HCV envelope protein E2 with deleting hypervariable region 1 and use thereof
A technology of hypervariable region and envelope, applied in the field of biomedical engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Amplification of the 1-6 genotype HCV envelope E2 protein gene containing HVR1 and deleting HVR1:
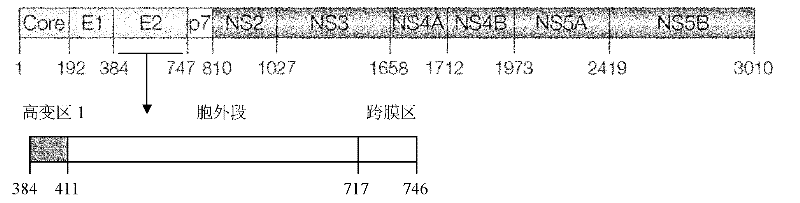
[0049] HCV envelope protein E1E2 genes of types 1a, 1b, and 2a were donated by Professor C.M.Rice of Rockefeller University in the United States; HCV envelope protein E1E2 genes of types 3a, 4, 5, and 6 were donated by Professor J.K.Ball of Nottingham University in the United Kingdom. See Table 1 for the gene sequence. Design and synthesize primers according to the corresponding sequences of 7 HCV envelope E2 genes of 1-6 genotypes (1a, 1b, 2a, 3a, 4, 5, 6), and amplify E2 protein by polymerase chain reaction (PCR) Extracellular segment gene (corresponding to 364-661 amino acid residues of HCV polyprotein, 364-383 amino acid residues as secretory signal peptide of E2 protein). The sequences of the amplification primers (upstream primer FP1, downstream primer RP) are shown in Table 2. Reagents for PCR amplification are products of Promega Corporation, USA.
...
Embodiment 2
[0058] Embodiment 2: Identification of 1-6 genotype HCV envelope E2 protein expression product:
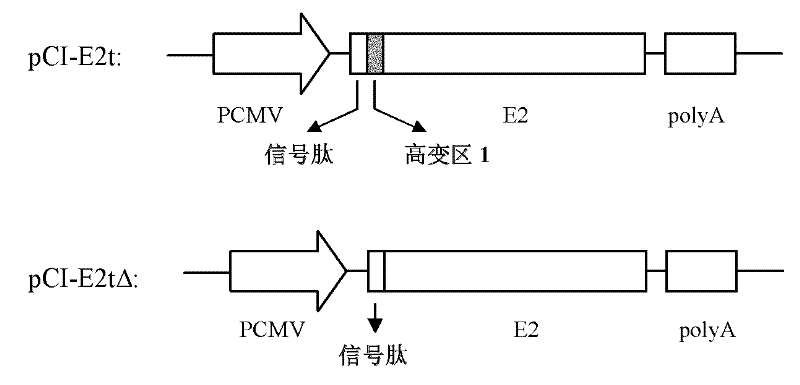
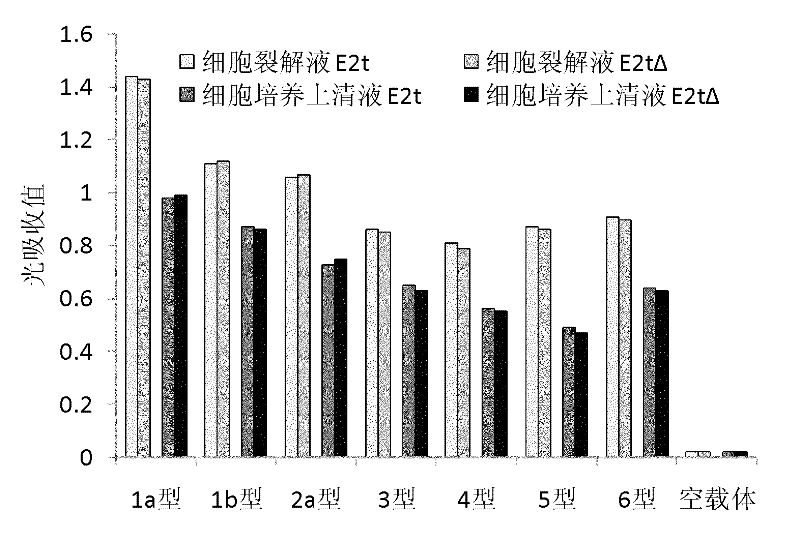
[0059] Human embryonic kidney (HEK) 293T cells were subcultured with DMEM medium containing 10% fetal bovine serum. When the cells were 80% confluent, the cells were digested with 0.25% trypsin, subcultured and inoculated on 35mm cell culture dishes, and cultured overnight. Combine 4 μg of plasmids constructed above with 7 strains of HCV envelope E2 protein expression plasmids pCI-E2t (including HVR1), pCI-E2tΔ (deleted HVR1) and empty vector pCI-neo with 10 μl of transfection reagent lipid Plastid Lipofectamine (product of Invitrogen, USA) was mixed evenly, and transfected into HEK 293T cells, and the operation was performed according to the instruction. After adding the plasmid-liposome mixture to the cell culture dish, place the cell culture dish in a cell culture incubator at 37°C, 5% carbon dioxide, and saturated humidity, and add 1ml of complete DMEM culture solution contain...
Embodiment 3
[0061] Embodiment 3: Mouse gene immunization and antibody detection:
[0062] Use a plasmid extraction kit (product of Qiagen, Germany) to prepare and purify a large number of HCV envelope E2 protein expression plasmids pCI-E2t, pCI-E2tΔ and empty vector pCI-neo of the above 1-6 genotypes, and dissolve them in PBS buffer (pH 7.4), adjust the concentration to 100 μg / ml, immunize Balb / C mice, immunize 10 mice with each kind of plasmid, inject 0.1 ml of the plasmid solution into the tibialis anterior muscle on each side, and the equivalent dose is 20 μg of the plasmid per mouse. A total of three immunizations were performed with an interval of two weeks. One day before each immunization (the time is 0, 2, 4 weeks respectively, the time of first blood collection is set as 0 week) and every two weeks after the last immunization (a total of 7 times, the time is 6, 8, 10, 12, 14, 16, 18 weeks) Blood was collected from the orbit of the mice, and E2t (including HVR1), E2tΔ (deleted HV...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com