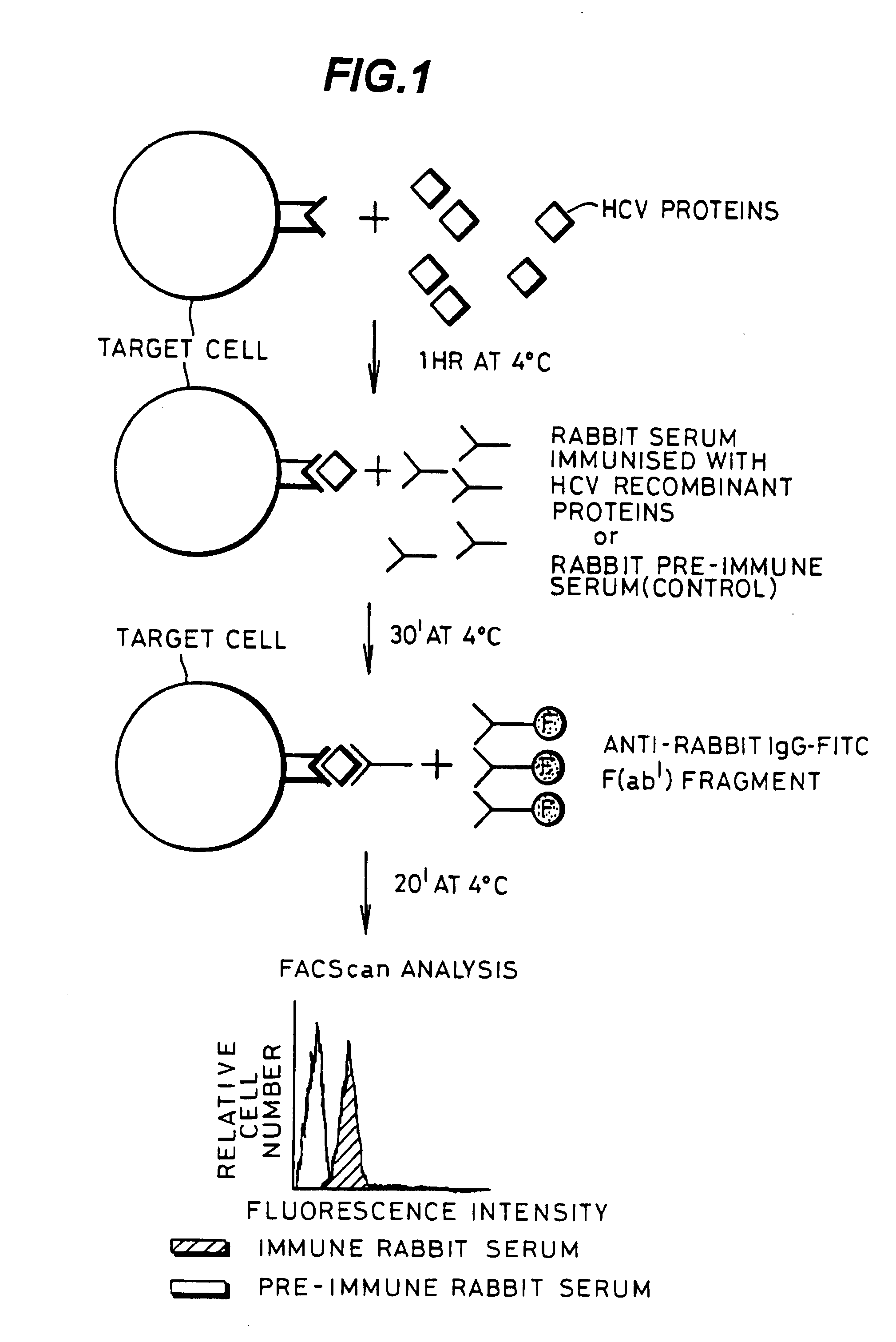
InactiveUS20080176219A1Facilitates ready screening of possible HCV receptor-bindingPrognostic valueMicrobiological testing/measurementBiological testingAssayReceptor
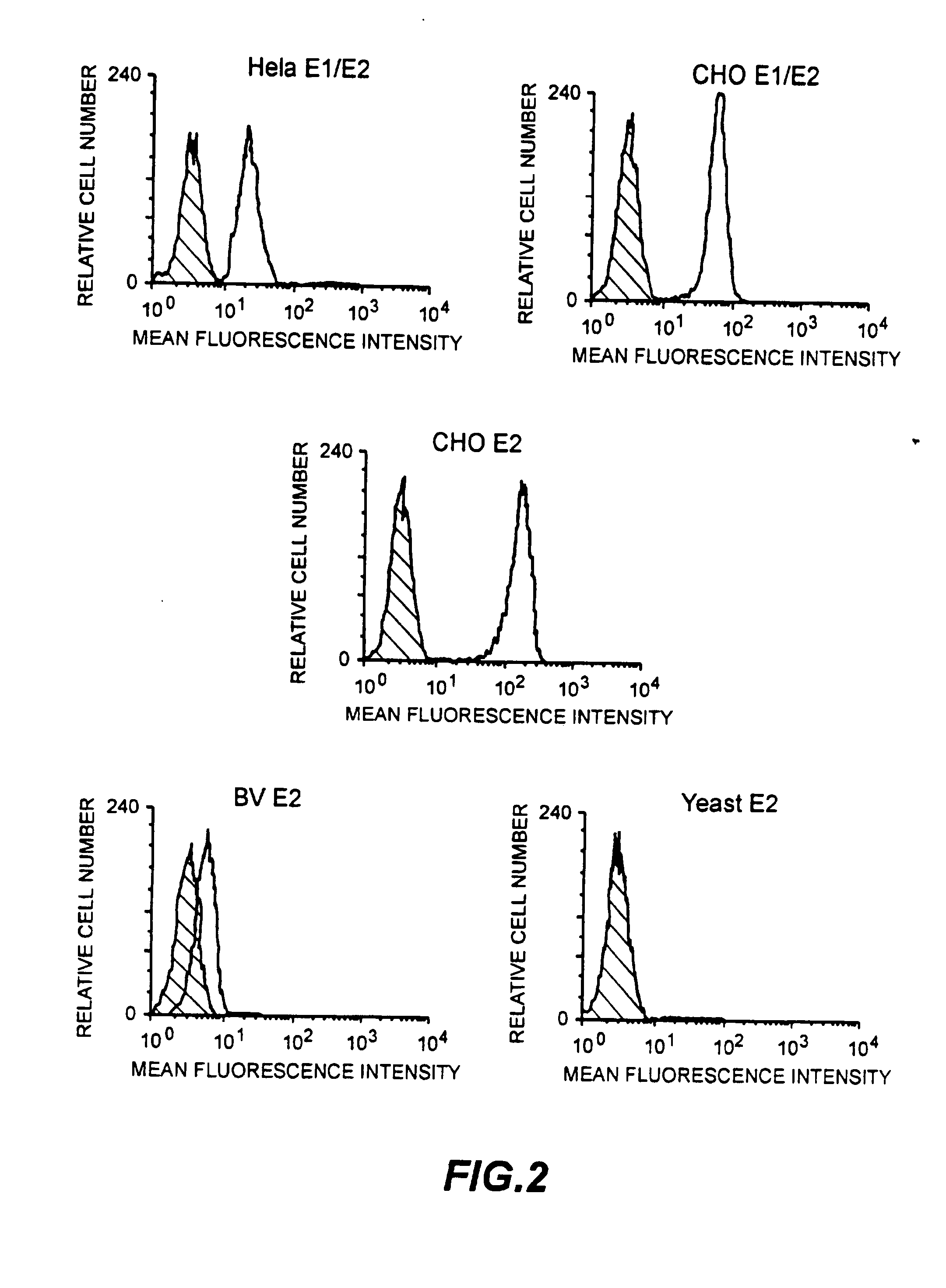
Identification of HCV receptor target cells using HCV receptor-binding ligands and cell separation by flow cytofluorimetry is described. HCV receptor target cells are employed to conduct assays for HCV receptor-binding ligands in order to identify potential HCV vaccine candidates. HCV receptor target cells are used to measure antibody neutralisation to monitor vaccine development, as a diagnostic of HCV infection and to develop neutralising antibodies for passive immunisation.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Assay to detect HCV receptor binding
InactiveUS20070298410A1Prognostic valueFacilitates ready screening of possible HCV receptor-bindingMicrobiological testing/measurementBiological testingFluorescencePassive immunisation
Identification of HCV receptor target cells using HCV receptor-binding ligands and cell separation by flow cytofluorimetry is described. HCV receptor target cells are employed to conduct assays for HCV receptor-binding ligands in order to identify potential HCV vaccine candidates. HCV receptor target cells are used to measure antibody neutralisation to monitor vaccine development, as a diagnostic of HCV infection and to develop neutralising antibodies for passive immunisation.
