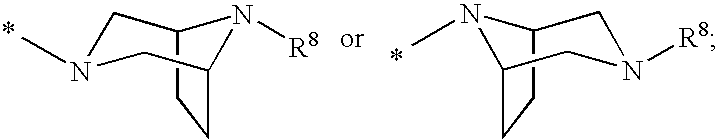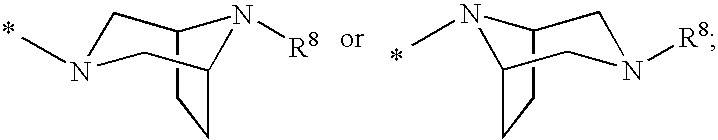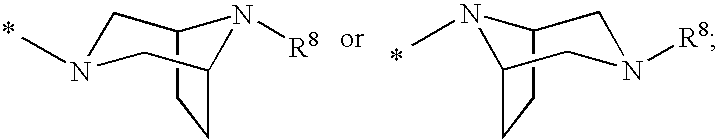Patents
Literature
120 results about "NS5B" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nonstructural protein 5B (NS5B) is a viral protein found in the hepatitis C virus (HCV). It is an RNA-dependent RNA polymerase, having the key function of replicating HCV's viral RNA by using the viral positive RNA strand as a template to catalyze the polymerization of ribonucleoside triphosphates (rNTP) during RNA replication. Several crystal structures of NS5B polymerase in several crystalline forms have been determined based on the same consensus sequence BK (HCV-BK, genotype 1). The structure can be represented by a right hand shape with fingers, palm, and thumb. The encircled active site, unique to NS5B, is contained within the palm structure of the protein. Recent studies on NS5B protein genotype 1b strain J4’s (HC-J4) structure indicate a presence of an active site where possible control of nucleotide binding occurs and initiation of de-novo RNA synthesis. De-novo adds necessary primers for initiation of RNA replication.
Modified Human Hepatitis C Virus Genomic RNA That can be Autonomously Replicated
ActiveUS20090176200A1Easy to useSsRNA viruses positive-senseGenetic material ingredientsNucleotideGenetics
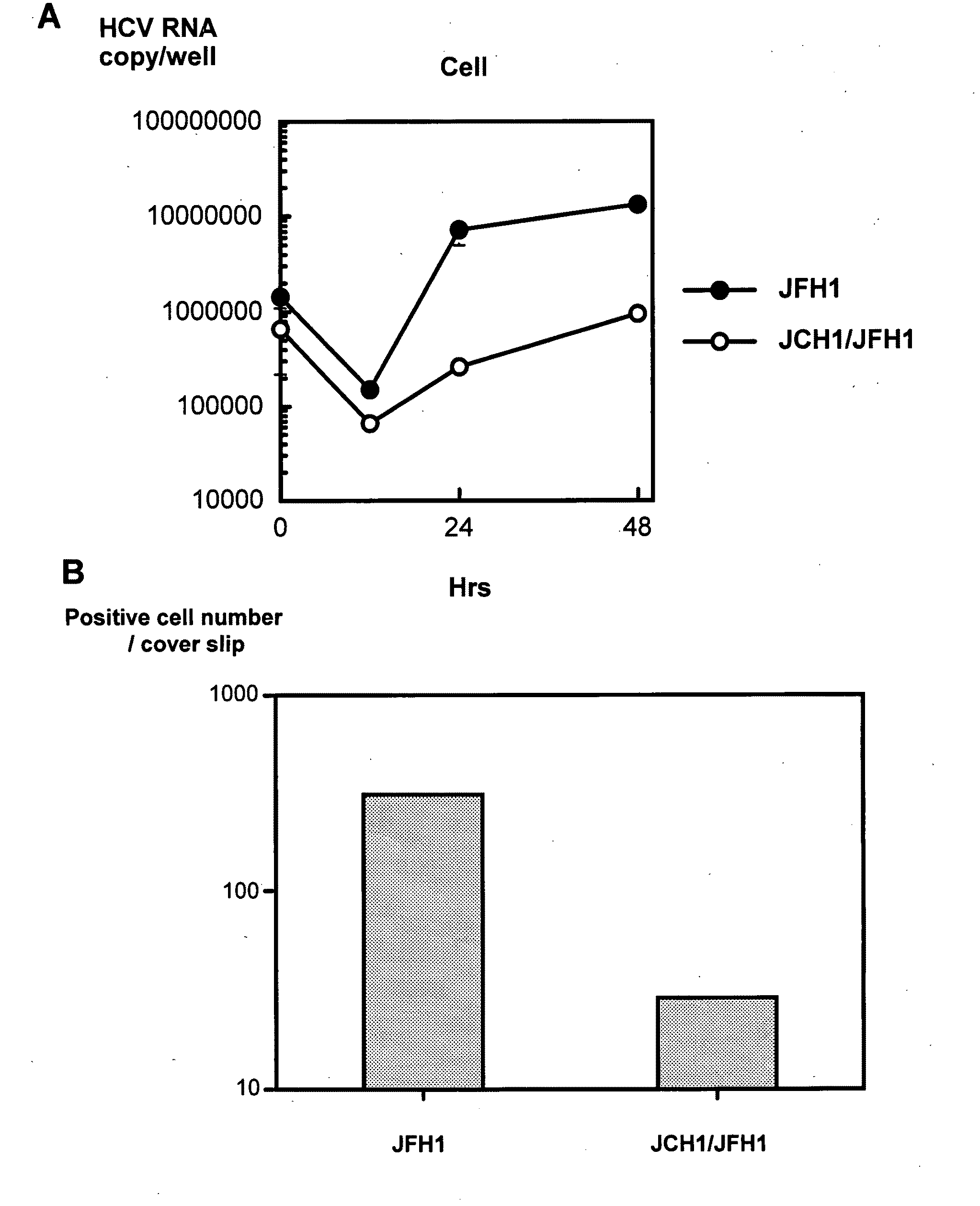
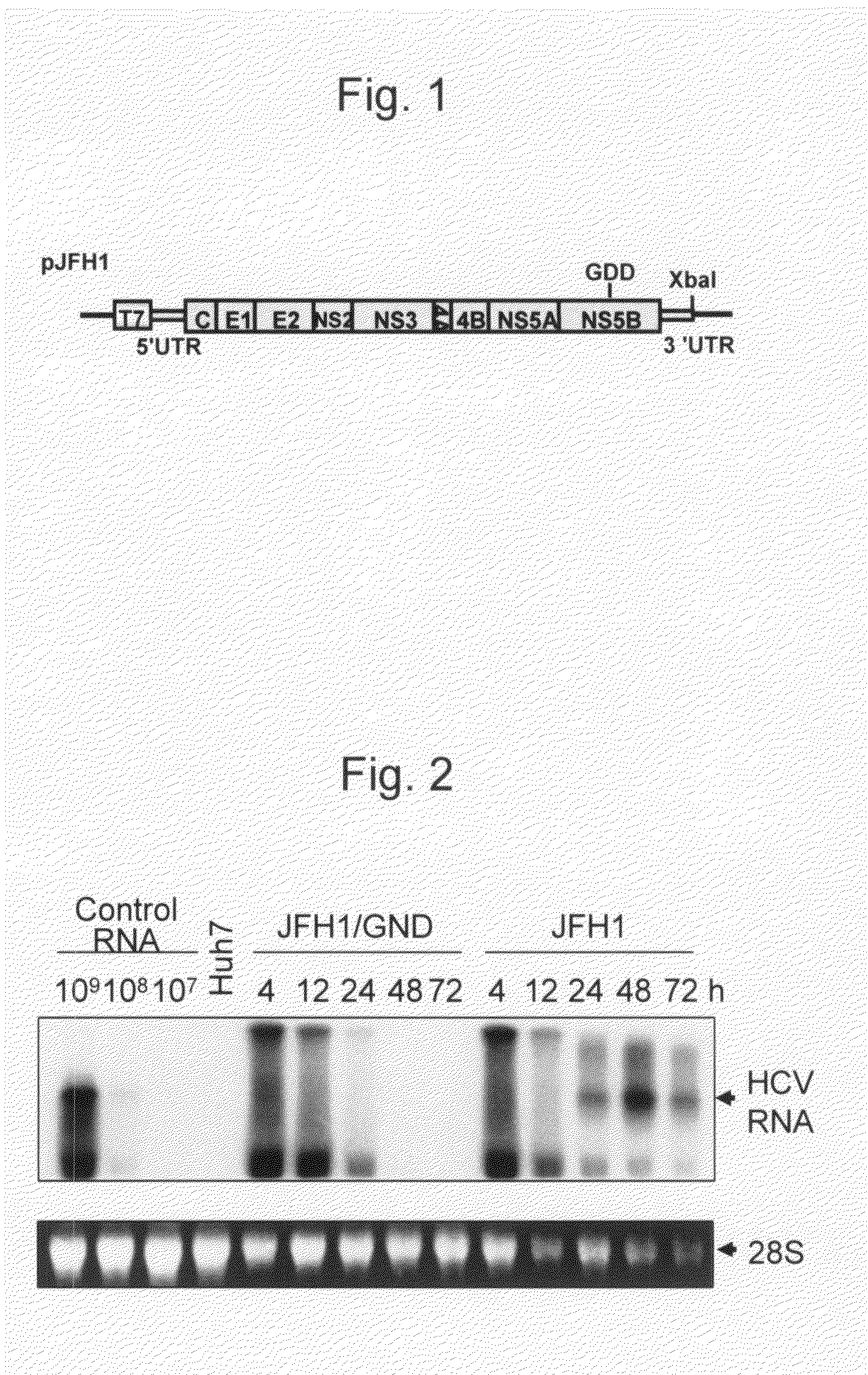
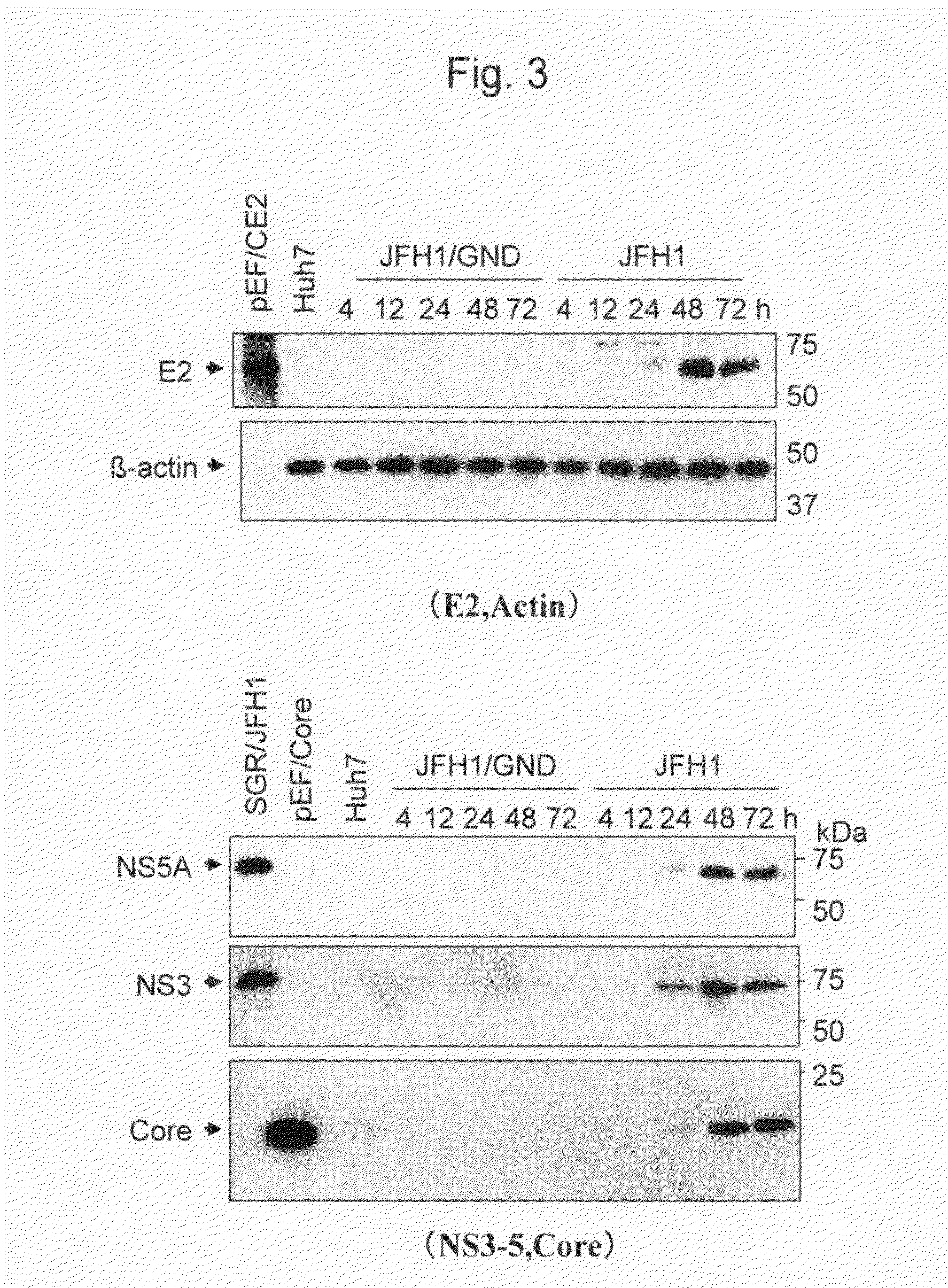
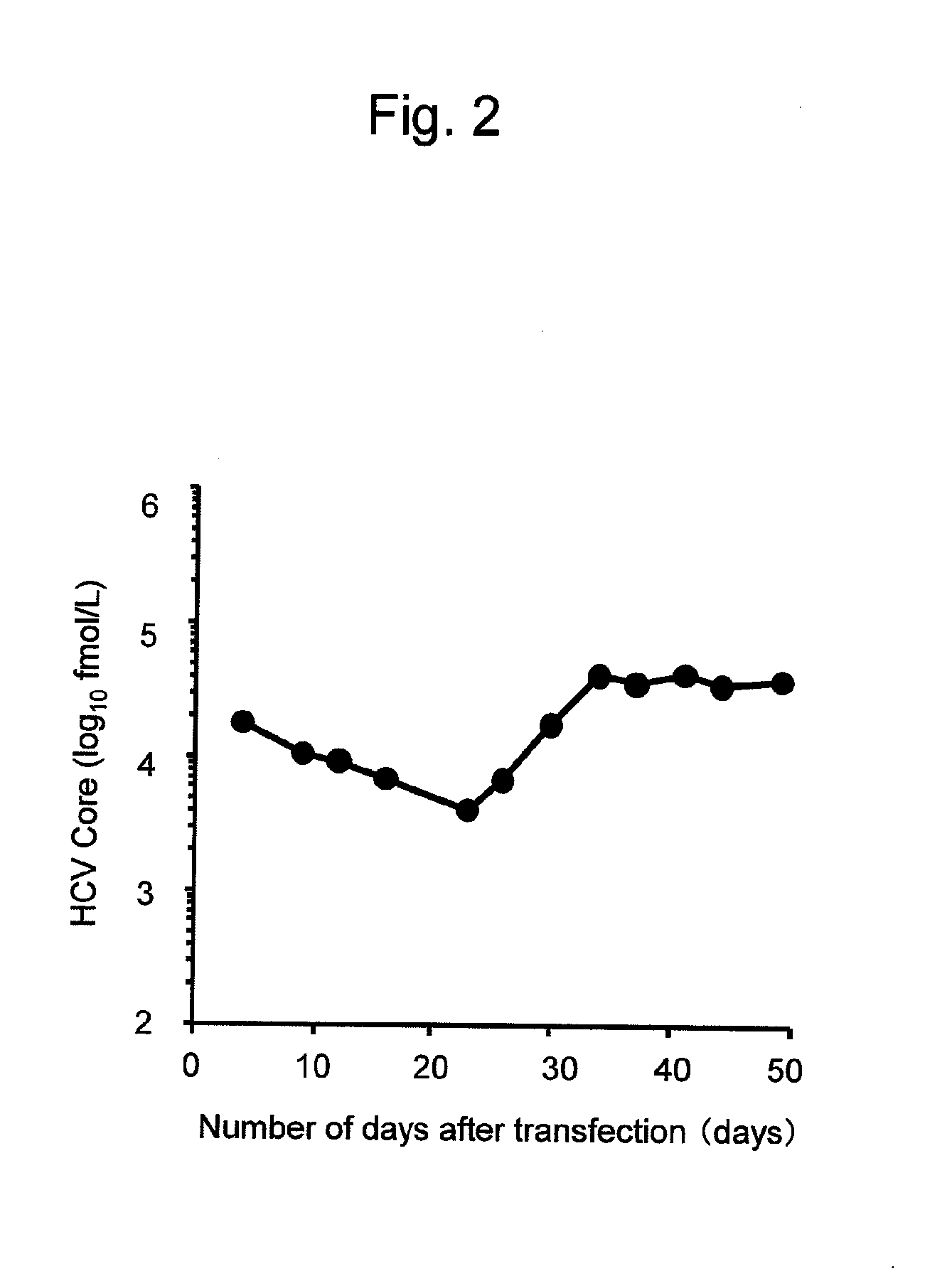
