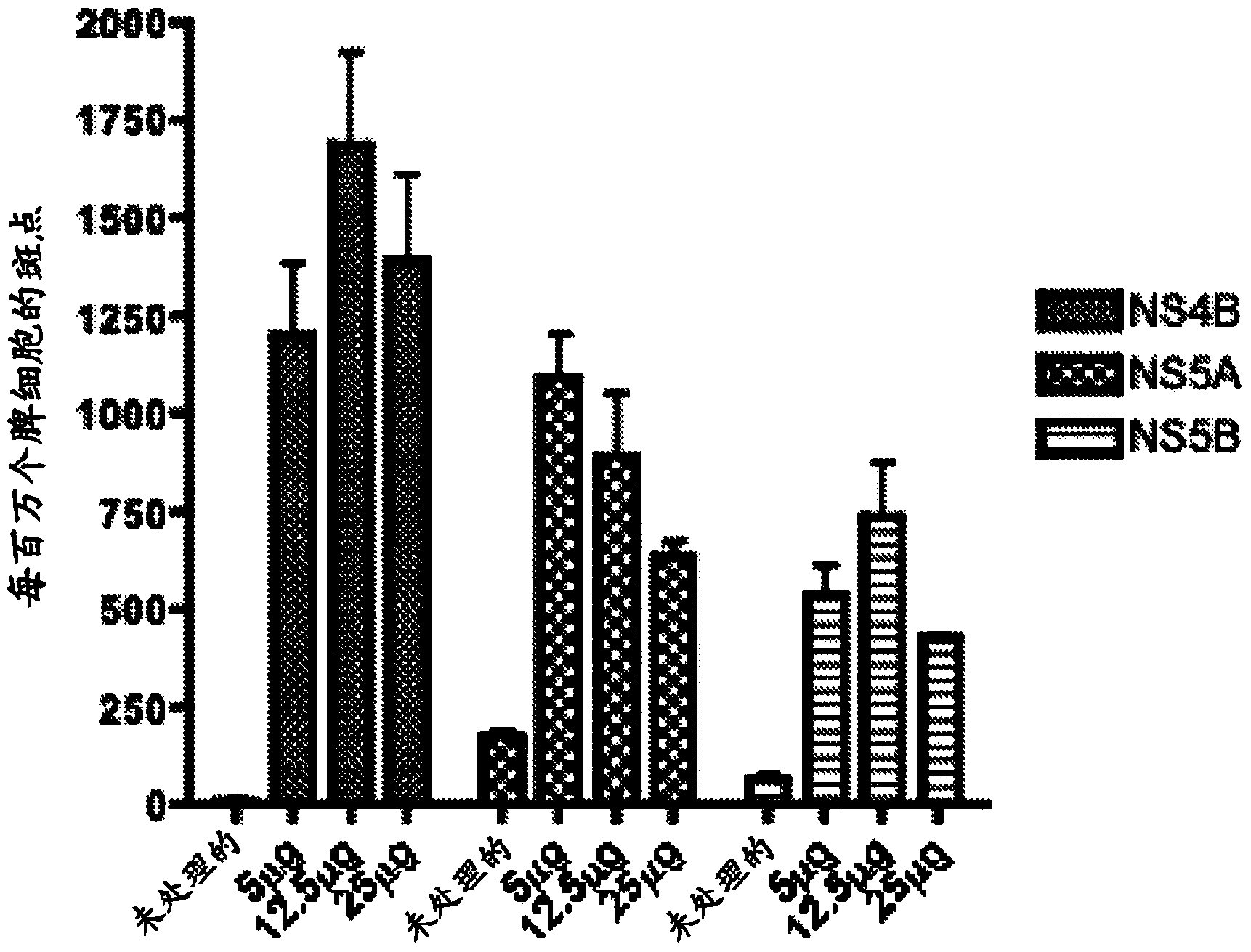Improved HCV vaccines and methods for using the same
A technology of immunogenic fragments and proteins, applied in vaccines, chemical instruments and methods, biochemical equipment and methods, etc., can solve the problem of platform immunogenicity reduction and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Design and expression of pConNS4B, pConNS5A and pConNS5B
[0088] The HCV genotype la consensus sequence for HCV proteins NS4B, NS5A and NS5B was generated from 170 different sequences obtained from the Los Alamos National Laboratory HCV sequence database. Several modifications were then made to the consensus constructs in order to enhance their expression and detection, including the addition of the leader sequence IgE at the C-terminal and N-terminal HA-tags of each construct. Alternatively, use GeneOptimizer with codon and RNA optimization TM Each construct was further modified (GENEART, Germany) and subcloned into the clinical expression vector pVAX under the control of the CMV promoter. The final constructs were called pConNS4B, pConNS5A and pConNS5B (plasmid maps are shown in Figure 8A-Figure 8C ).
