Antiviral compounds inhibitors of HCV NS5B
A compound and selected technology can be applied in the direction of antiviral agents, medical preparations containing active ingredients, drug combinations, etc., and can solve problems such as limiting practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0230] In one embodiment, compounds of general formula (I) are provided:
[0231] E. 1a -V 1a –C(=O)-P 1a -W 1a -P 1b -C(=O)-V 1b -E 1b (I)
[0232] in:
[0233] W 1a yes
[0234]
[0235] and W 1a is optionally substituted with one or more groups independently selected from halogen, alkyl, haloalkyl, optionally substituted aryl, optionally substituted heterocycle, and cyano;
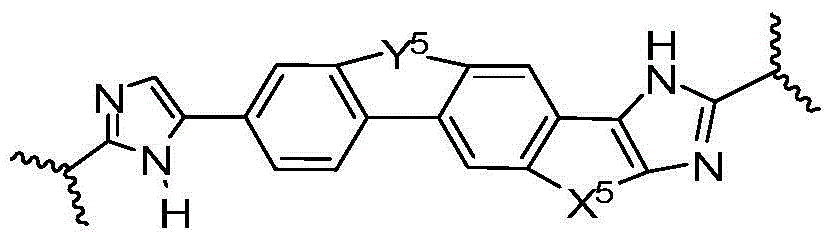
[0236] Y 5 Yes-O-CH 2 -, -CH 2 -O-, -O-C(=O)- or -C(=O)-O-; X 5 is-CH 2 -CH 2 -or-CH=CH-;
[0237] E. 1a is -N(H)(alkoxycarbonyl), -N(H)(cycloalkylcarbonyl), or -N(H)(cycloalkoxycarbonyl); or E 1a -V 1a together is R 9a ;
[0238] E. 1b is -N(H)(alkoxycarbonyl), -N(H)(cycloalkylcarbonyl), or -N(H)(cycloalkoxycarbonyl); or E 1b -V 1b together is R 9b ;
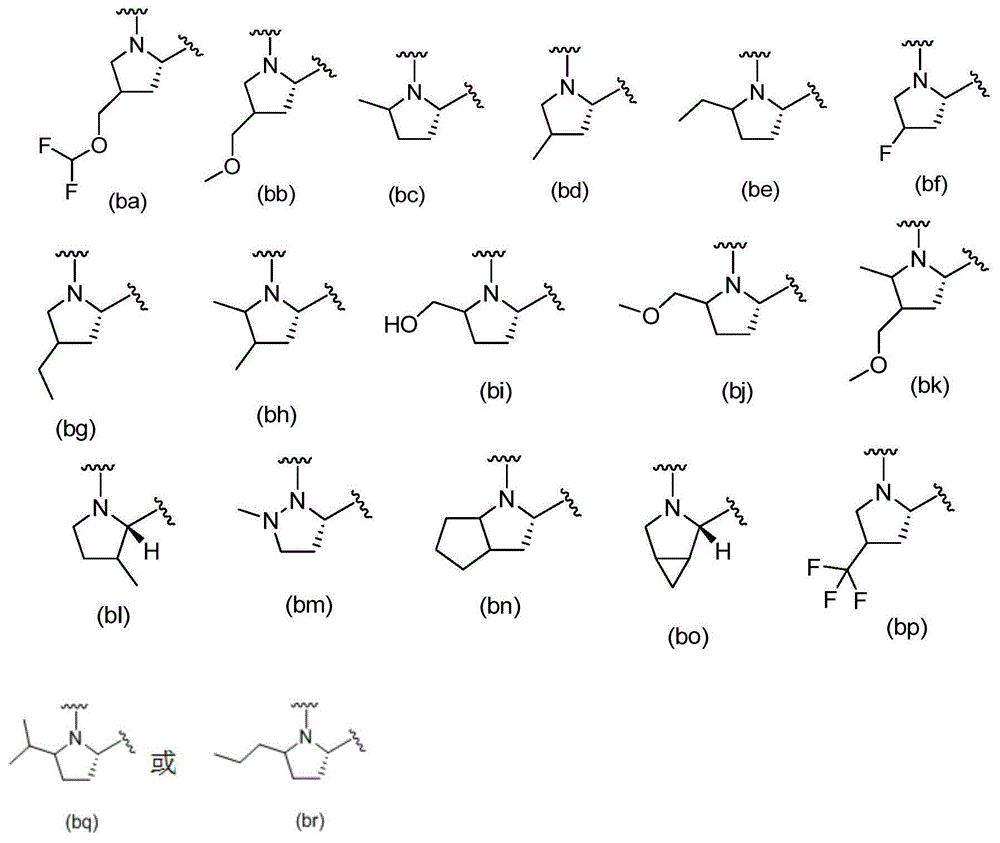
[0239] V 1a and V 1b each independently selected from:
[0240]
[0241] P 1a selected from:
[0242]
[0243]
[0244] P 1b selected from:
[0245]
[0246] with
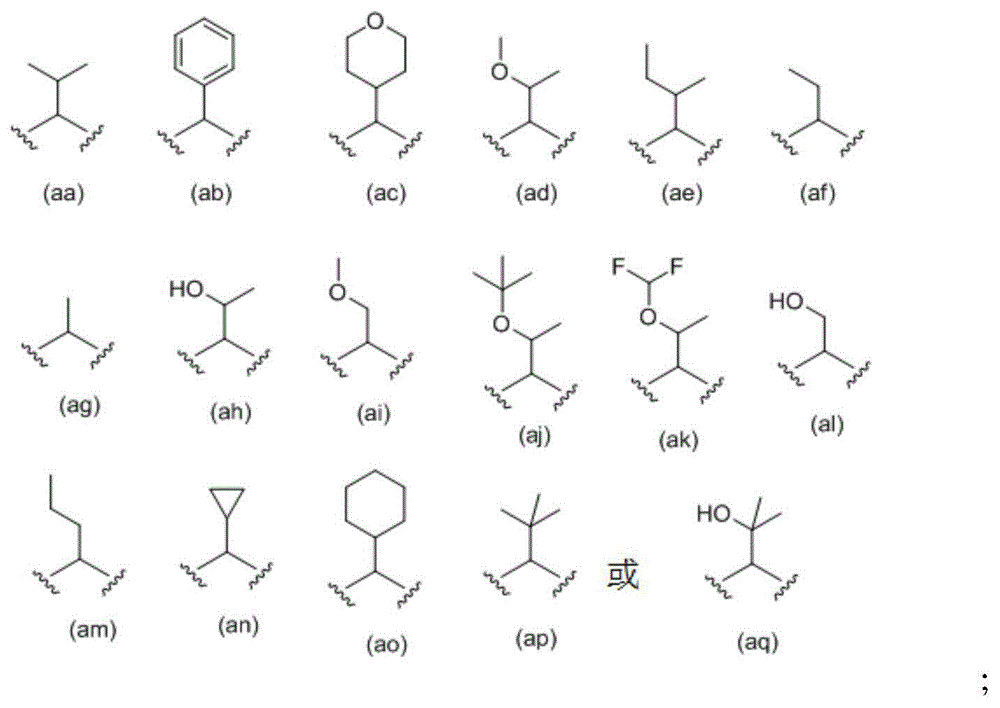
[0247] R 9a and R 9b each independent...
Embodiment AA
[0316]
[0317]
[0318] 3,4-Dihydronaphthalen-1(2H)-one: Dissolve 7-hydroxy-1-tetralone (13.9 g, 85.7 mmol) and 1-bromo-2- To a stirred solution of (bromomethyl)-4-chlorobenzene (25.6 g, 90.0 mmol), potassium carbonate (24 g, 172 mmol) was added. The reaction was stirred under argon for 18 hours, then diluted with ethyl acetate (1 