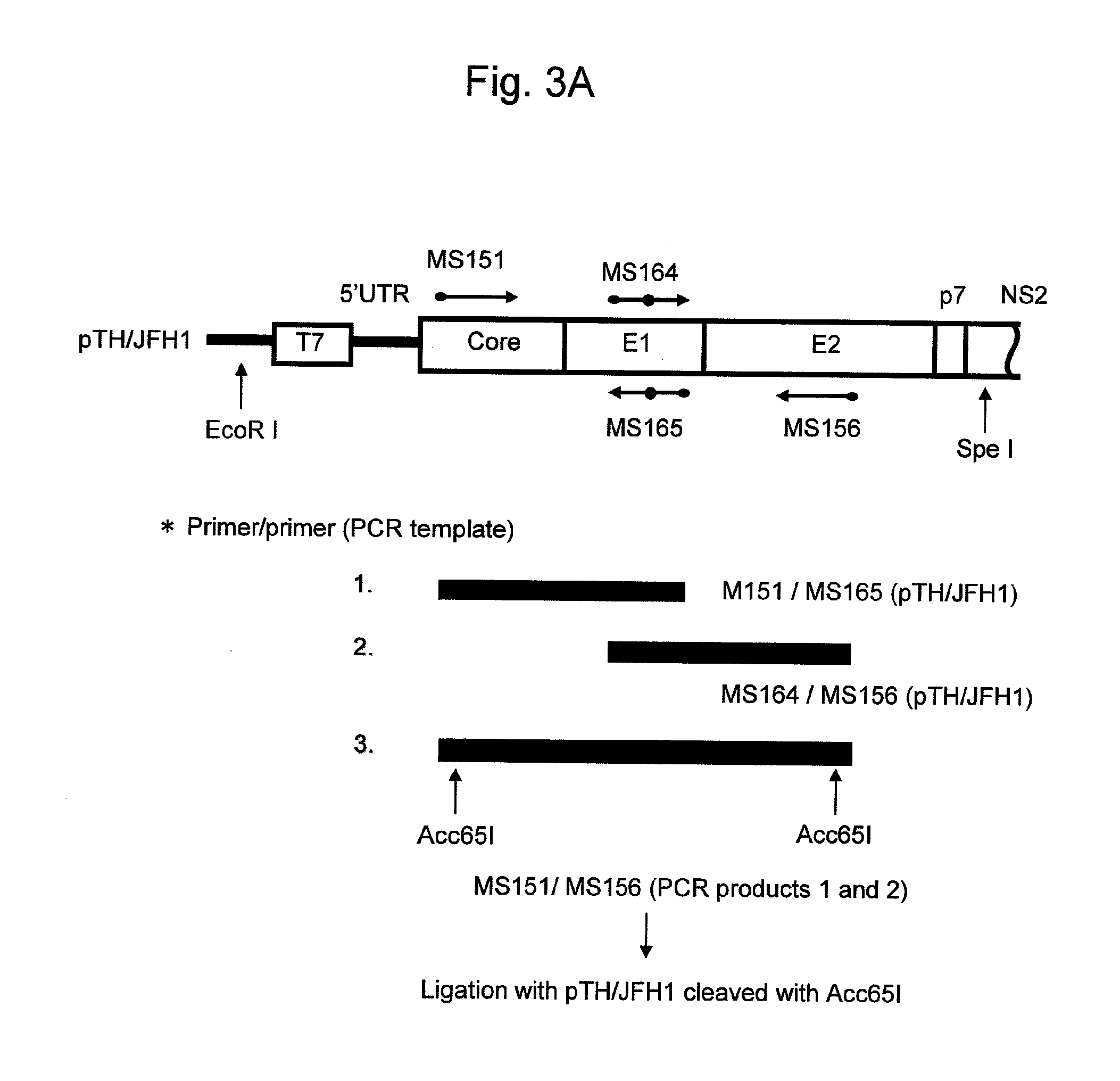
The present invention provides modified hepatitis C virus genomic RNA, comprising nucleotide sequences of genomic RNA portions of two or more types of hepatitis C viruses, which comprises a 5′ untranslated region, a core protein coding sequence, an E1 protein coding sequence, a p7 protein coding sequence, an E2 protein coding sequence, an NS2 protein coding sequence, an NS3 protein coding sequence, an NS4A protein coding sequence, an NS4B protein coding sequence, an NS5A protein coding sequence, an NS5B protein coding sequence, and a 3′ untranslated region, and which can be autonomously replicated. In particular, the present invention relates to modified hepatitis C virus genomic RNA, which can be autonomously replicated by substitution of the RNA sequence portion encoding NS3, NS4, NS5A, and NS5B proteins of hepatitis C virus genomic RNA with a partial RNA sequence encoding NS3, NS4, NS5A, and NS5B proteins of a JFH1 strain shown in SEQ ID NO: 1.
Owner:TORAY IND INC +1
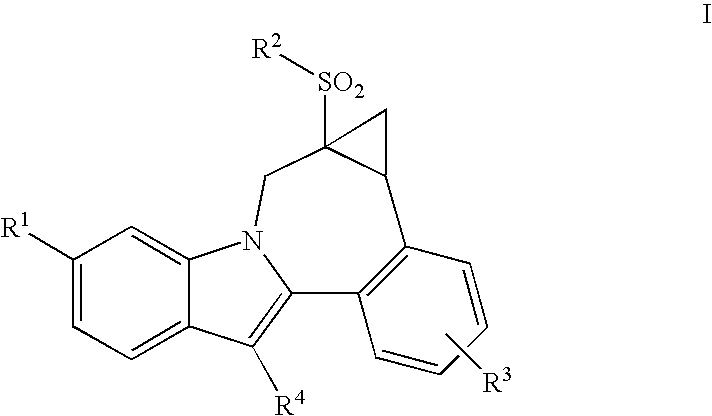
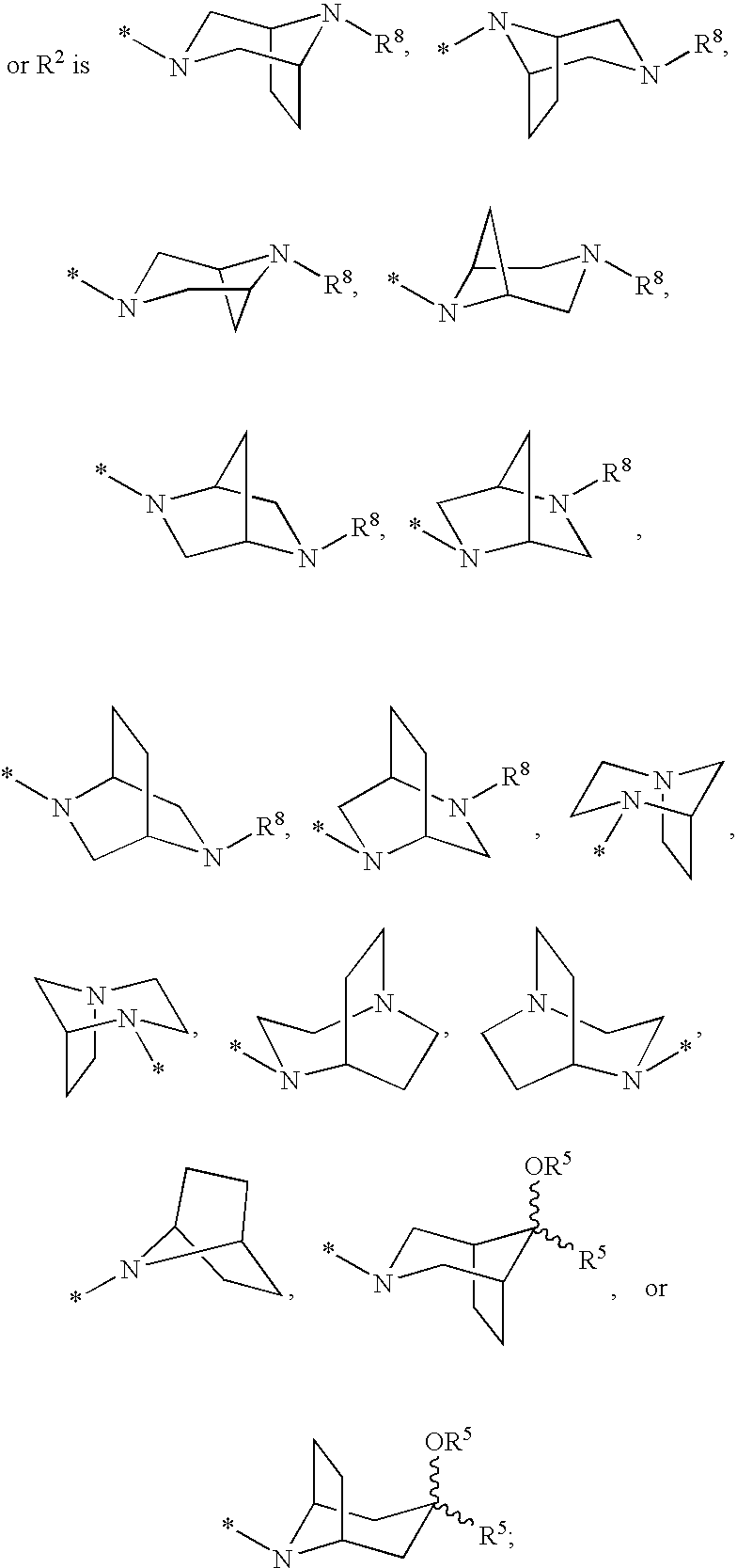
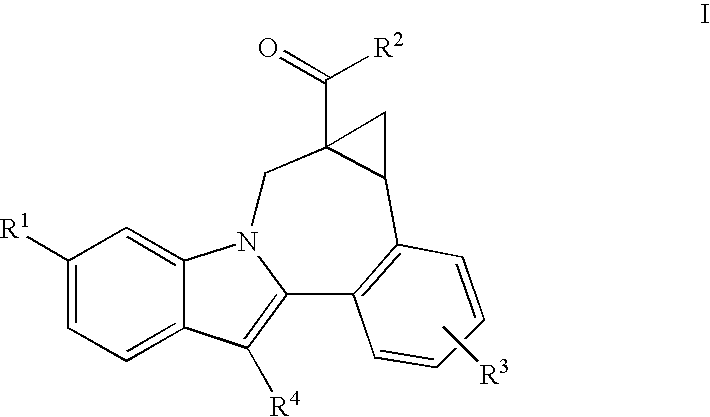
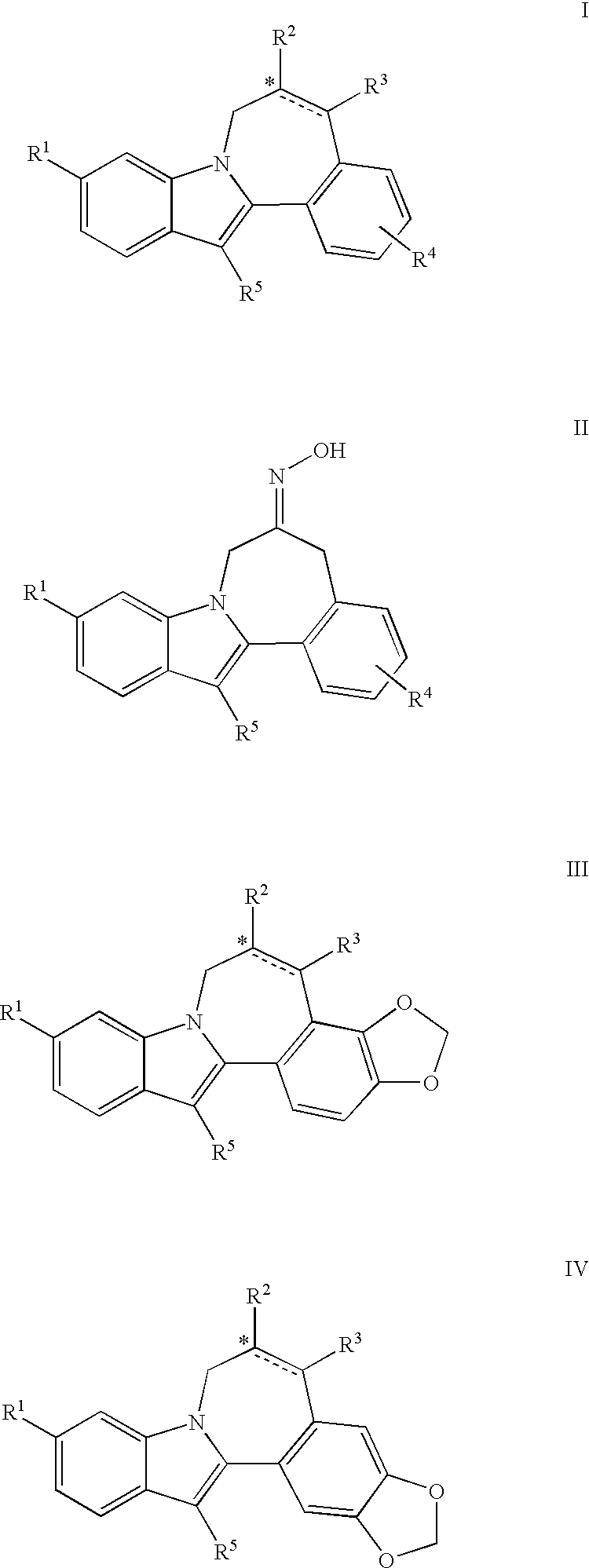
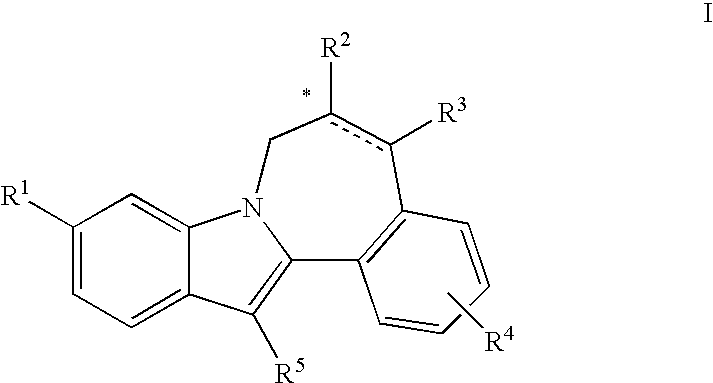
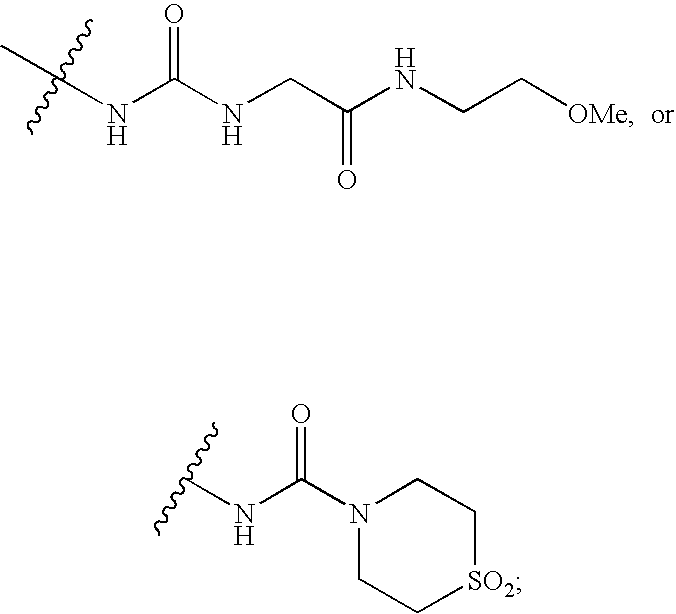
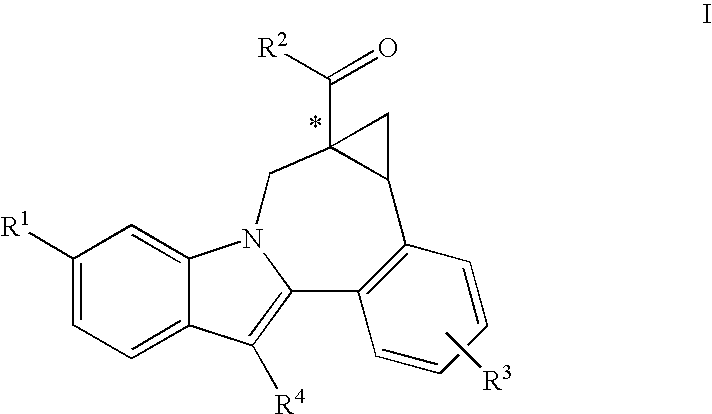
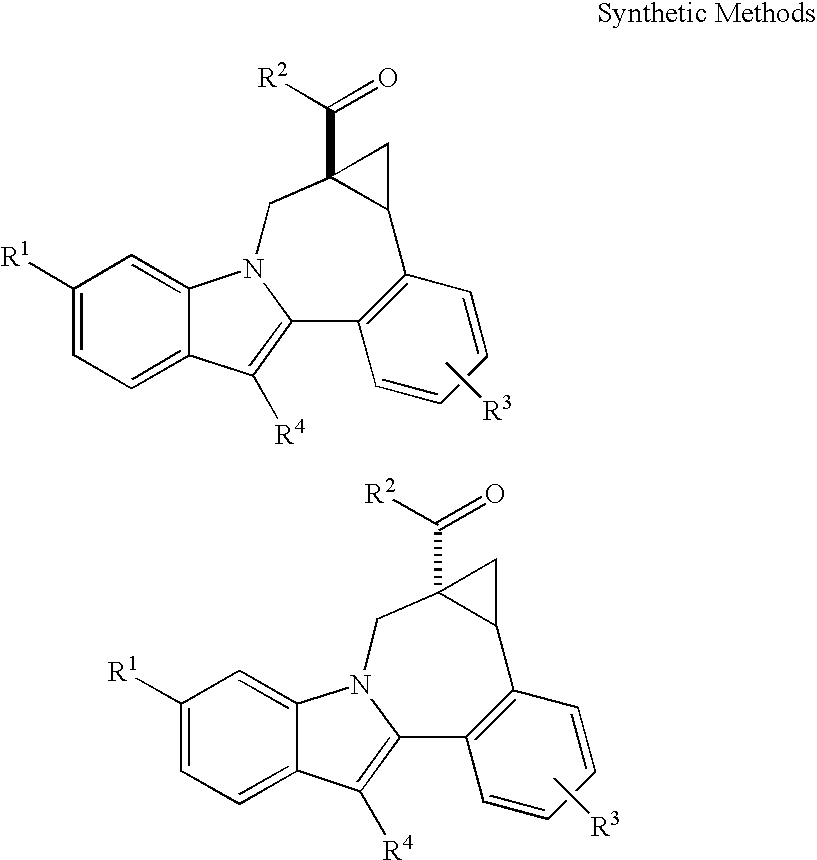
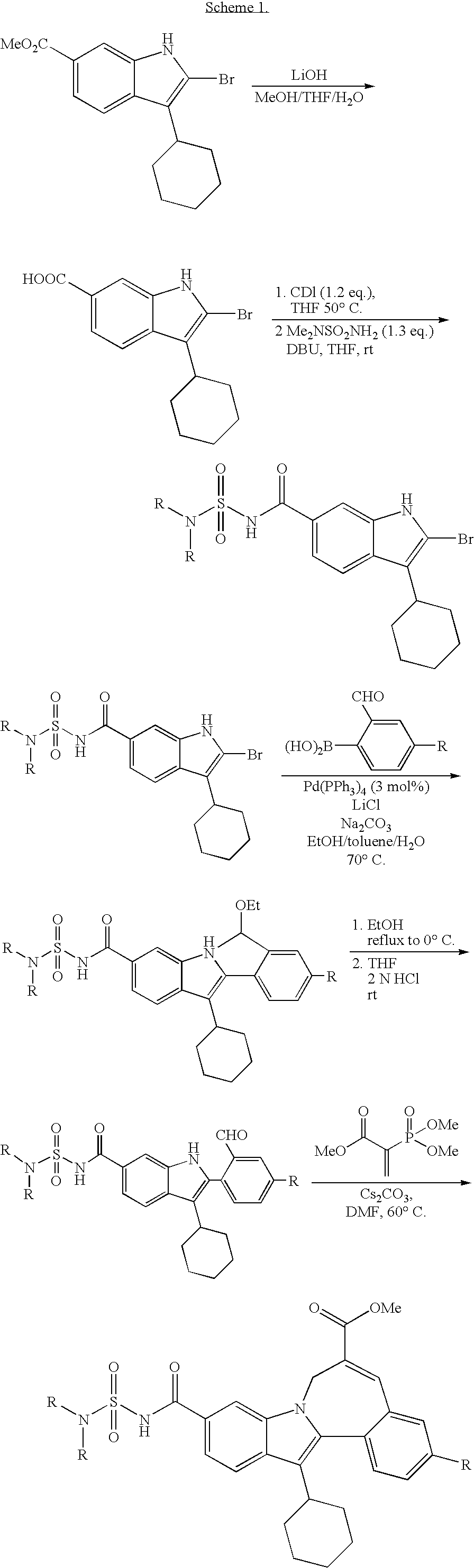
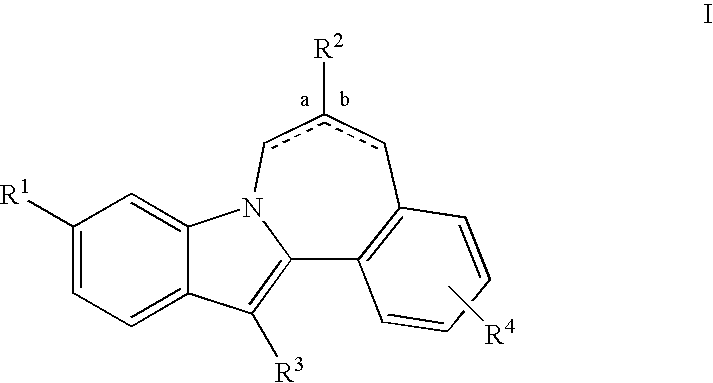
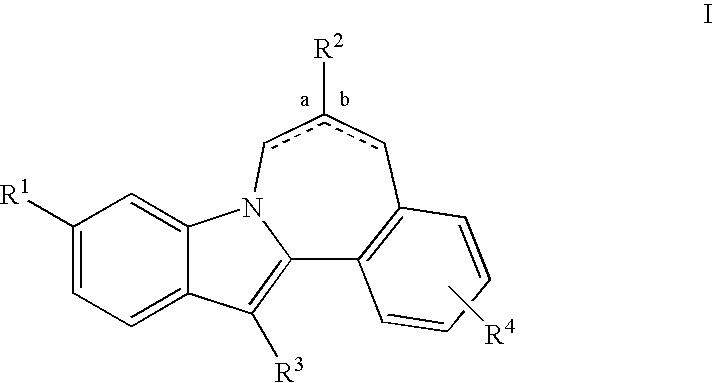
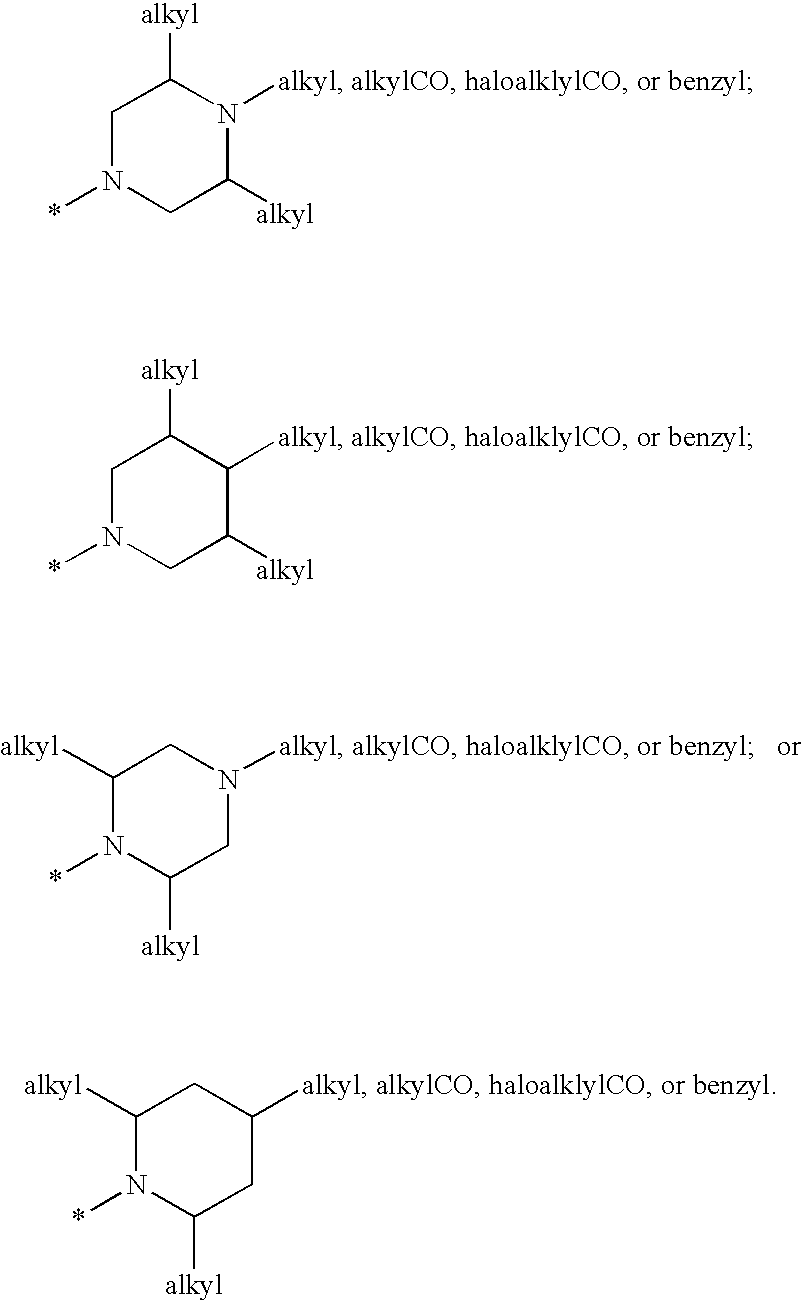