Owner:CHIRON CORP
Improved hcv vaccine and method of use thereof
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Anti-hcv vaccine and preparation methods and uses thereof
InactiveUS20100310605A1Strong cellular immune responseStrong immune responseSsRNA viruses positive-senseViral antigen ingredientsGeneVirology
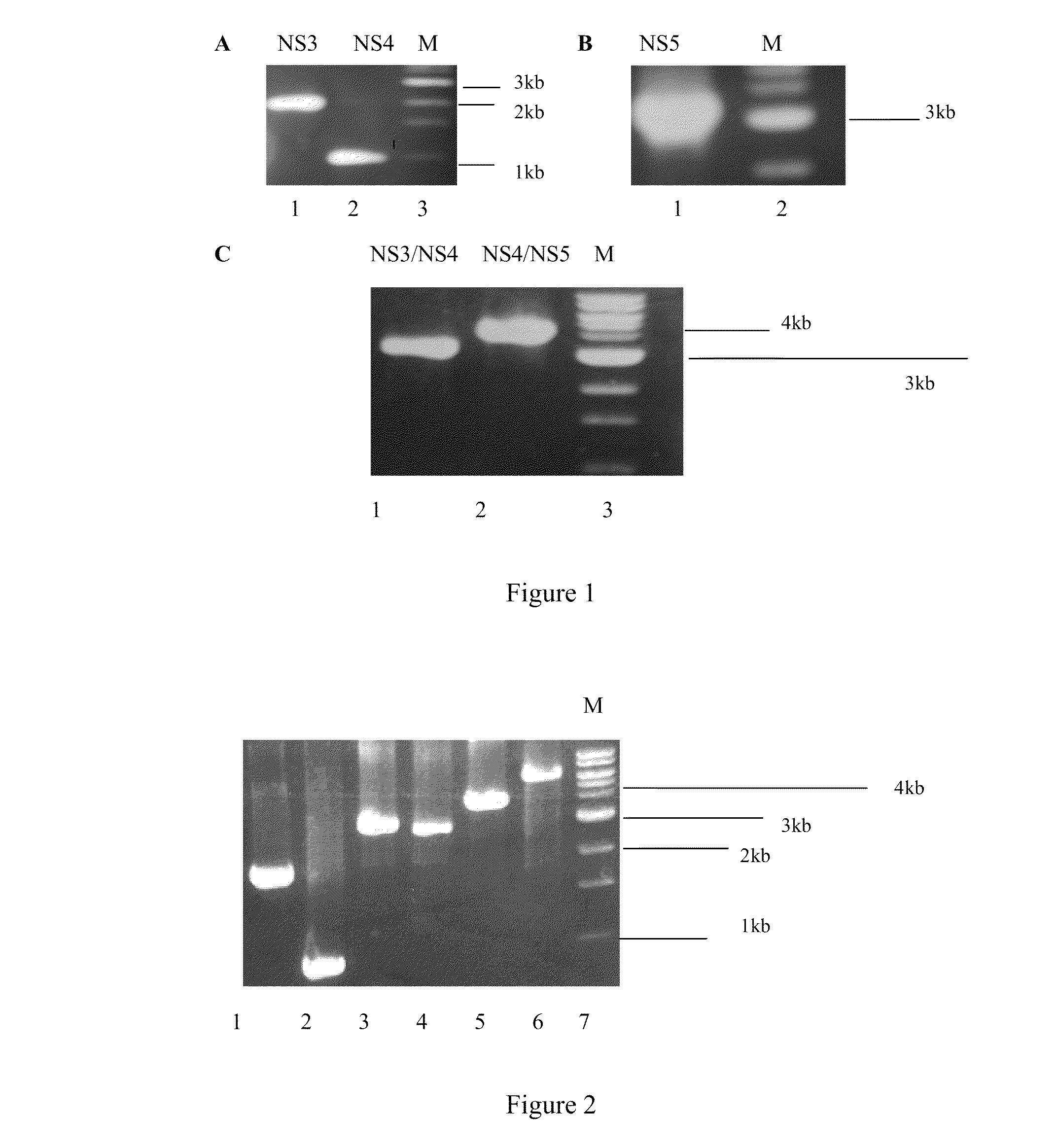
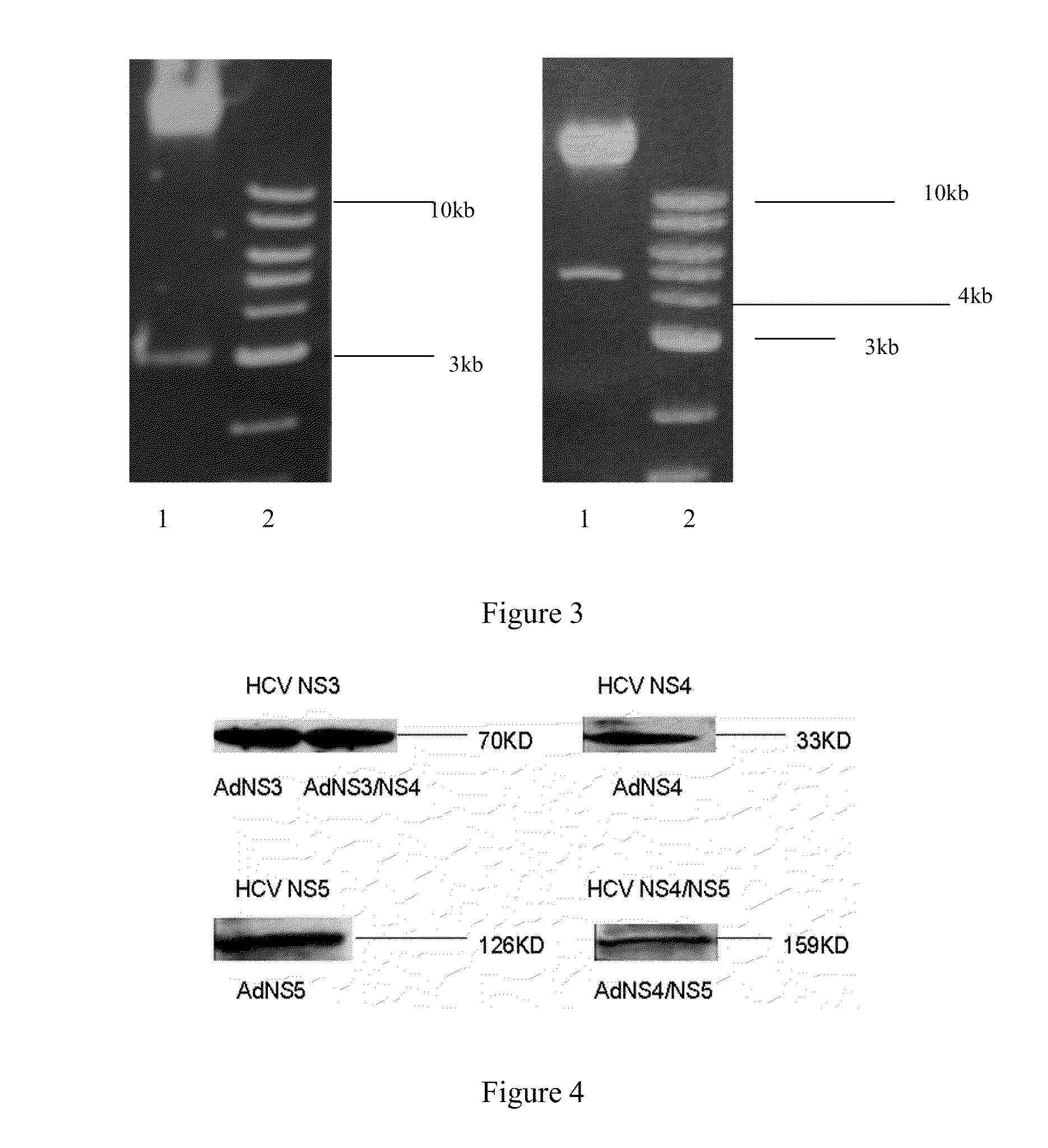
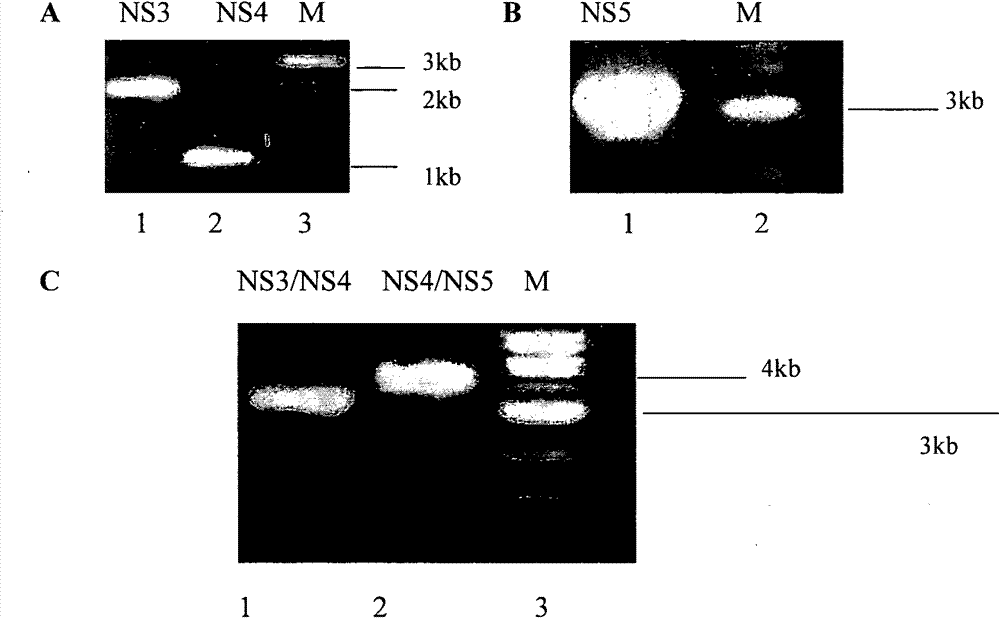
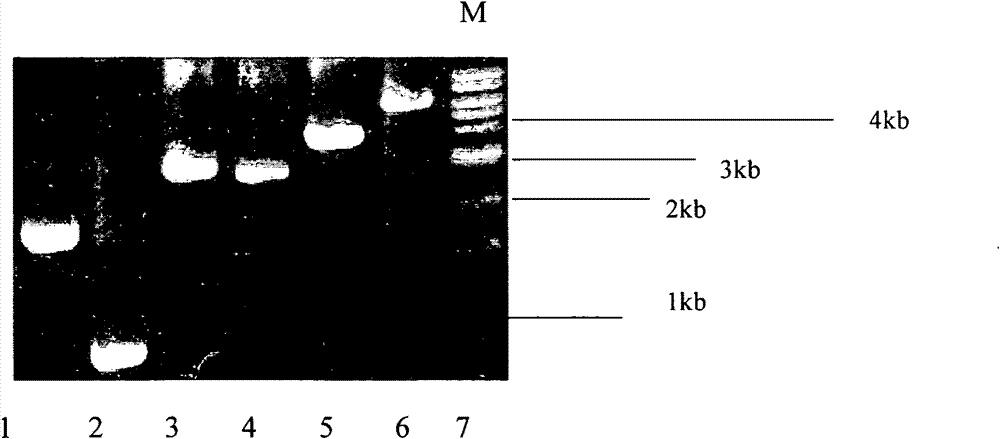
An anti-HCV vaccine, which is prepared through recombination of an NS gene in series, including NS3 / NS4 and NS4 / NS5, with an adenovirus vector. The preparation methods and uses of the anti-HCV vaccine.
Owner:PEOPLES HOSPITAL PEKING UNIV
Improved HCV vaccines and methods for using the same
Improved anti-HCV immunogens and nucleic acid molecules that encode them are disclosed. Immunogens disclosed include those having consensus HCV genotype 1a, including for example, NS4B, NS5A and NS5B. Pharmaceutical composition, recombinant vaccines comprising and live attenuated vaccines are disclosed as well methods of inducing an immune response in an individual against HCV are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
An anti-hcv vaccine and preparation methods and uses thereof
InactiveCN101663399BOvercoming the deficiencies of tandem immunityStrong immune responseSsRNA viruses positive-senseGenetic material ingredientsD'Aguilar virusGene
An anti-HCV vaccine, which is prepared through recombination of an NS gene in series, including NS3 / NS4 and NS4 / NS5, with an adenovirus vector. The preparation methods and uses of the anti-HCV vaccine.