L). The organics were washed three times with water and once with brine. The organic layer was then dried over magnesium sulfate, filtered and concentrated. To the resulting oil was added methanol (500 mL), and the suspension was stirred for thirty minutes. 7-(2-Bromo-5-chlorobenzyloxy)-(27.8 g, 89% yield) was isolated by filtration.
[0319] 3-Chloro-10,11-dihydro-5H-dibenzo[c,g]chromen-8(9H)-one: Add palladium(II) pivalate (1.18g, 3.8mmol), tri( In a 1L flask of 4-fluorophenyl)phosphine (1.20g, 3.8mmol), pivalic acid (2.33g, 22.8mmol) and potassium carbonate (31.8g, 228mmol), add dimethylacetamide (380mL) A solution of 7-(2-bromo-5-chlo...
Embodiment AB
[0327]
[0328]
[0329] (2S,4S)-1-tert-butyl 2,4-dimethylpyrrolidine-1,2,4-tricarboxylate. To a solution of (2S,4S)-1-tert-butyl 2-methyl 4-cyanopyrrolidine-1,2-dicarboxylate (9.0 g, 35.4 mmol) in MeOH (196 mL) was added HCl ( 4M in 1,4-dioxane, 100 mL, 403 mmol). The solution was stirred at room temperature for 16 h, and concentrated in vacuo. The crude intermediate was dissolved in ethyl acetate (180 mL) and basified with aqueous bicarbonate (sat.). Di-tert-butyl bicarbonate (8.5 g, 38.9 mmol) was added and the biphasic solution was stirred at room temperature for 12 h. The layers were then separated, and the aqueous layer was back extracted with ethyl acetate. The combined organic layers were washed with brine, passed through Na 2 SO 4 Dried and concentrated. The crude oil was purified by silica gel chromatography (15% to 40% to 100% ethyl acetate / hexanes) to provide (2S,4S)-1-tert-butyl 2,4-dimethylpyrrolidine-1,2 , 4-Tricarboxylate (9.56 g, 94%).
[0330] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com