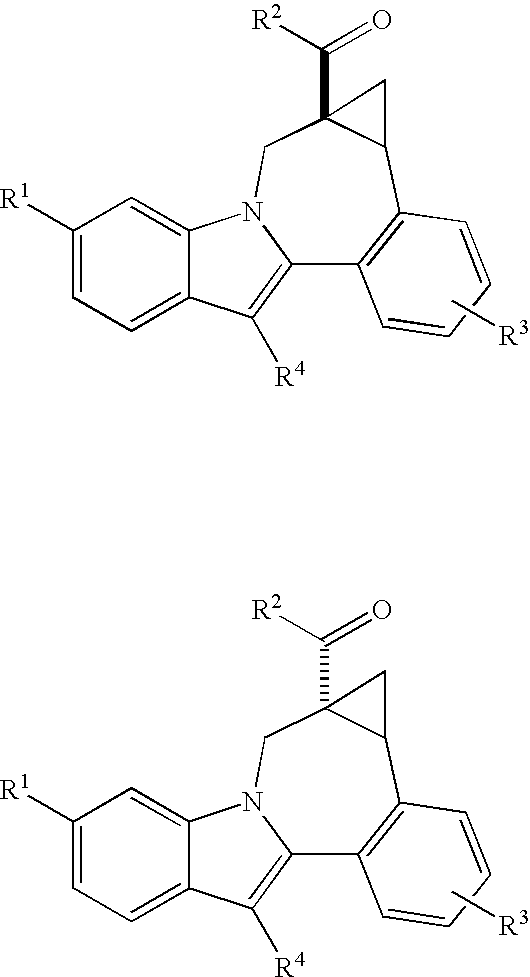
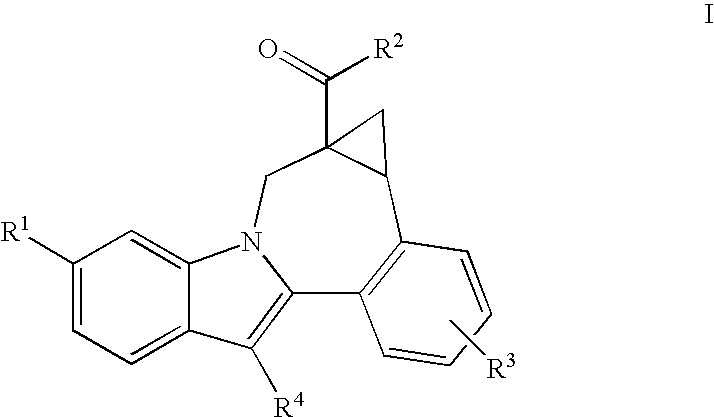
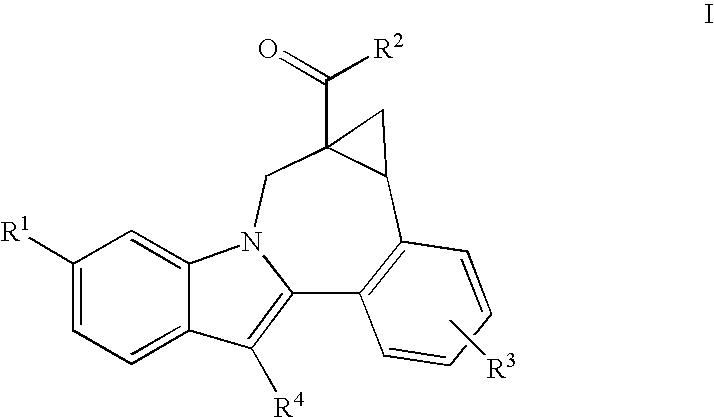
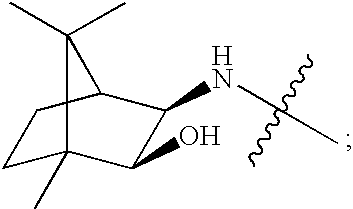
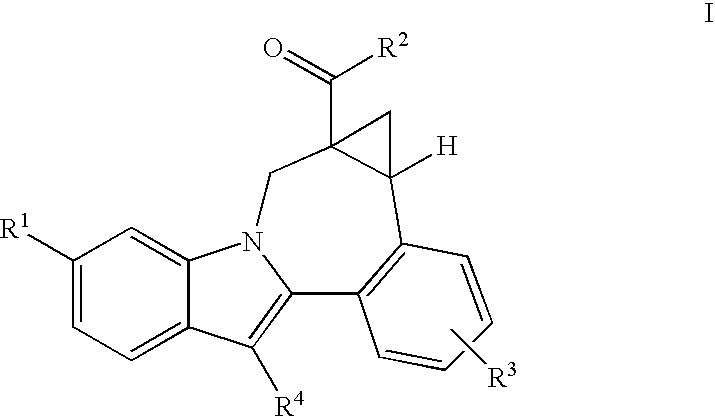
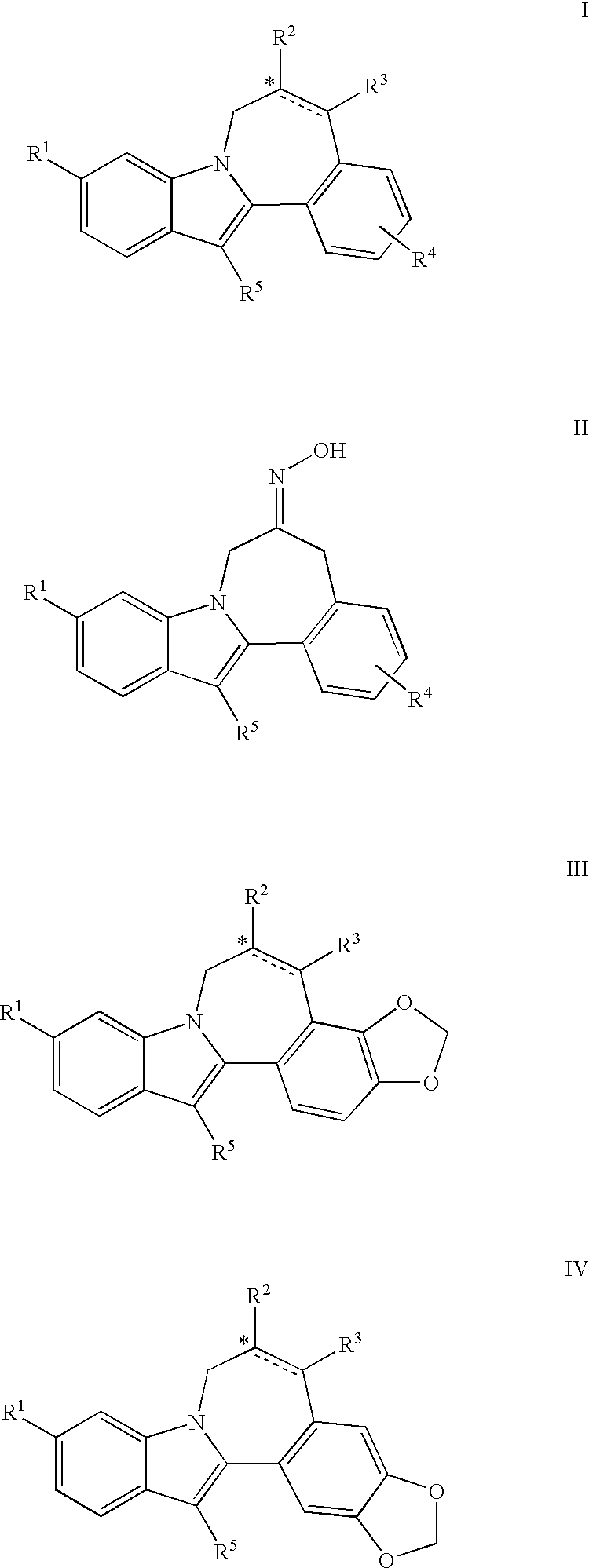
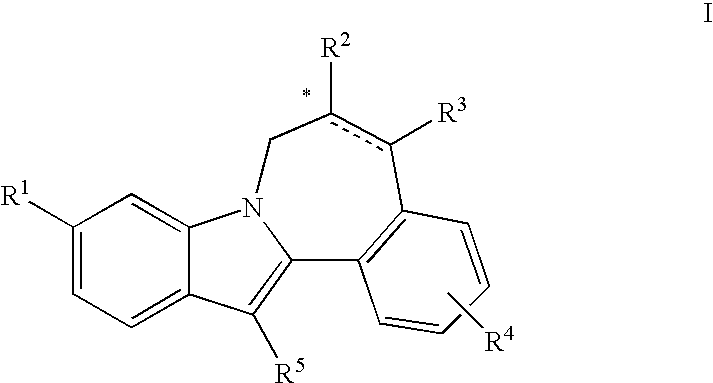
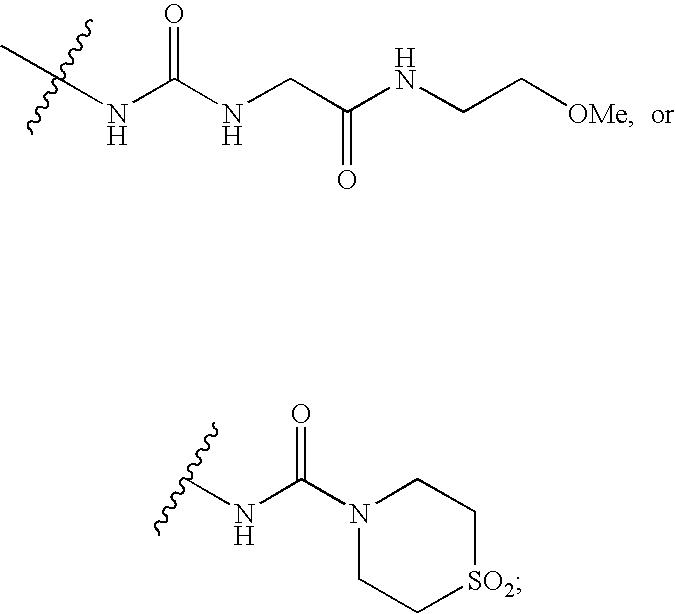
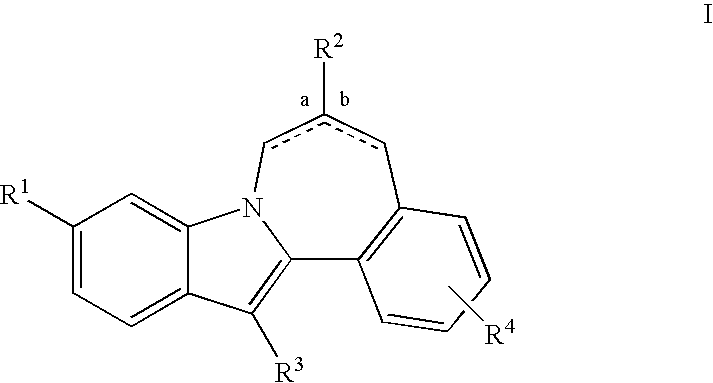
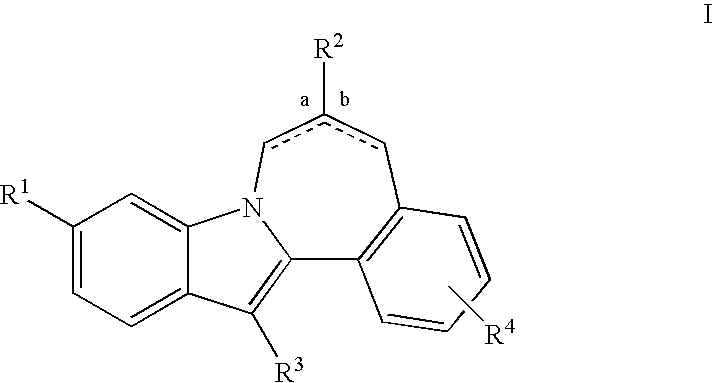
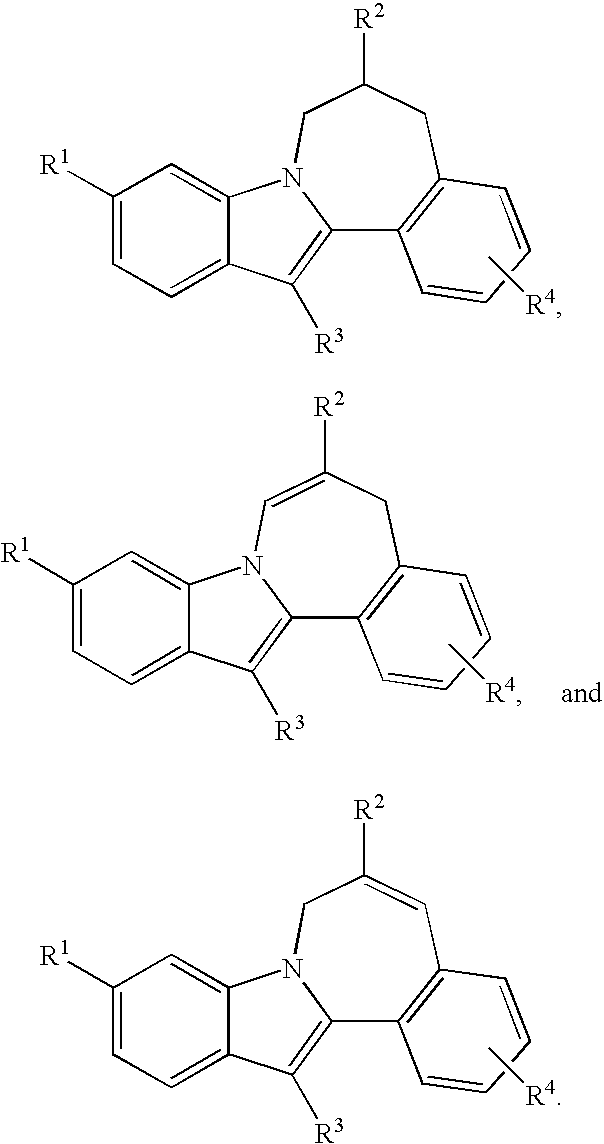
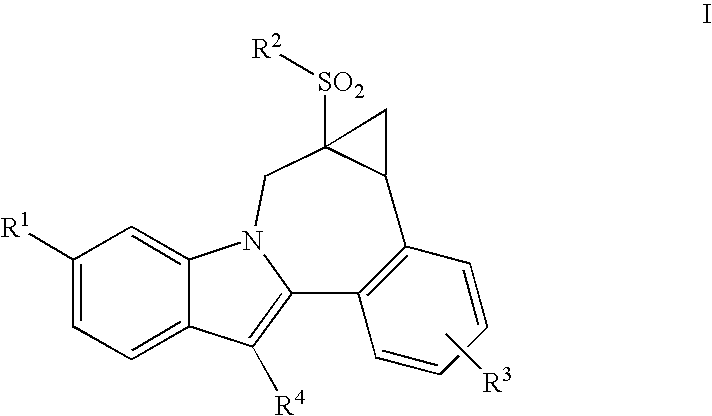
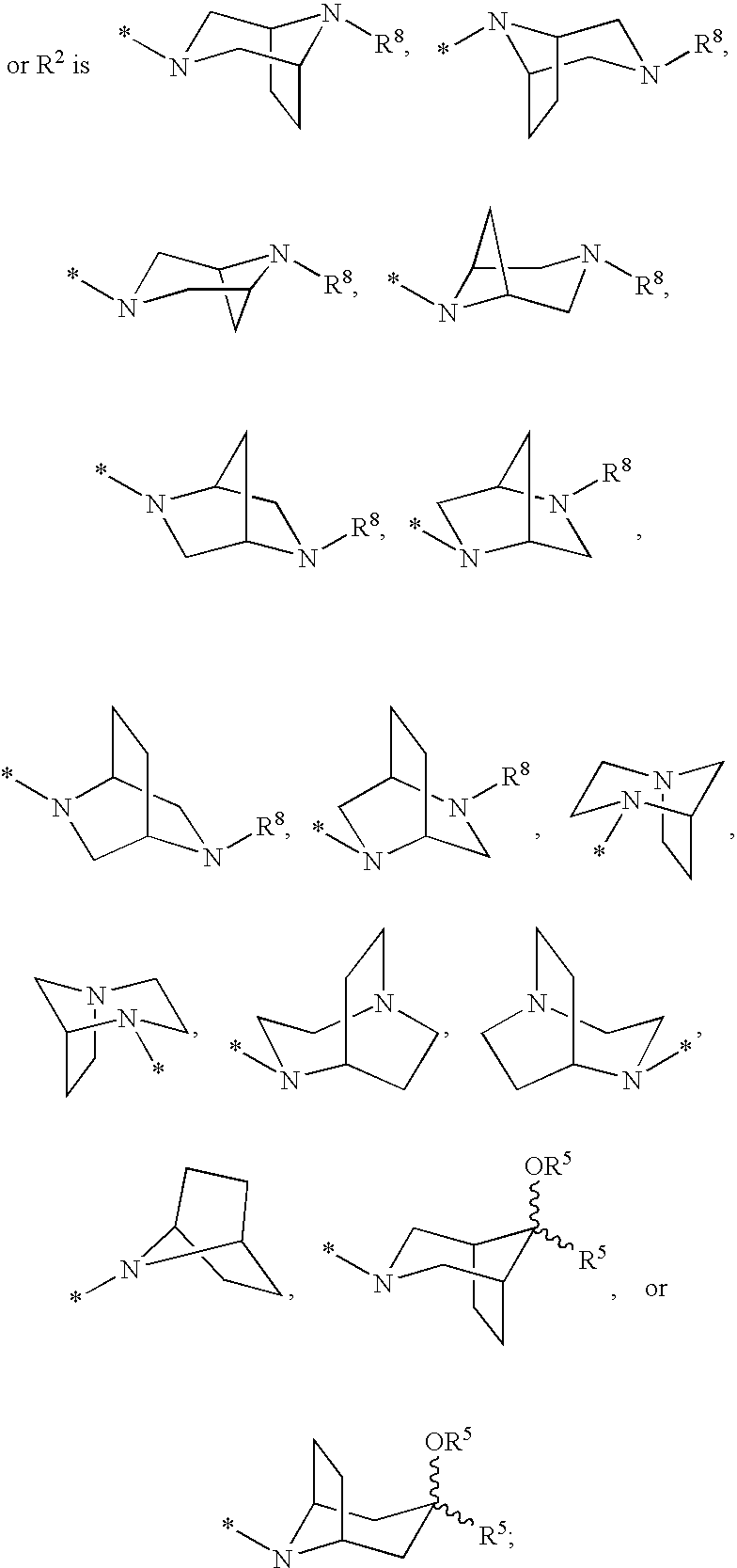
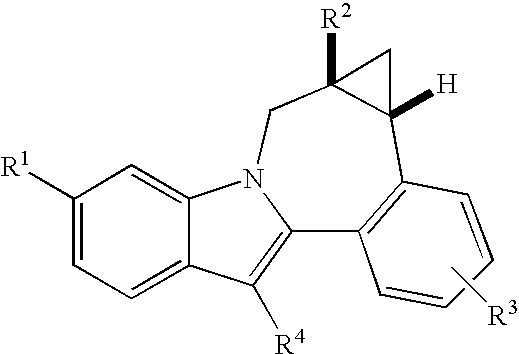
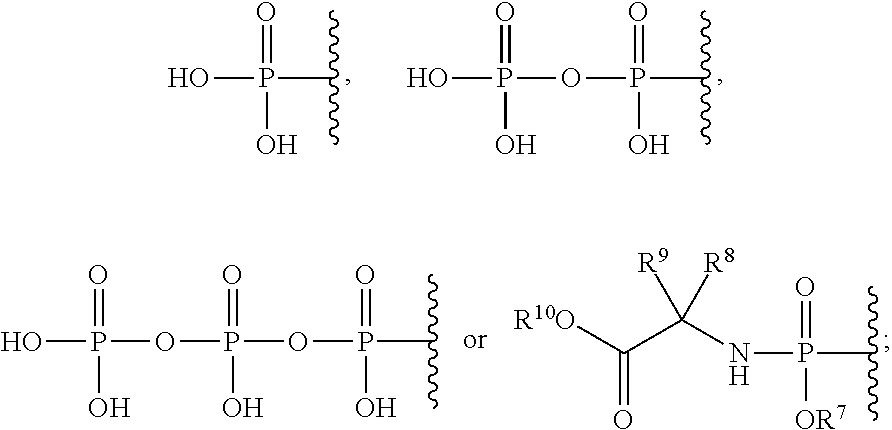
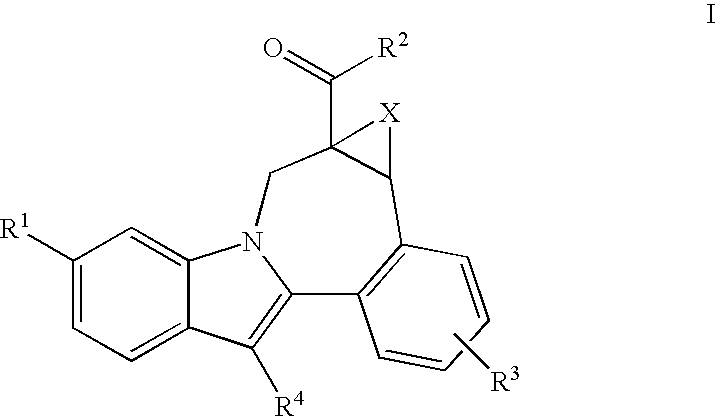
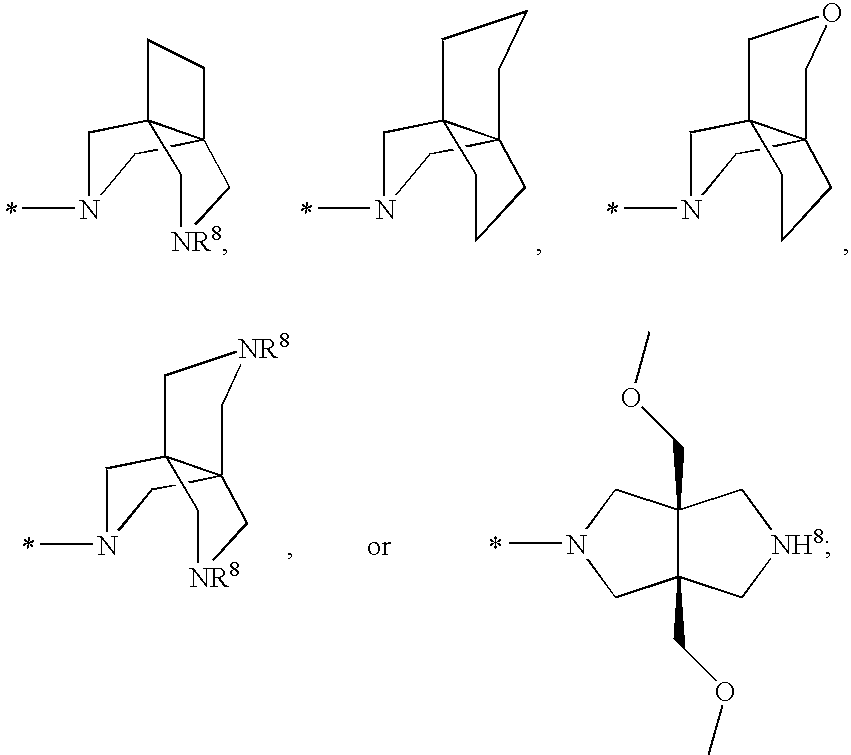
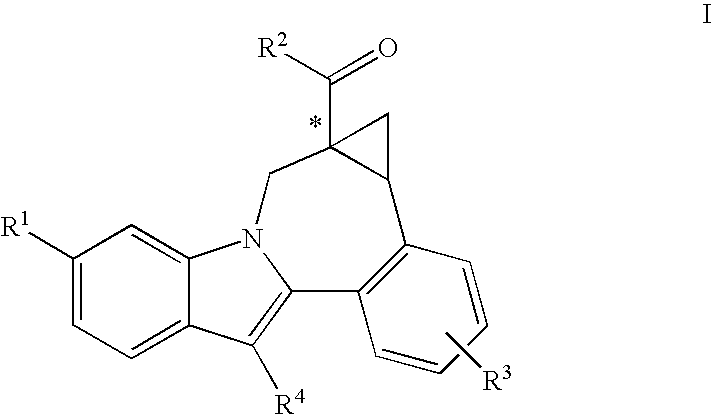
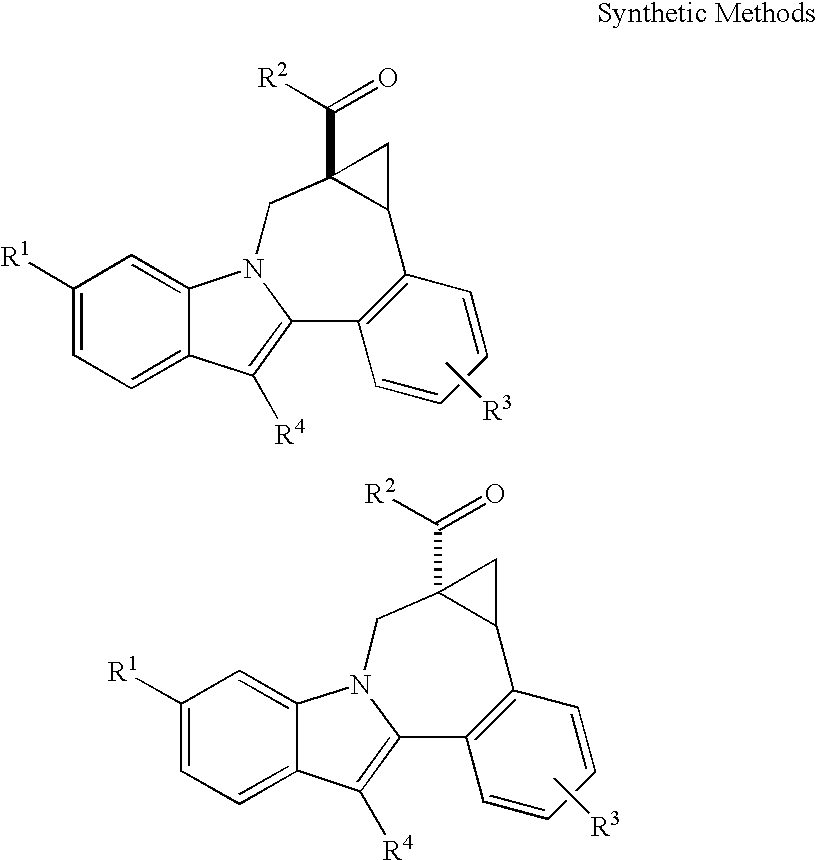
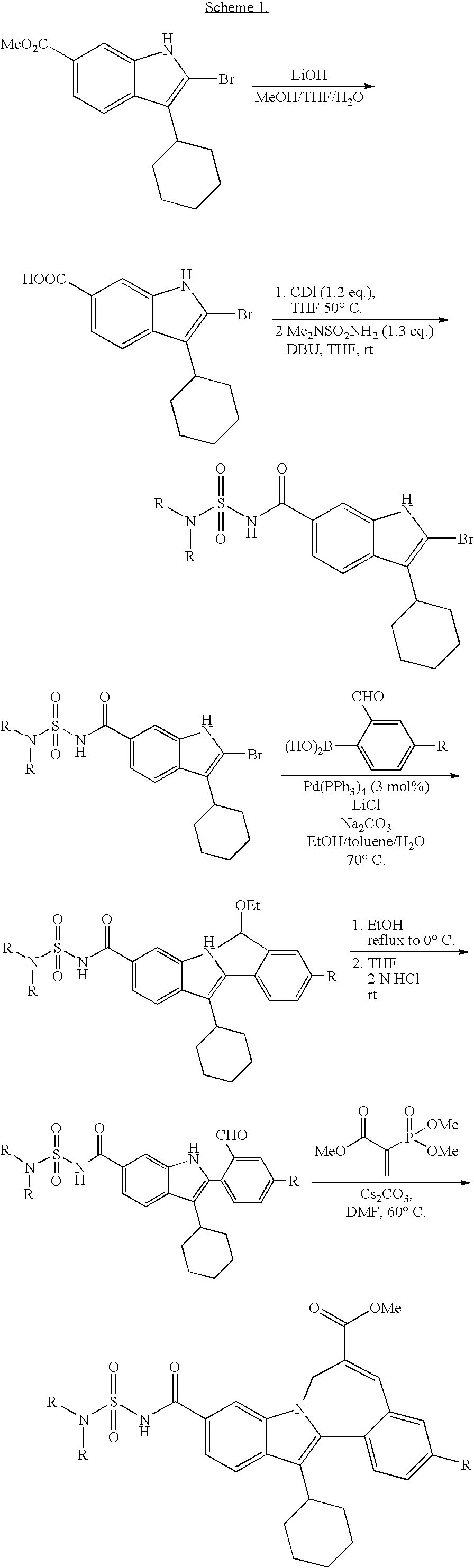
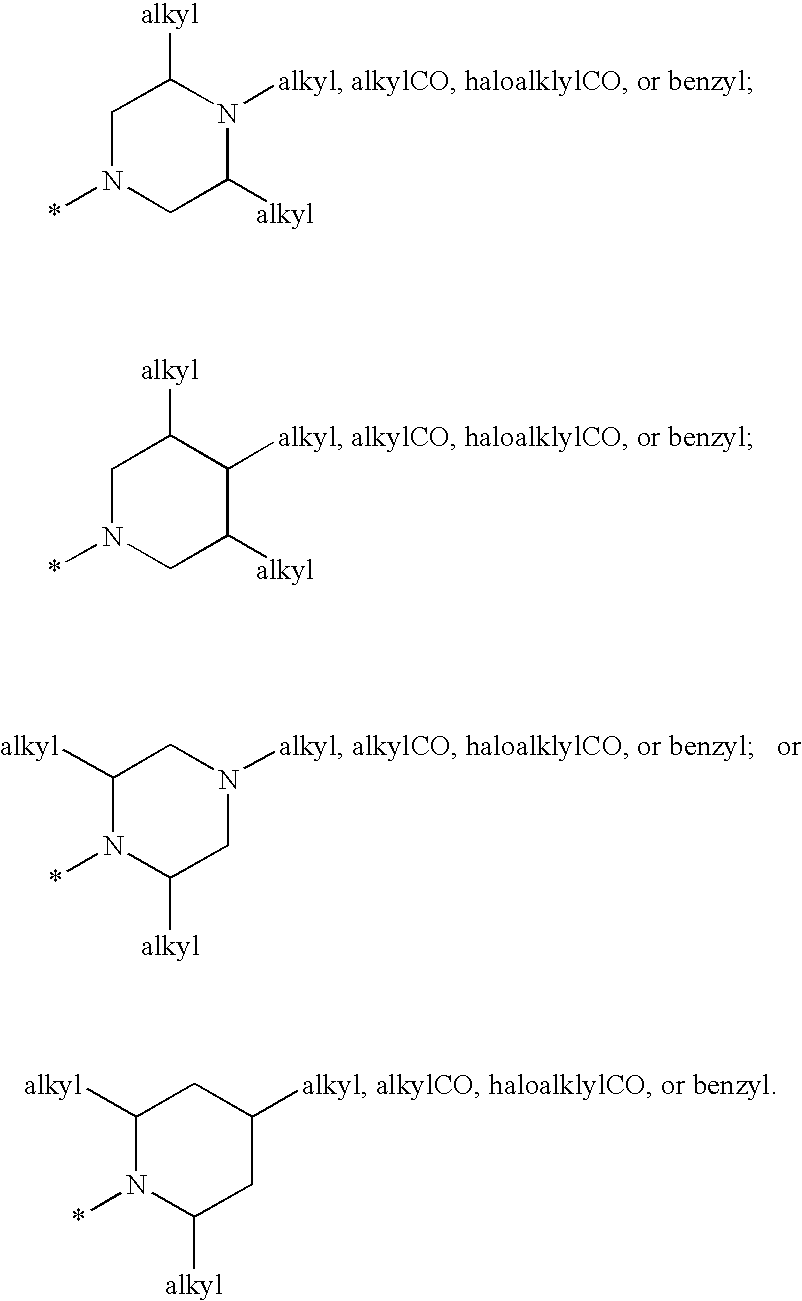
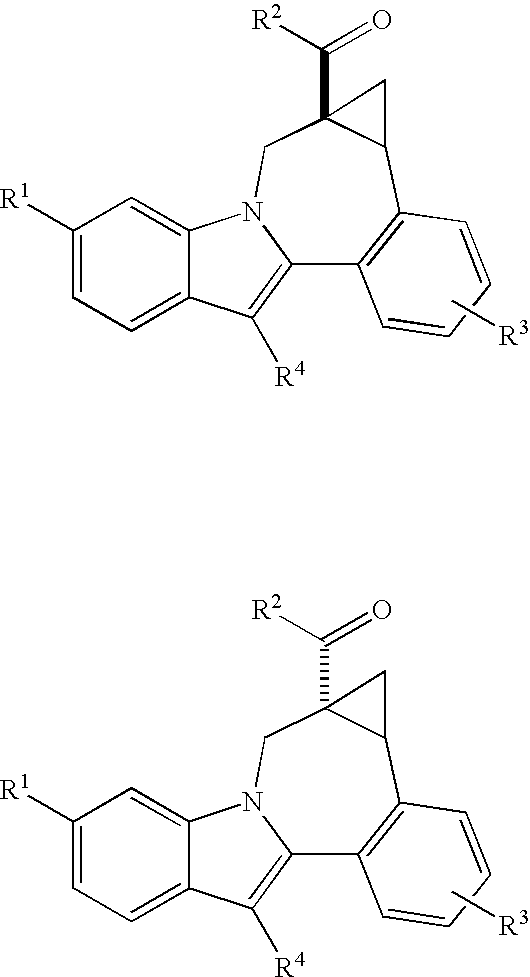
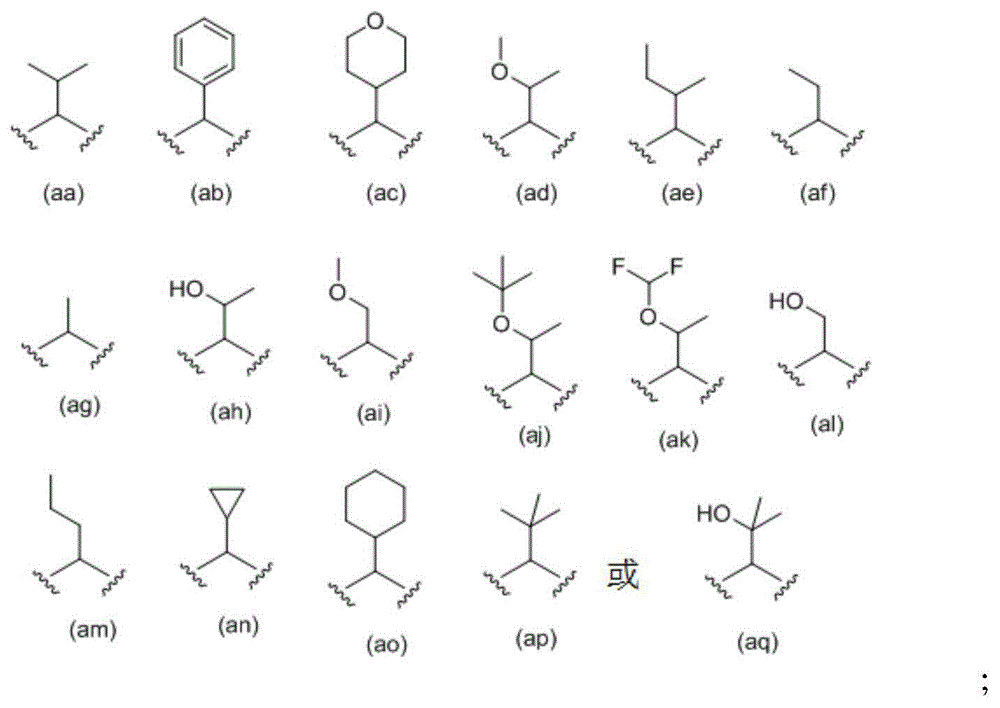
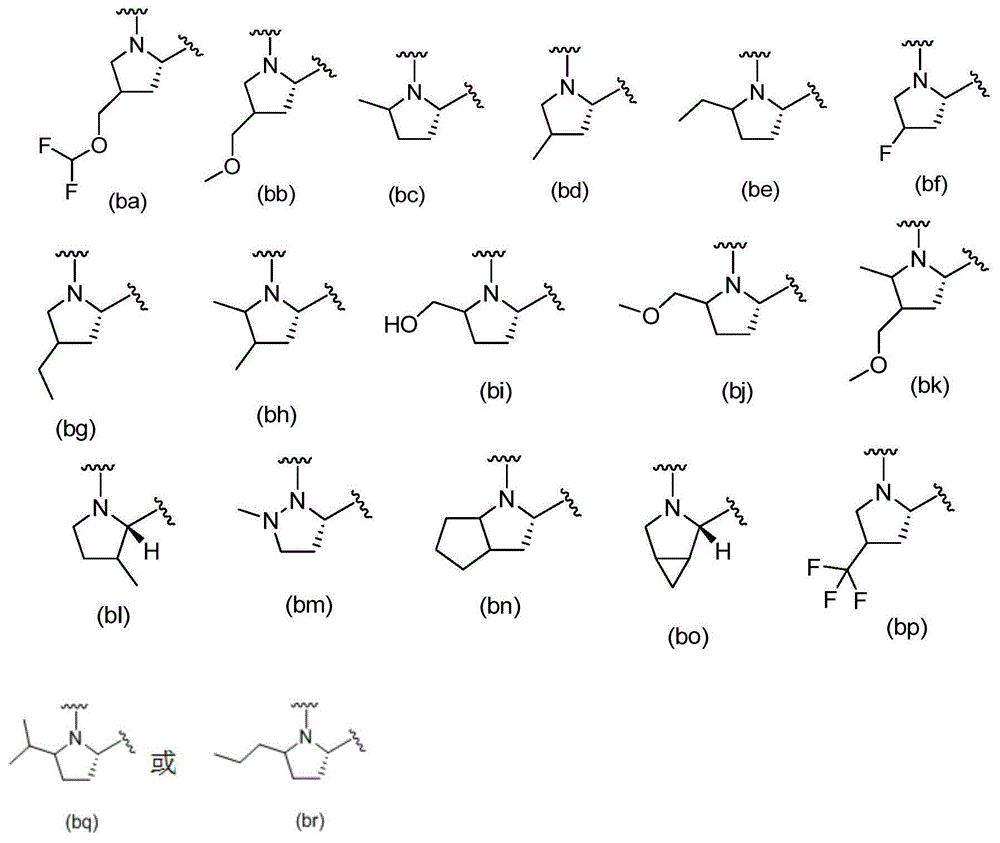
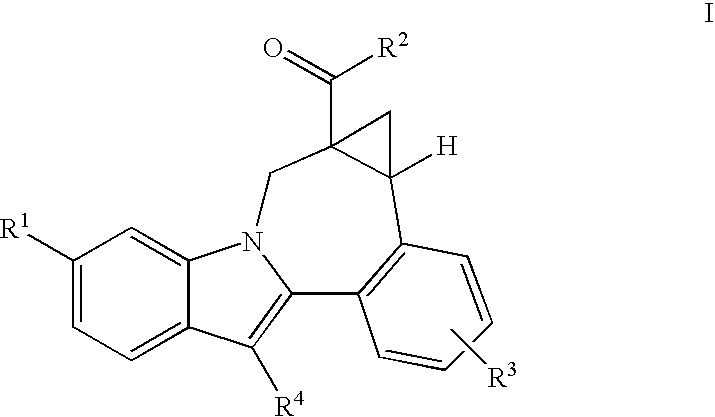
Cyclopropyl fused indolobenzazepine HCV NS5B inhibitors
Owner:BRISTOL MYERS SQUIBB CO
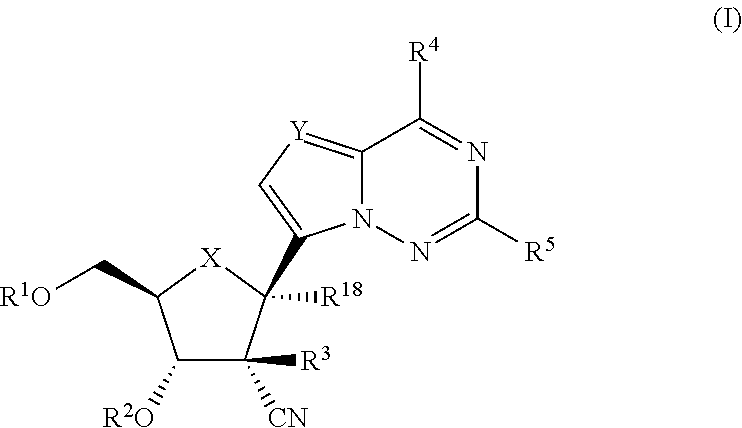
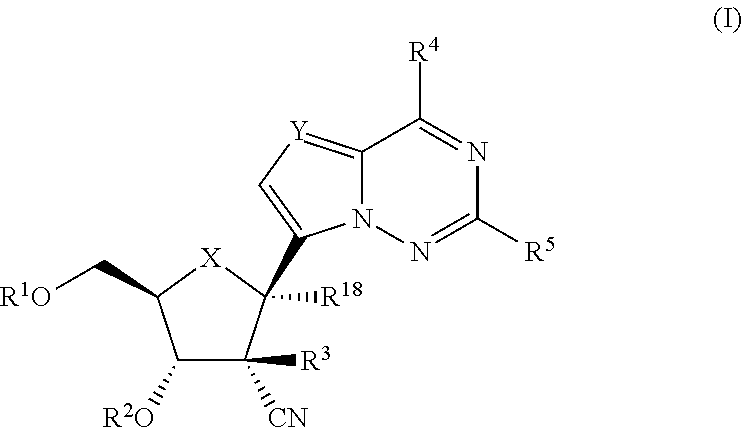
2′-cyano substituted nucleoside derivatives and methods of use thereof for the treatment of viral diseases
ActiveUS9242988B2Organic active ingredientsSugar derivativesImmunomodulating AgentPharmaceutical Substances
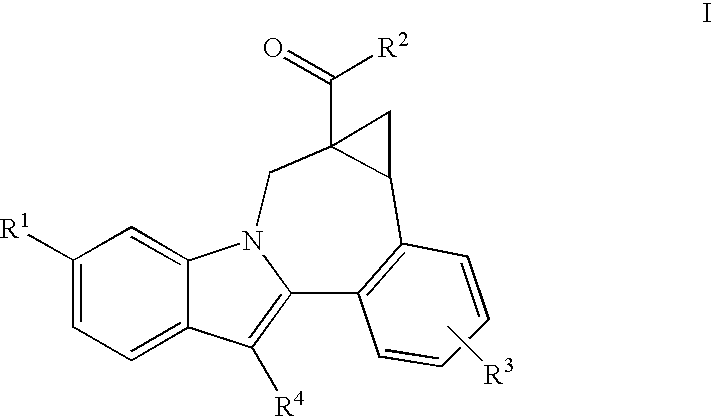
