Adenovirus vector and its application in preparing HCV cell and mouse models
A technology of adenovirus and recombinant adenovirus, applied in the biological field, can solve the problems of HCV vaccine evaluation, pathogenic mechanism, and research that cannot be used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the acquisition of HDAd-HCV recombinant adenoviral vector
[0038] The third-generation adenovirus vector HDAd-HCV, which recombines the HCV type 2a JFH1 genome, is to insert sequence 1 in the sequence listing into the adenovirus vector HDAd (Prolonged Peritoneal Gene Expression Using AHelper-Dependent Adenovirus.Peritoneal Dialysis International, 2009; 29:508-516 , the public can obtain from the Institute of Microbial Epidemiology, Academy of Military Medical Sciences of the Chinese People's Liberation Army) The vector obtained between the I-Ceu I and I-Sce I sites.
[0039] The specific preparation is as follows:
[0040] 1. Construction of pMD18T-HCV500-Ribo:
[0041] The pJFH1 plasmid (Production of Infectious Hepatitis C Virus in Tissue Culture from ACloned Viral Genome. Nat. Med, 2005; 11(7): 791-796, available to the public from the Institute of Microbial Epidemiology, Academy of Military Medical Sciences of the Chinese People's Liberation Army) as...
Embodiment 2
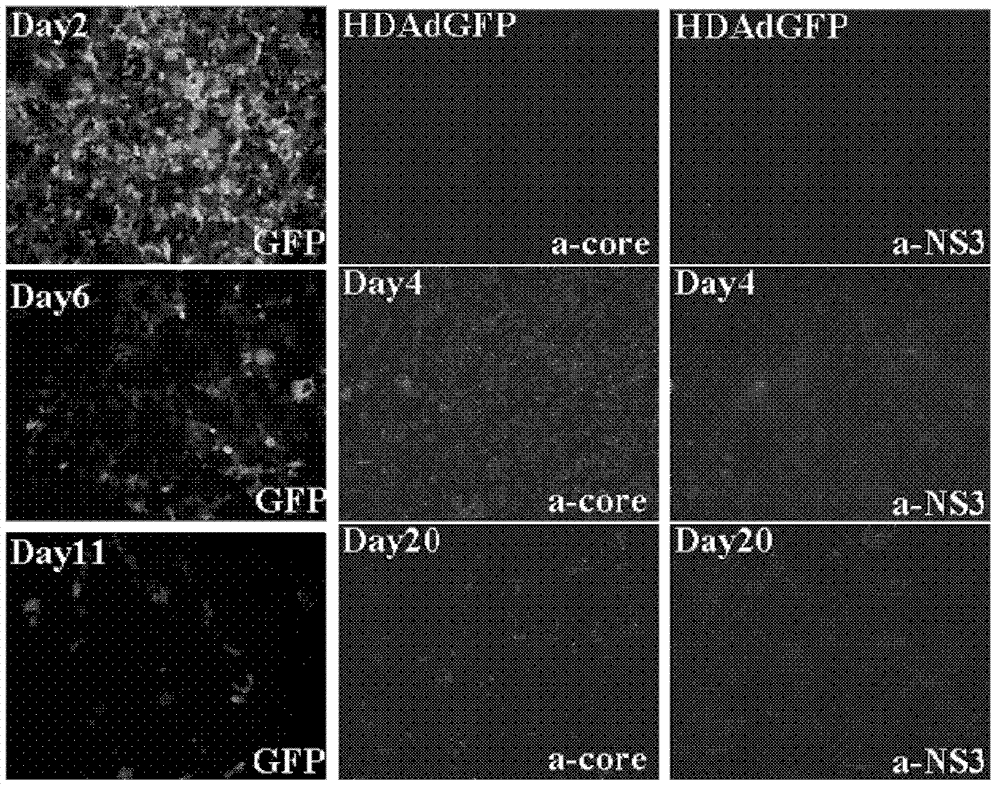
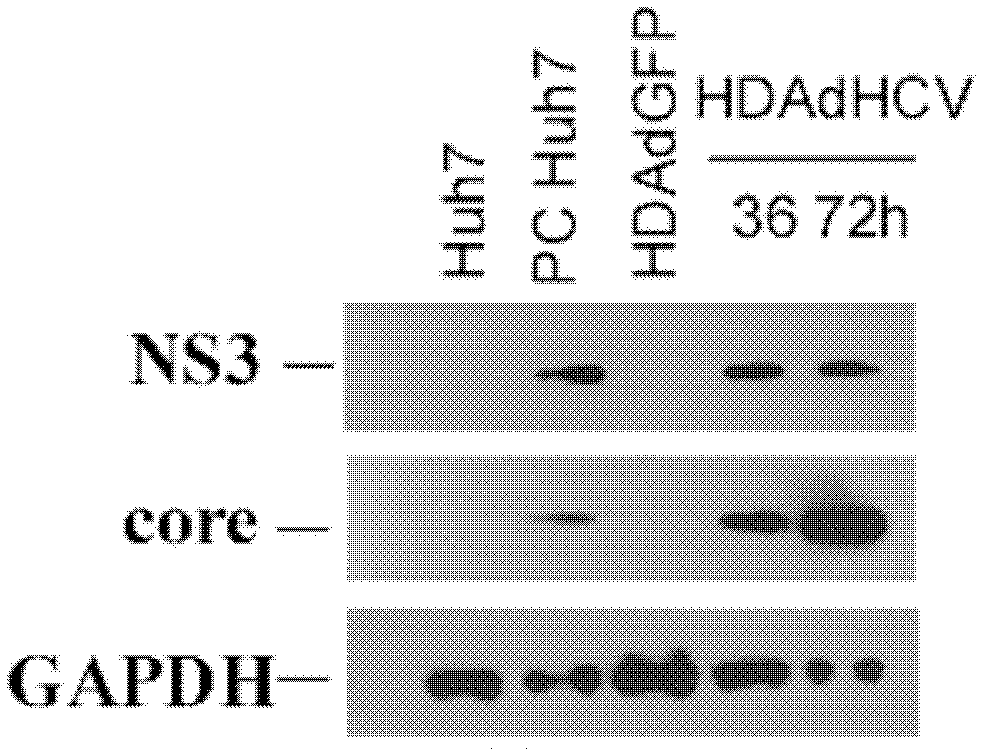
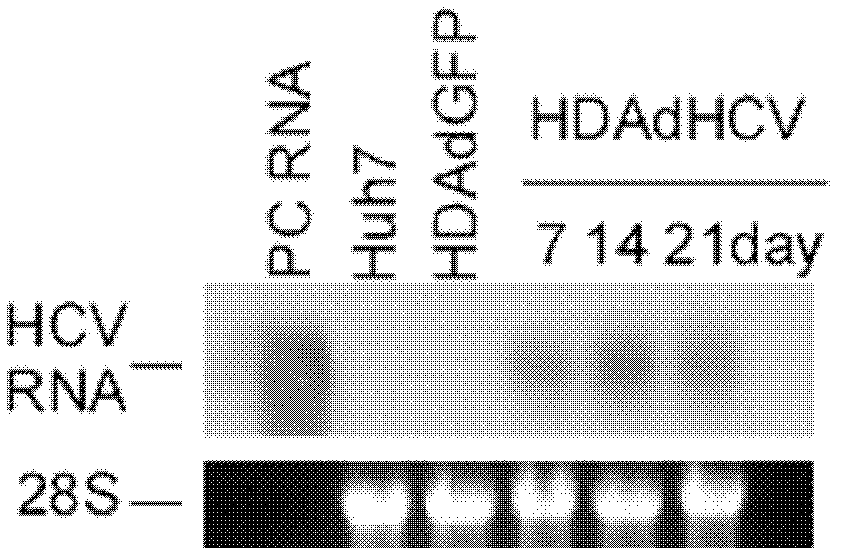
[0053] Example 2, Application of HDAd-HCV Recombinant Adenoviral Vector in Preparation of Cell Model and Mouse Model
[0054] I, the acquisition of HDAd-HCV recombinant adenovirus
[0055] After the vector HDAd-HCV and HDAd empty vector obtained by Example 1 were all linearized with Pme I, they were mixed with the helper virus AdNG163 (Prolonged Peritoneal Gene Expression Using A Helper-Dependent Adenovirus.Peritoneal Dialysis International, 2009; 29:508-516, public (available from the Institute of Microbial Epidemiology, Academy of Military Medical Sciences of the Chinese People's Liberation Army) co-transfected 293 cells stably expressing Cre recombinase to package and activate recombinant HDAd-HCV, and then continuously added AdNG163 helper virus, respectively, through 60mm cell culture dishes, 150mm cell culture dishes and 3L cell culture flasks were expanded and cultured (37°C 60rpm shake flask culture for 3 days), the cells were collected and lysed and then subjected to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com