Owner:PEOPLES HOSPITAL PEKING UNIV
HCV (hepatitis c virus) envelope protein gene and application
InactiveCN104480123AInactivation/attenuationMicroorganism based processesNucleotideNeutralizing antibody
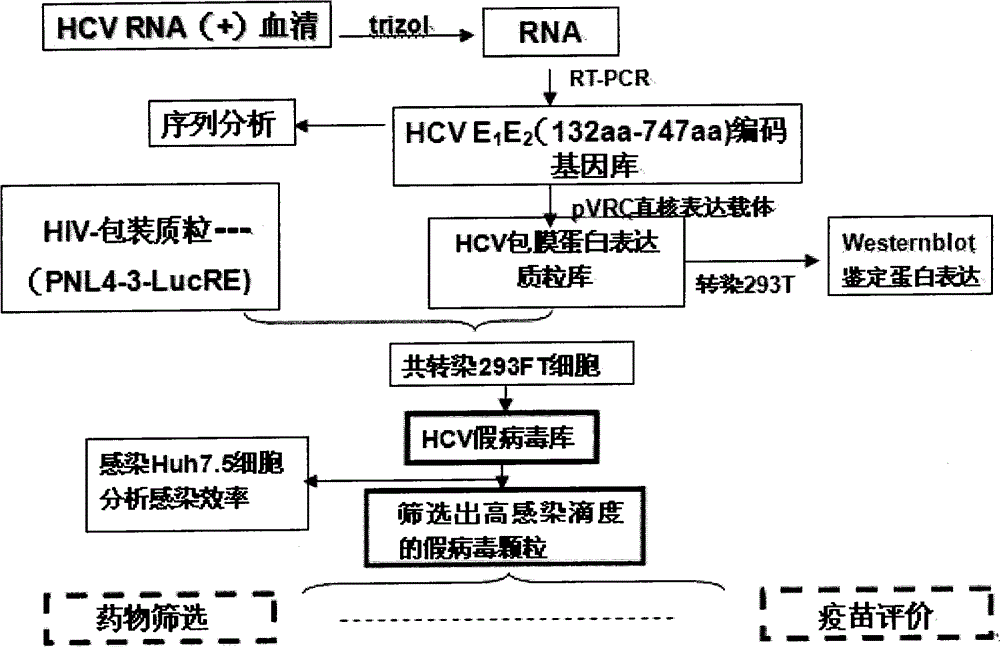
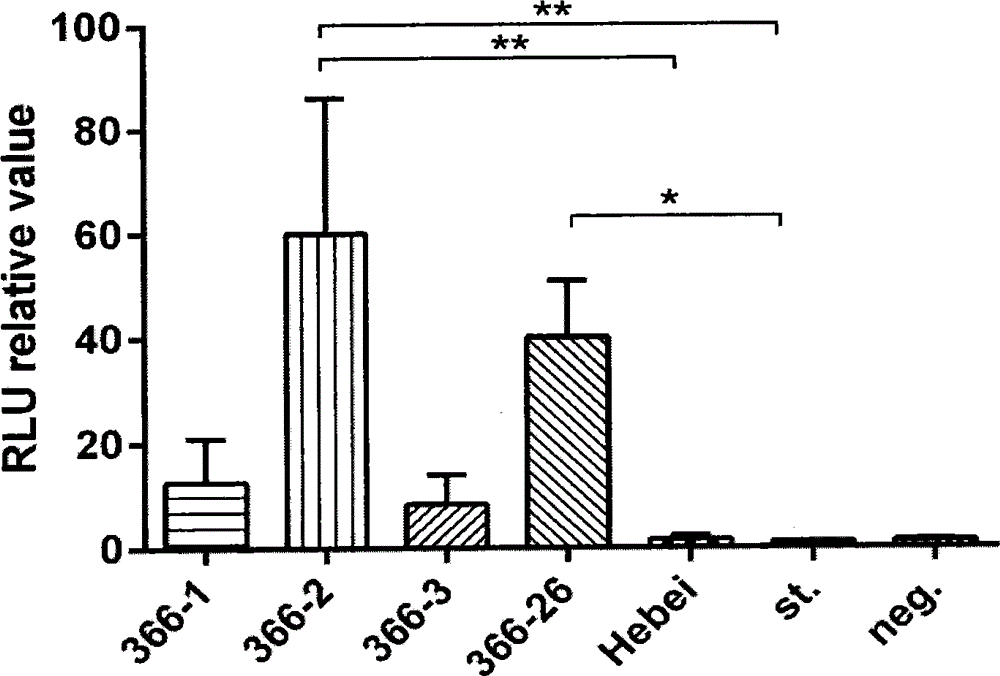
The invention discloses an HCV (hepatitis c virus) envelope protein gene and an application. The nucleotide sequence of the HCV envelope protein gene is represented as SEQ ID NO.1. RT-PCR (reverse transcription-polymerase chain reaction) and nested PCR technologies are adopted to obtain HCV 1b subtype envelope protein genes popular in China, an expression vector provided with different HCV 1b subtype envelope protein genes is constructed, a two-plasmid cotransfection method is adopted to establish a pseudovirus database, high-infectivity pseudovirion is screened out, and an effective model is provided for studying screening of anti-HCV medicine and immune effect evaluation of HCV vaccines, meanwhile, early characteristics of HCV infection can be further studied, and a technology platform can be provided for evaluation of response of a serum neutralizing antibody from an HCV patient and an antibody of an experimental vaccine and the like.