Compounds of Formula (I) are disclosed, wherein R1, R2, R3, R4, R5, and R18 are defined herein. The compounds encompassed by Formula (I) include compounds which are HCV NS5B inhibitors and other compounds which can be metabolized in vivo to HCV NS5B inhibitors. The compounds and their pharmaceutically acceptable salts are useful for the prophylaxis or treatment of infection by HCV and the prophylaxis, treatment, or delay in the onset of disease caused by HCV. The compounds and their salts can be employed as ingredients in pharmaceutical compositions, optionally in combination with other antivirals, immunomodulators, antibiotics or vaccines.
Owner:MERCK SHARP & DOHME LLC
Hepatitis C virus nucleic acid vaccine
ActiveUS9056090B2Reduce capacityReduce loadSsRNA viruses positive-senseViral antigen ingredientsMolecular biologyNucleic Acid Vaccines
The present invention features nucleic acid constructs that can be used as a HCV nucleic acid vaccine, vaccine component, or in the production of a HCV vaccine. Described constructs include those: (1) encoding for a chimeric HCV polypeptide containing a NS3-4A region based on a first HCV strain and an NS3-NS4A-NS4B-NS5A or an NS3-NS4A-NS4B-NS5A-NS5B region based on a second strain; and (2) a chimpanzee based adenovector encoding an HCV polypeptide.
Owner:MSD ITAL
Antibody having inhibitory activity on infection with hepatitis c virus (HCV) and use thereof
The objection of the invention is to provide an antibody that inhibits infection with hepatitis C virus (HCV). To this end, this invention provides an antibody that recognizes the hepatitis C virus (HCV) particle obtained from the hepatitis C virus (HCV) genome comprising the following (i) and (ii) ligated to each other as an antigen and has an inhibitory activity on infection with hepatitis C virus (HCV): (i) (a) the 5′-untranslated region, the core protein-encoding sequence, the E1 protein-encoding sequence, the E2 protein-encoding sequence, and the p7 protein-encoding sequence of the JFH-1 strain of the hepatitis C virus (HCV) or (b) the 5′-untranslated region, the core protein-encoding sequence, the E1 protein-encoding sequence, the E2 protein-encoding sequence, and the p7 protein-encoding sequence of the J6CF strain the hepatitis C virus (HCV); and (ii) the NS2 protein-encoding sequence, the NS3 protein-encoding sequence, the NS4A protein-encoding sequence, the NS4B protein-encoding sequence, the NS5A protein-encoding sequence, the NS5B protein-encoding sequence, and the 3′-untranslated region of the JFH-1 strain.
Owner:TORAY IND INC +1
Antiviral compounds inhibitors of HCV NS5B
ActiveCN104487442AEfficacy of significantly resistant variantsOrganic active ingredientsOrganic chemistryStereochemistryAnti virals
Owner:GILEAD SCI INC
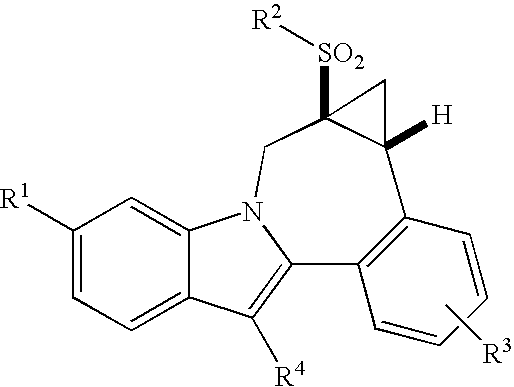
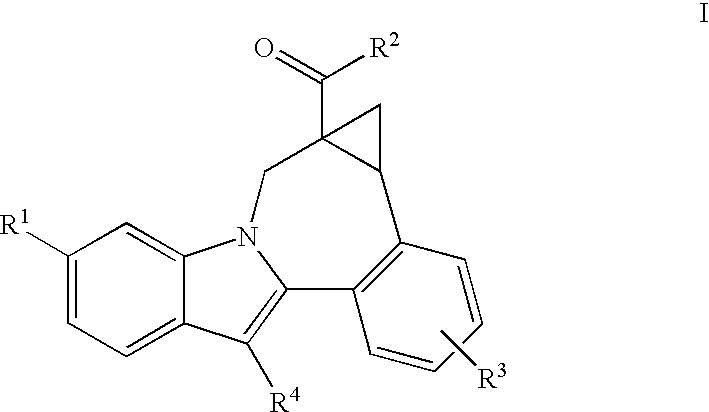
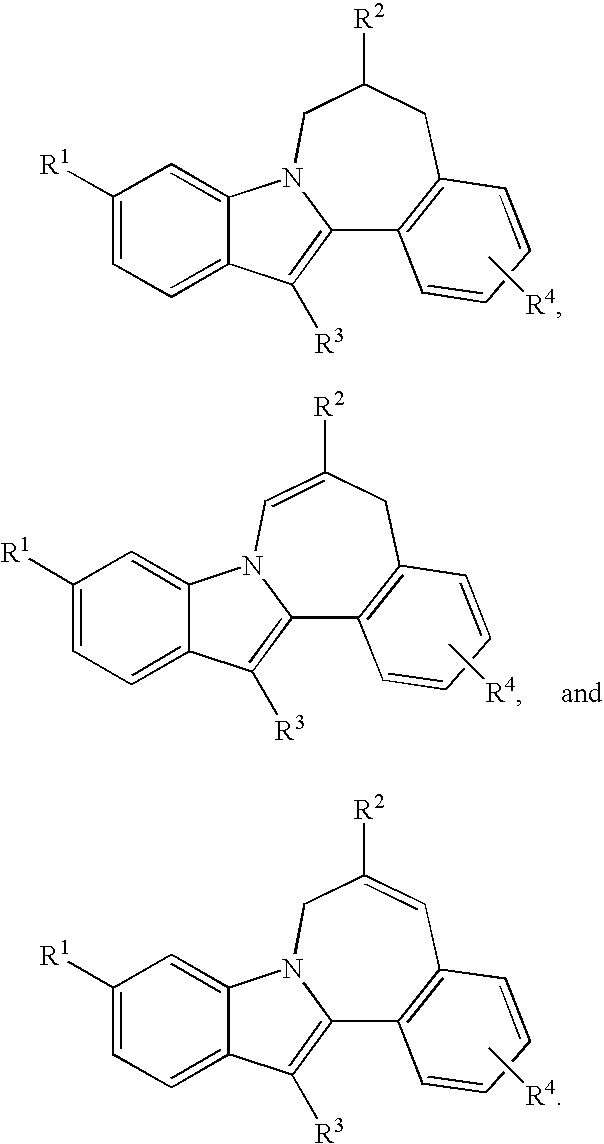
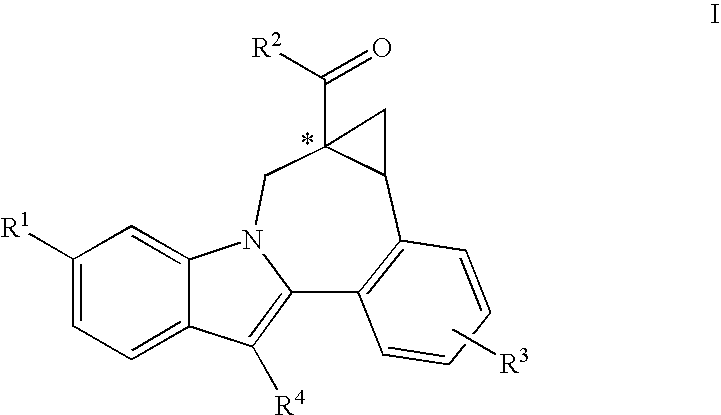
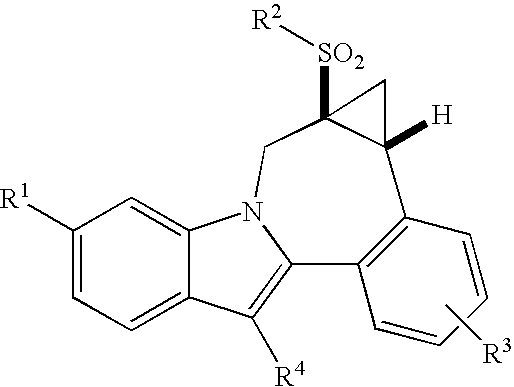
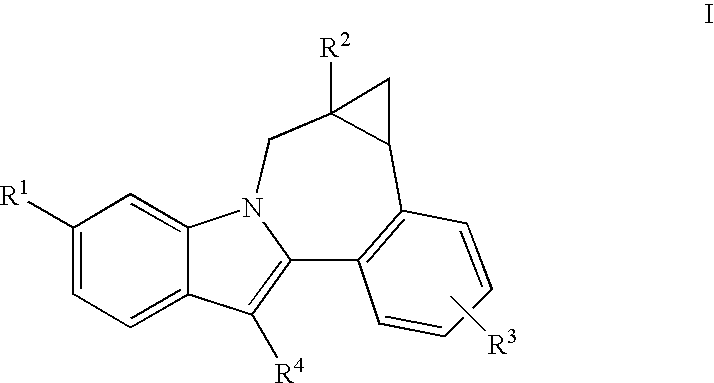
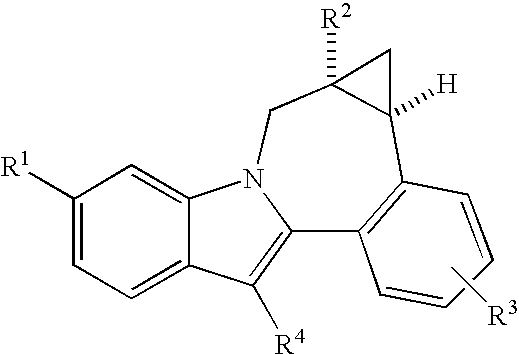
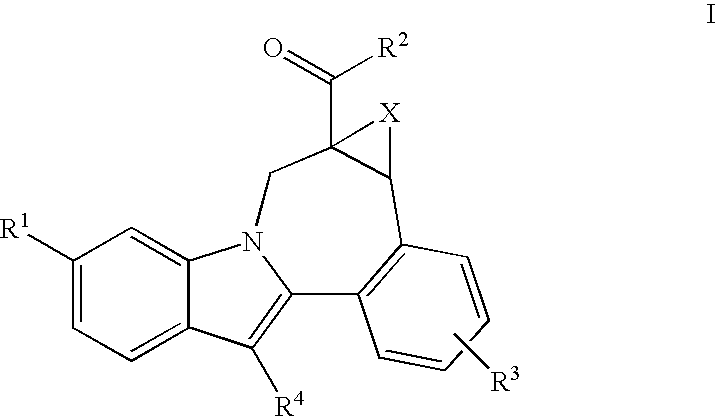