Owner:陶格斯
Method for expressing hepatitis C virus envelope protein E2 by mammal cell with high efficient secretion
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
HCV Vaccinations
InactiveUS20090186047A1Promote migrationImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsVaccinationHepacivirus
The invention relates to a method for preventing or treating Hepatitis C Virus (HCVi) infections, wherein a HCV vaccine comprising an effective amount of at least one HCV T-cell antigen and a polycationic compound comprising peptide bonds is administered to a human individual bi-weekly at least 3 times.
Owner:INTERCELL AG
Adenovirus vector and its application in preparing HCV cell and mouse models
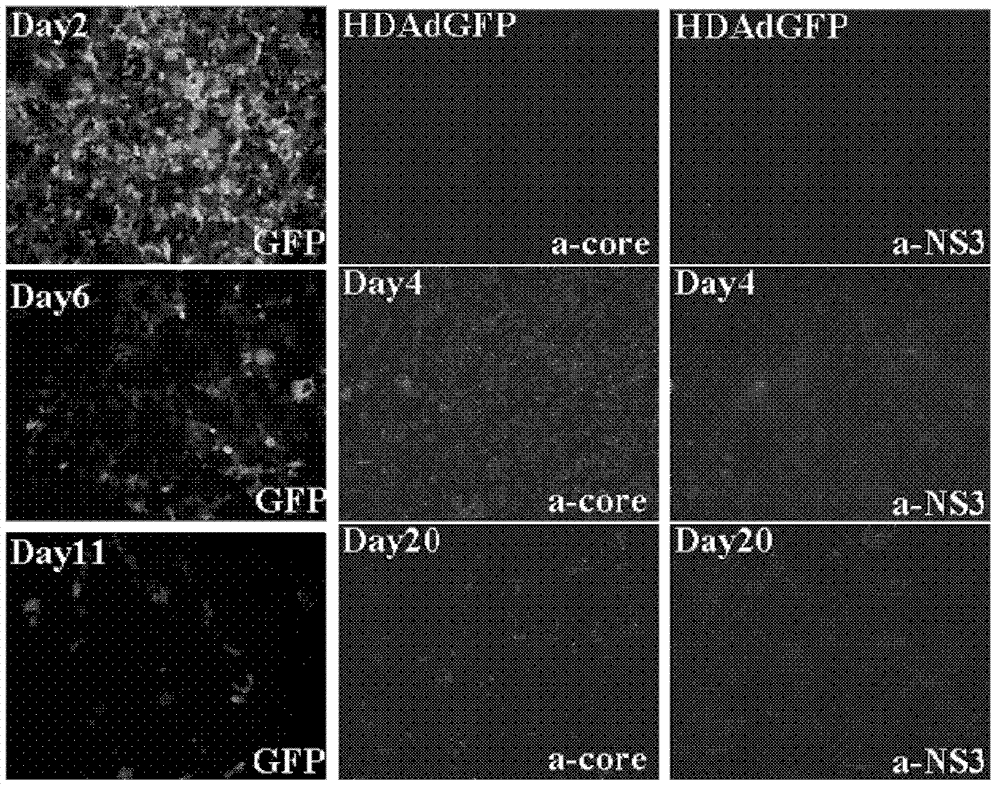
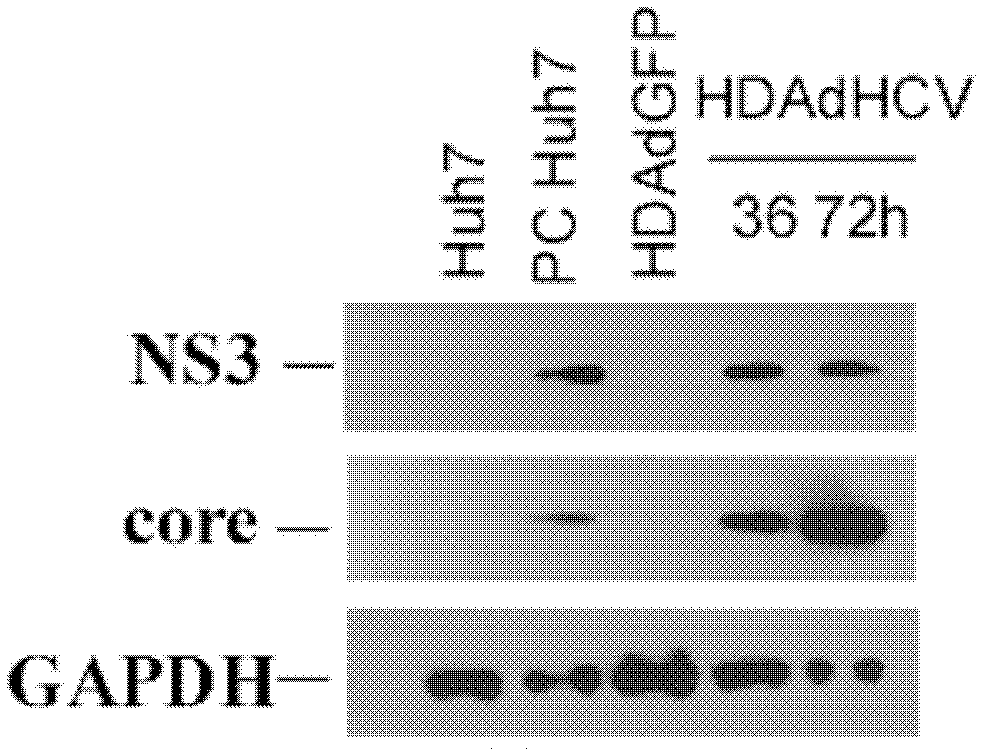
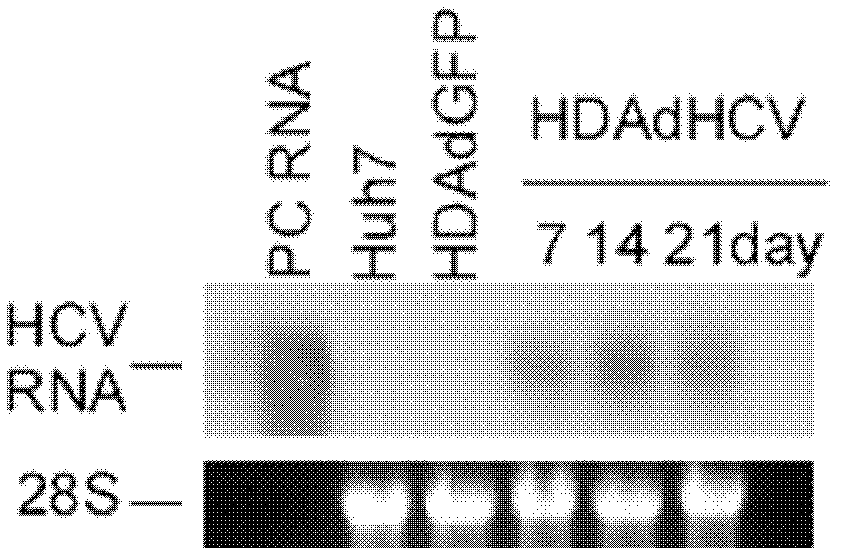
The invention discloses an adenovirus vector and its application in preparing HCV (hepatitis C virus) cells and mouse models. The invention provides a recombinant adenovirus vector which is obtained by inserting nucleotides shown by the sequence 1 in the sequence table in the adenovirus vector HDAd (helper-dependent adenovirus). The experiments prove that the invention provides a third generation adenovirus vector of a recombinant HCV 2a type full-length genome, the prepared adenovirus can be used for preparing HCV whole-genome cell models or mouse models, and the obtained models can be used for drug screening of anti-HCV drugs, so as to be used for HCV vaccine evaluation and researches of the pathogenic mechanism.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
E1e2 hcv vaccines and methods of use
InactiveUS20160067332A1SsRNA viruses positive-senseViral antigen ingredientsHCV GenotypingImmunogenicity
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
E1e2 hcv vaccines and methods of use
InactiveUS20210145963A9SsRNA viruses positive-senseViral antigen ingredientsHCV GenotypingImmunity response
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
HCV composite multi-epitope transgene plant oral vaccine
Owner:QINGDAO UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com