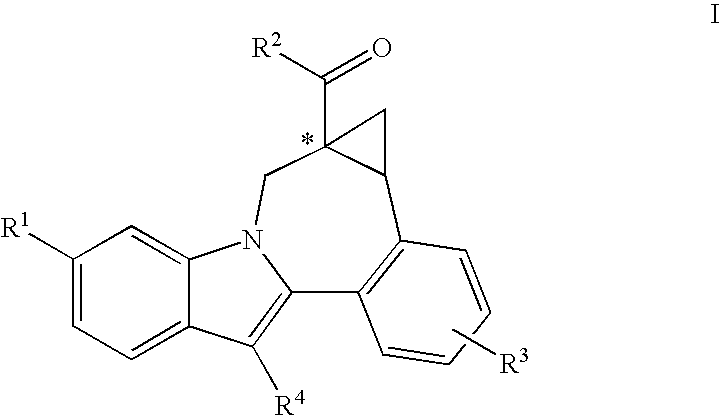
Cyclopropyl fused indolobenzazepine HCV NS5B inhibitors
Owner:BRISTOL MYERS SQUIBB CO
Hepatitis C virus vaccine
ActiveUS7598362B2Facilitates cleavageFacilitates ribosome bindingFungiSsRNA viruses positive-senseAntigenRNA-dependent RNA polymerase
Owner:MERCK SHARP & DOHME LLC +1
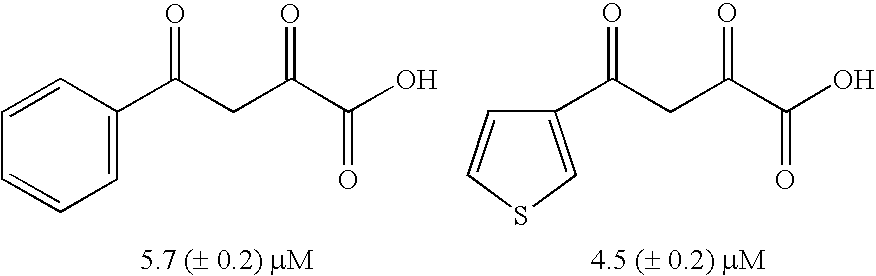
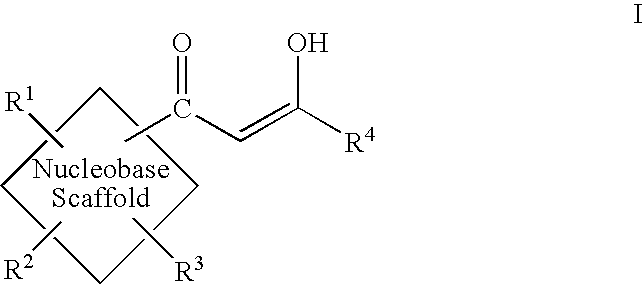
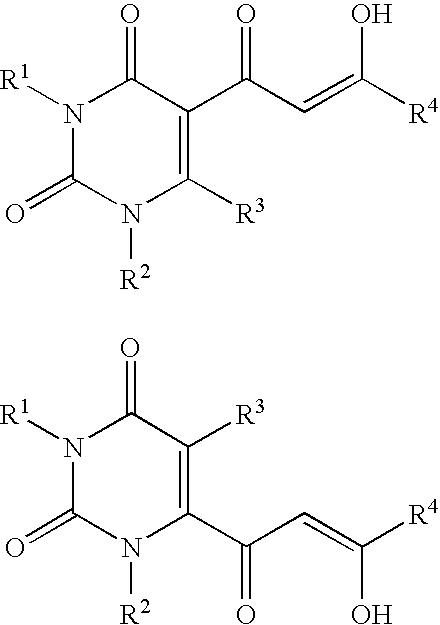
Diketo acids on nucleobase scaffolds as inhibitors of Flaviviridae
A new class of diketo acids constructed on nucleobase scaffolds, designed as inhibitors of HCV replication through inhibition of HCV NS5B RNA polymerase, is described. These compounds are useful in the prevention or treatment of infection by HCV and in the treatment of other Flaviviridae infections, either as the compounds, or as pharmaceutically acceptable salts, with pharmaceutically acceptable carriers, used alone or in combination with antivirals, immunomodulators, antibiotics, vaccines, and other therapeutic agents. Methods of treating HCV and methods of treating or preventing infection by HCV are also described.
Owner:UNIV OF GEORGIA RES FOUND INC
In vitro activity measuring method for hepatitis C virus RNA depending RNA polymerase and application thereof
InactiveCN101168781ASimple methodPractical methodMicrobiological testing/measurementLibrary screeningMagnetite NanoparticlesGenetic engineering
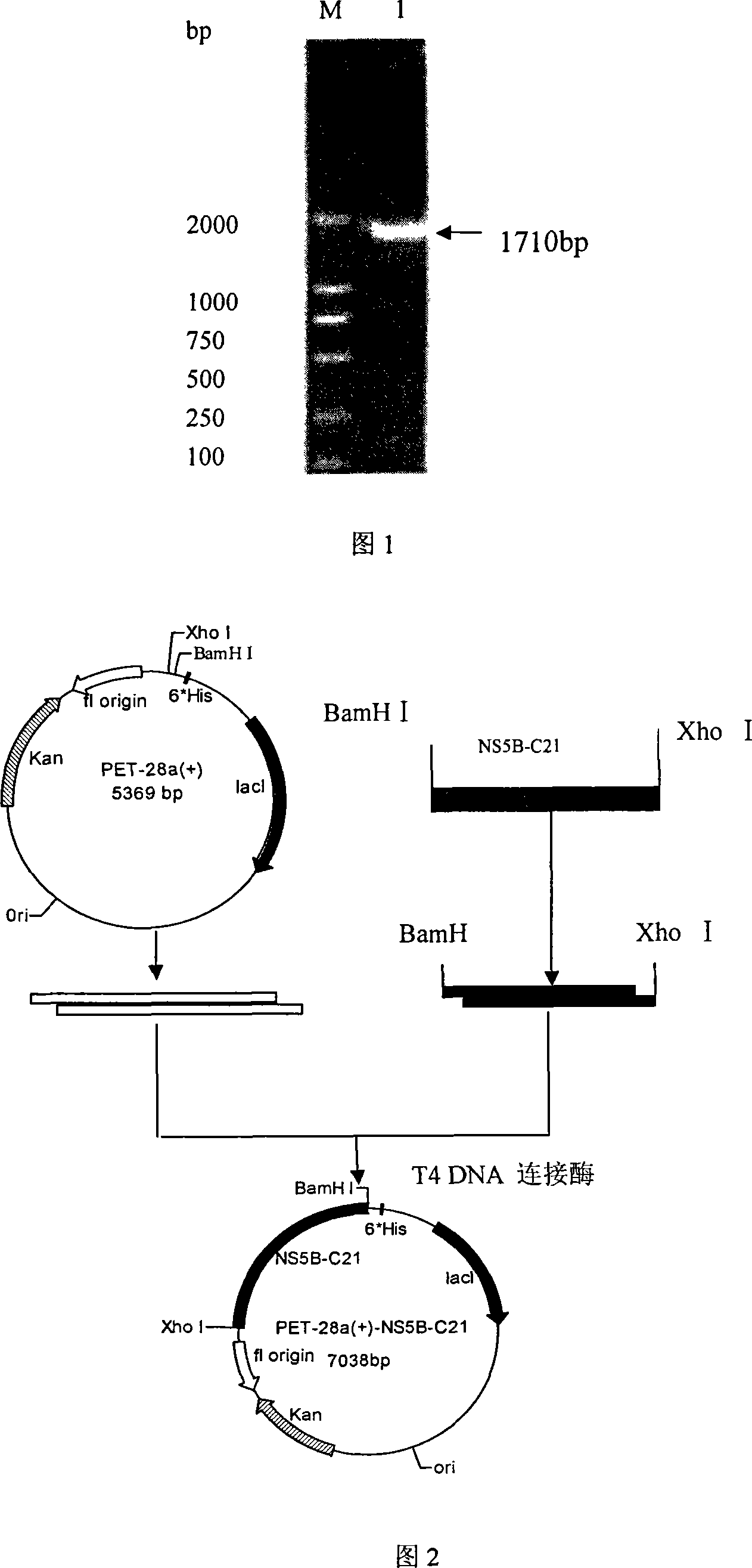
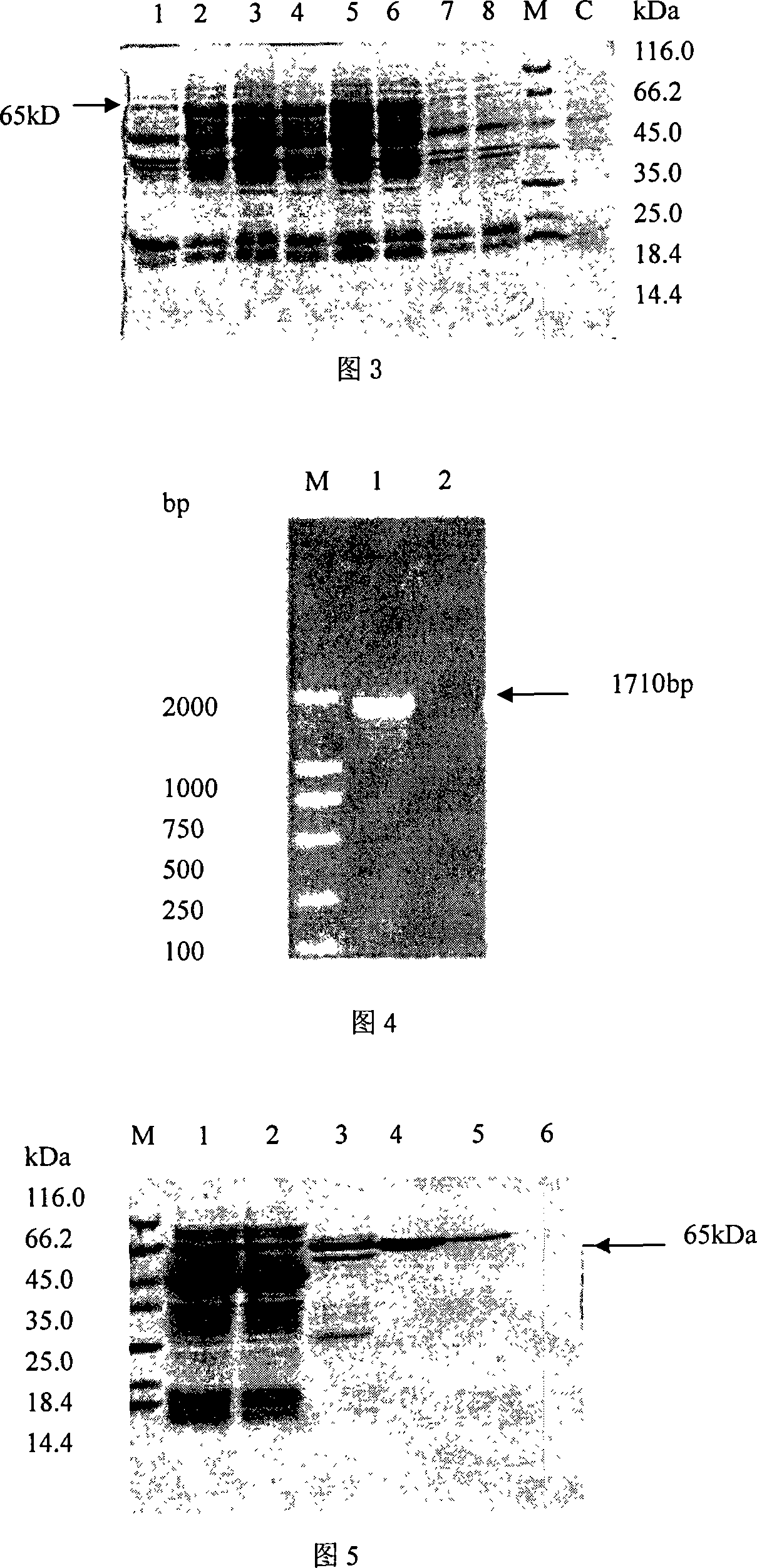
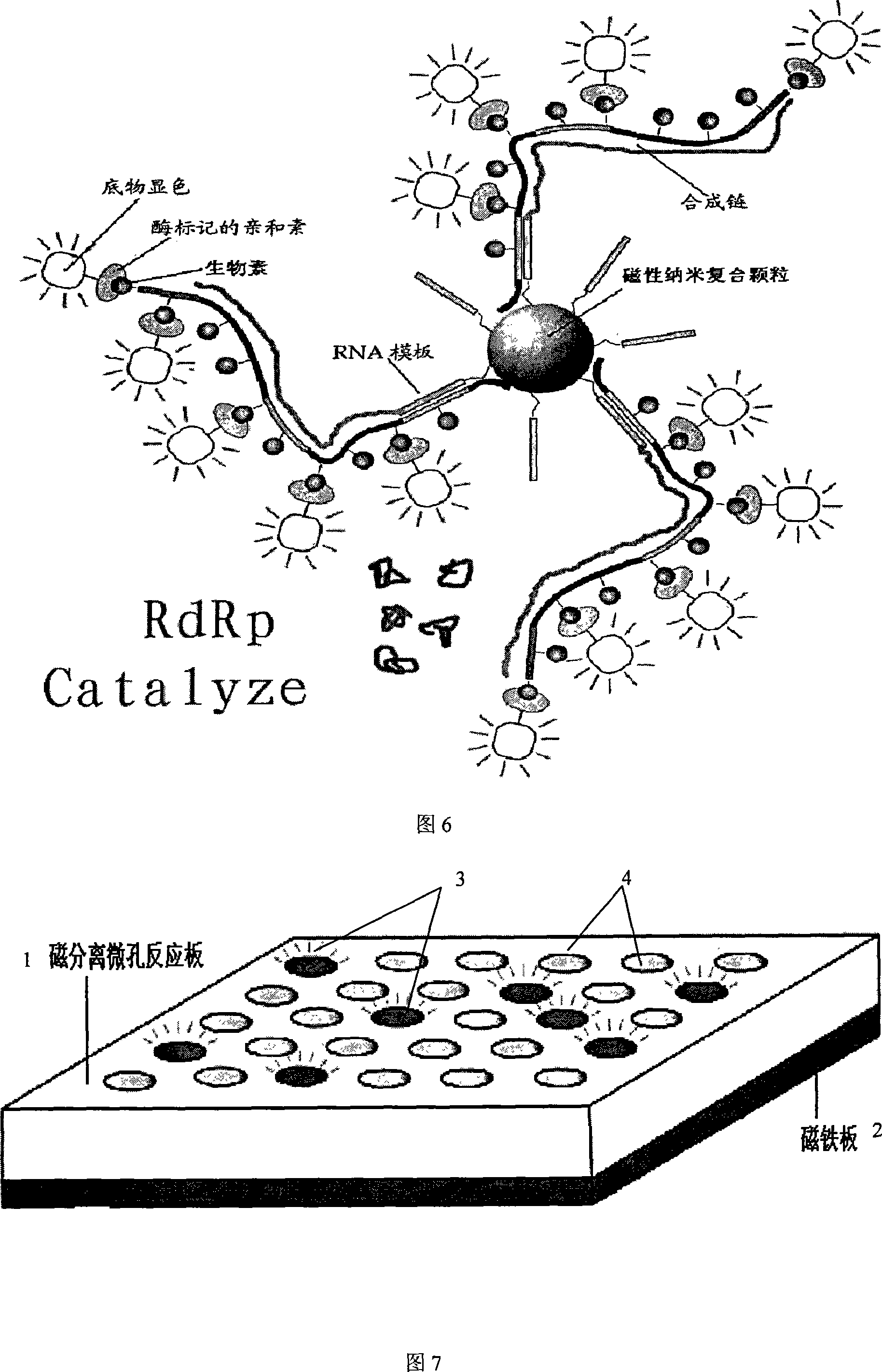
The invention provides a detecting method and an application of the extraneous activity of the RNA polymerase relied on by the hepatitis C virus RNA, which relates to an secure and practical establishing method for the detection of the activity extraneous detection of the RNA polymerase relied on by the hepatitis C virus RNA, and the application relates to the genetic engineering technology and the nanometer segregation enrichment and RNA related technology field. The invention expresses the soluble NS5B protein through the utilization of the genetic engineering technology, and prepares the nanometer magnetic particle. One end of a RNA template is fixed on the nanometer magnetic particle, and added in the 96 hole reaction plate micromesh, and added with NS5B protein to compose a RNA duplication system, and a duplicated RNA double chain on the nanometer magnetic particle is mixed with marked biological elements. After the RNA is duplicated, the nanometer magnetic particle is attached at the micromesh bottom through an external magnetic field magnet, after repeatedly cleaning and attaching, the nanometer magnetic particle is combined with the marked combining factor of the horse radish peroxidase, after attaching and cleaning, the substrate constant of the horse radish peroxidase is added to develop color. New NS5B protease activity inhibitor can be screened from a traditional Chinese storeroom and a compound storeroom by the established method.
Owner:李越希
HCV replicons containing NS5B from genotype 2B
The present invention features methods for enhancing the ability of a genotype 2b NS5B sequence to function in a replicon, for producing replicons containing a functional genotype 2b NS5B, and for using replicons to measure the ability of a compound to affect HCV replication that is sustained with the genotype 2b polymerase. Also featured is a genotype 1b NS4B adaptive mutation. The ability to produce replicons containing a functional genotype 2b NS5B is illustrated by the production of chimeric replicons based on HCV genotype 1b where substantially all the NS5B sequence is replaced with a genotype 2b NS5B.
Owner:MERCK SHARP & DOHME CORP +1
Nucleic acid comprising chimeric gene derived from hepatitis c virus
InactiveUS20110045020A1Improve productivityImprove abilitiesSsRNA viruses positive-senseAntibody mimetics/scaffoldsGeneProline
This invention provides infectious chimeric HCV particles that can be used for vaccines. This invention further provides a nucleic acid comprising a chimeric gene derived from the hepatitis C virus comprising regions each encoding Core protein, E1 protein, E2 protein and p7 protein derived from a hepatitis C virus strain other than JFH-1 strain; NS2 protein derived from JFH-1 strain or a hepatitis C virus strain other than JFH-1 strain, or a chimeric NS2 protein of NS2 protein derived from JFH-1 strain and NS2 protein derived from a hepatitis C virus strain other than JFH-1 strain; and NS3 protein, NS4A protein, NS4B protein, NS5A protein, and NS5B protein derived from JFH-1 strain in that order in 5′ to 3′ direction, wherein the 328th proline residue from the amino acid residue at N-terminus of the Core protein is substituted with an amino acid residue other than proline. This invention further provides chimeric HCV particles comprising such nucleic acid, and use of such HCV particles for vaccines.
Owner:TOKYO METROPOLITAN INST OF MEDICAL SCI +2
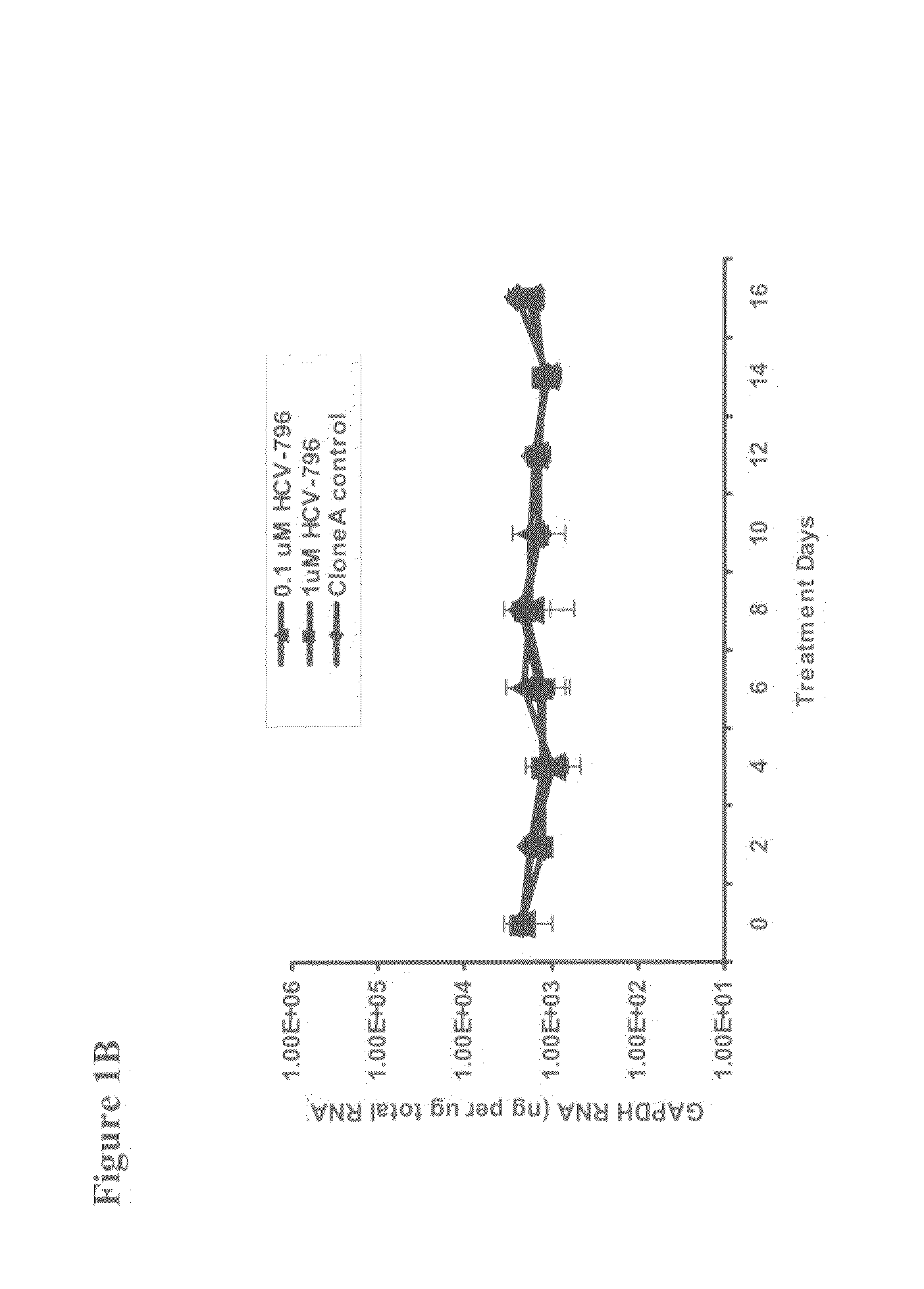
Identification and characterization of hcv replicon variants with reduced susceptibility to hcv-796, and methods related thereto
InactiveUS20080182895A1Reduce sensitivityReduced and minimal and no susceptibilityBiocideMicrobiological testing/measurementHepatitis c rnaTreatment resistant
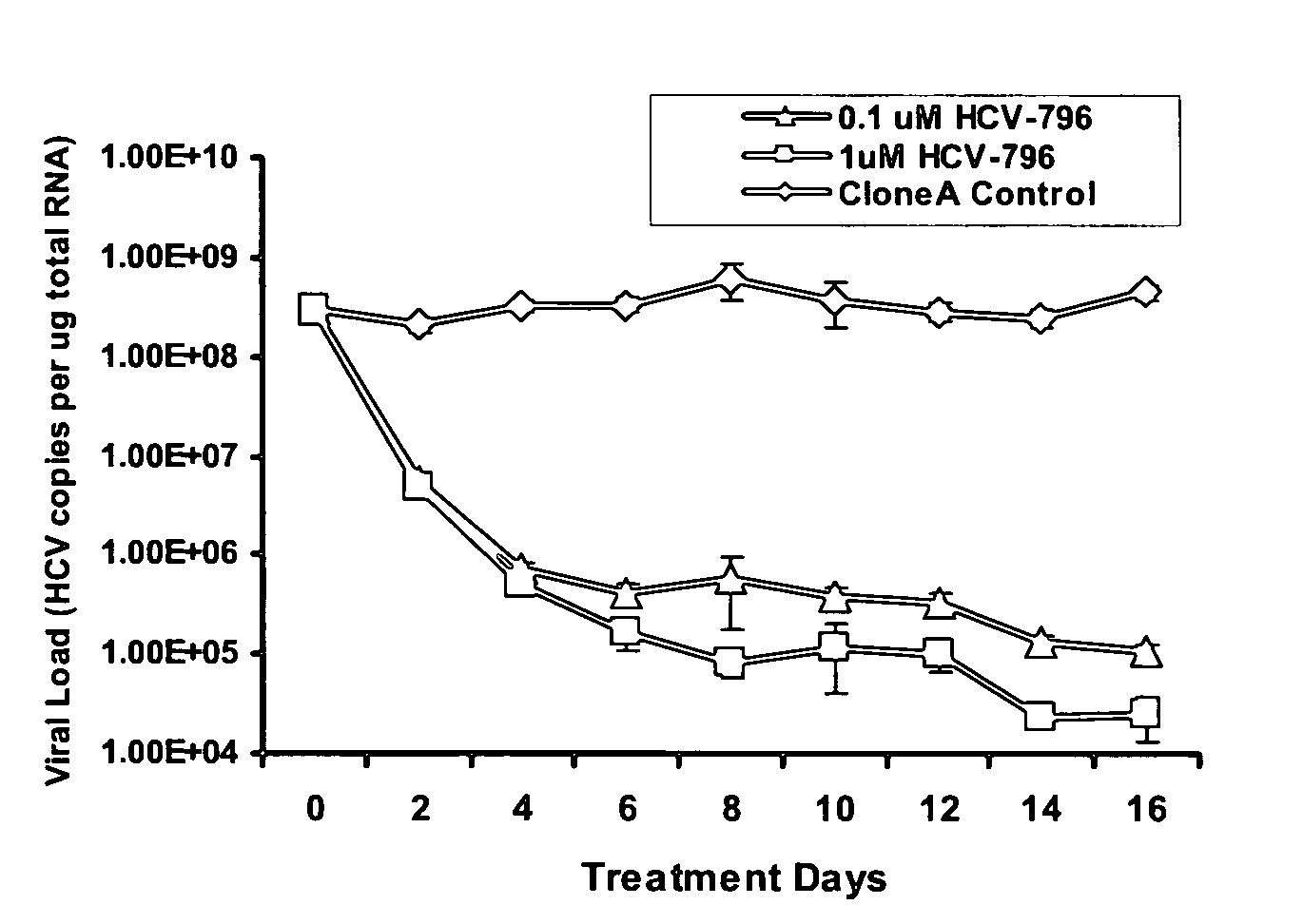
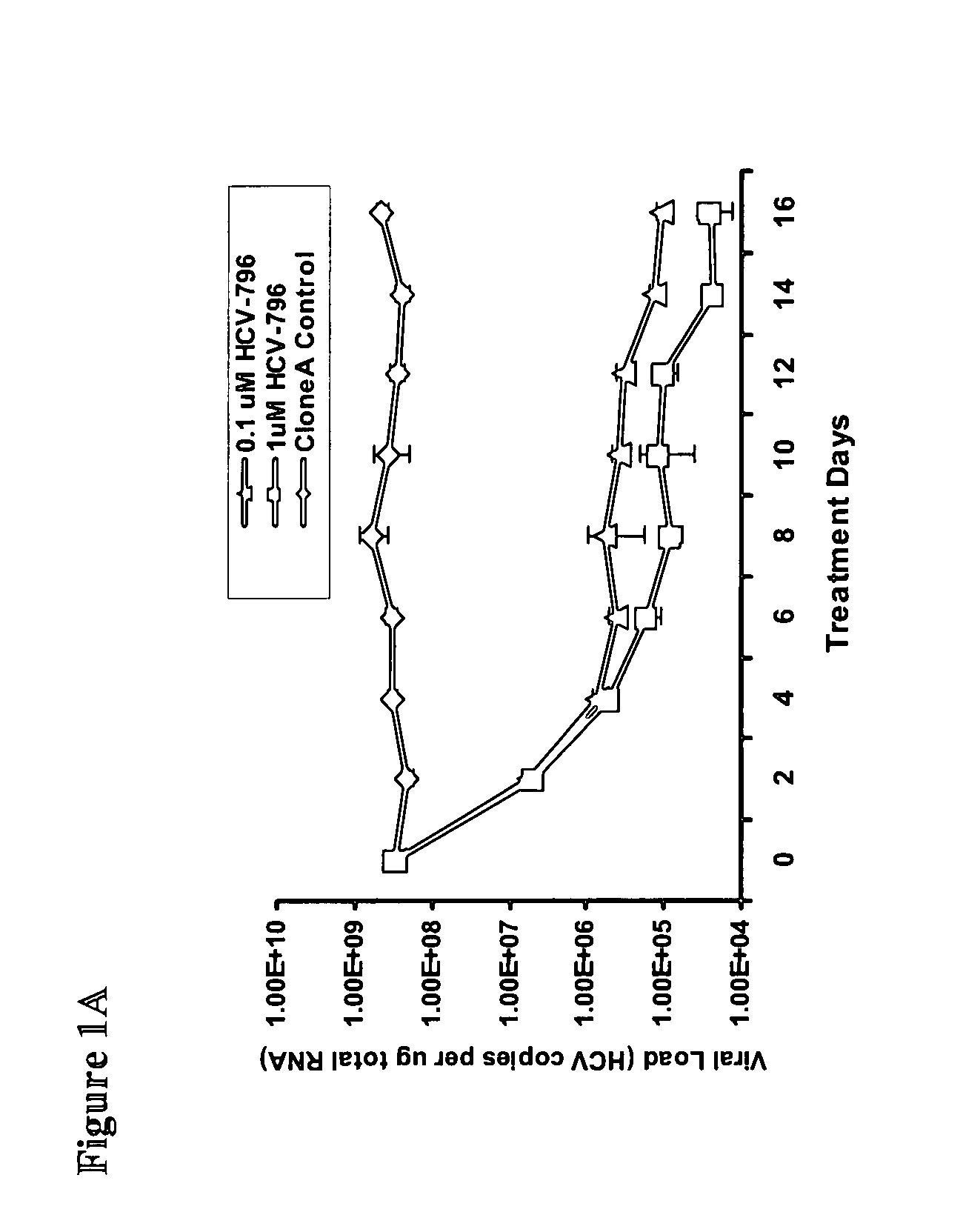
The present invention provides methods of decreasing the frequency of emergence, decreasing the level of resistance, and delaying the emergence of a treatment-resistant Hepatitis C viral infection, by administering to a subject, either in combination or in series, an inhibitor of the Hepatitis C RNA-dependent RNA polymerase NS5B, e.g., a benzofuran, such as 5-cyclopropyl-2-(4-fluorophenyl)-6-[(2-hydroxyethyl)(methylsulfonyl)amino]-N-methyl-1-benzofuran-3-carboxamide (HCV-796), and at least one additional anti-Hepatitis C agent, e.g., a ribavirin product or an immunomodulator, such as an interferon product. Additionally, the invention relates to methods of monitoring the course of treatment of a Hepatitis C viral infection, methods of monitoring and prognosing a Hepatitis C viral infection, and methods of identifying an individual with a decreased likelihood of responding to an anti-Hepatitis C viral therapy. These methods use the sequence and / or structure of the Hepatitis C RNA-dependent RNA polymerase NS5B to identify the emergence of a treatment-resistant Hepatitis C viral infection, particularly a benzofuran (e.g., HCV-796) treatment-resistant Hepatitis C viral infection.
Owner:WYETH LLC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com