[0089] Protein expression of each construct was determined by transiently transfecting human RD myocytes with each individual construct. Using Lipofectamine TM ...
Embodiment 2
[0092] Immunization of C57BL / 6 mice with pConNS4B, pConNS5A and pConNS5B induces strong cellular immune responses
[0093] Once the expression of the construct was determined, C57BL / 6 mice were immunized to determine the immunogenicity of the construct. 6- to 8-week-old female C57BL / 6 mice were purchased from the Jackson Laboratory and maintained according to the guidelines of the National Institutes of Health and the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. For each individual construct the animals were divided into three different feeding groups of five animals each. Animals were immunized intramuscularly with 5 μg, 12.5 μg or 25 μg of pConNS4B, pConNS5A or pConNS5B followed by electroporation.
[0094] Using CELLECTRA TM Electroporation was performed with an adaptive constant current electroporation device and electrode array (Inovio Pharmaceuticals, Inc., Blue Bell, PA).
[0095] Animals received a total of two immunizations...
Embodiment 3
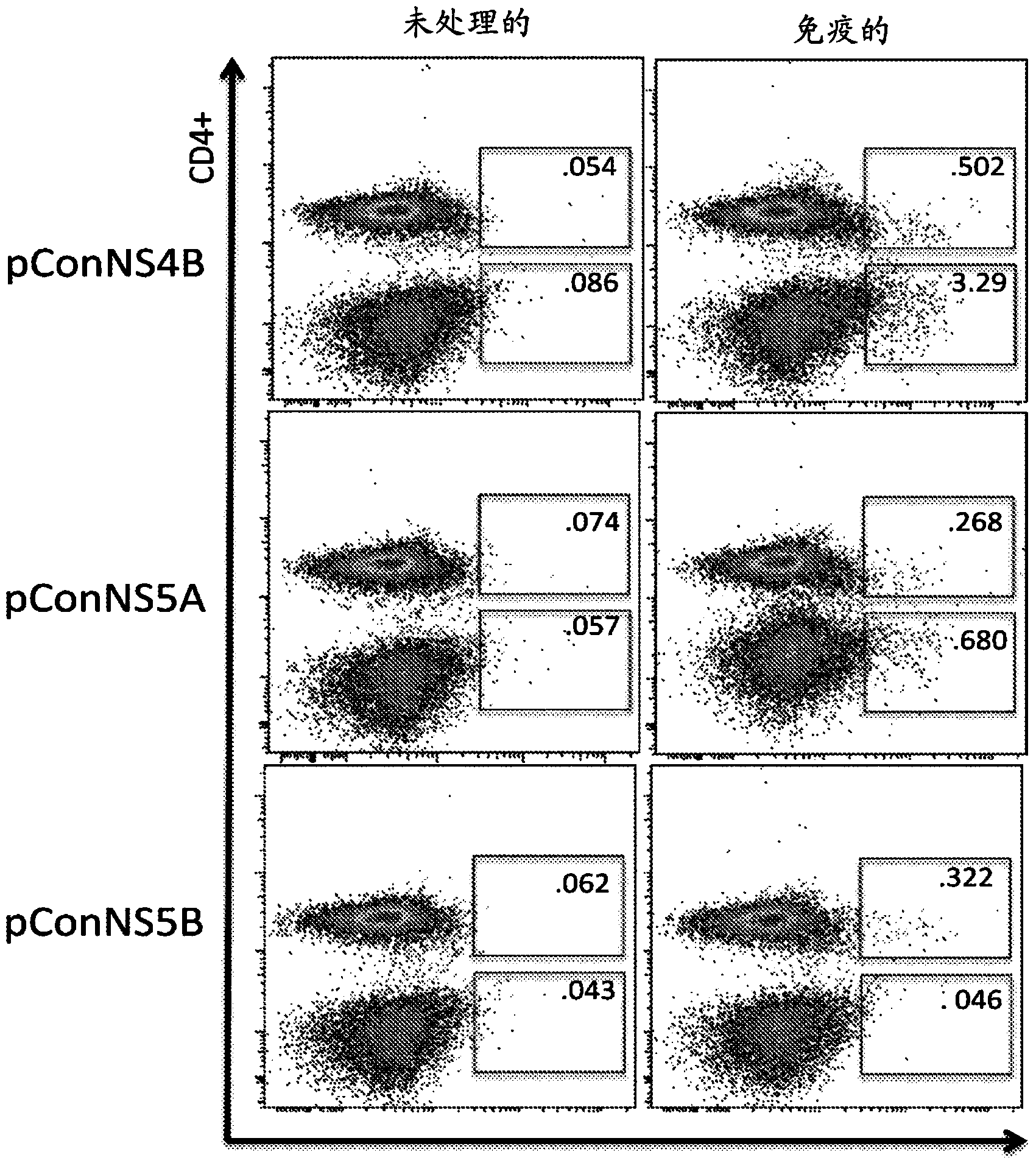
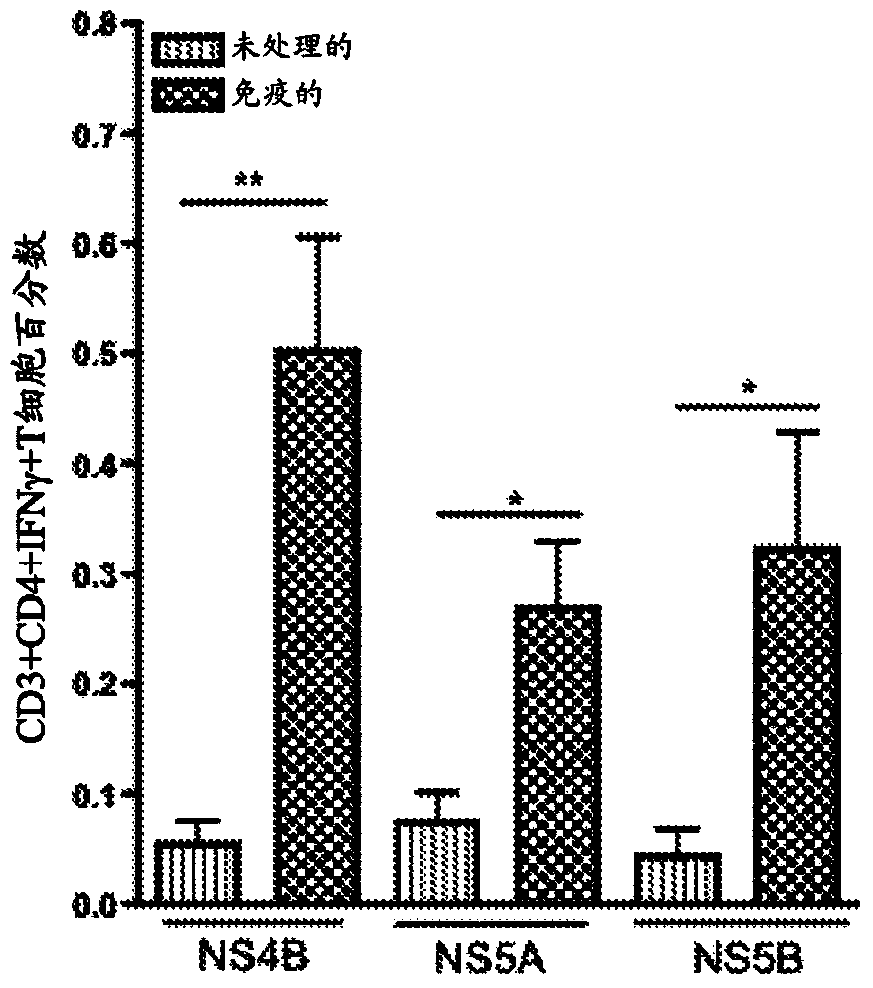
[0101] Immune-induced NS4B-, NS5A-, and NS5B-specific T cells were detected in the liver after intramuscular immunization
[0102] Mice were immunized as previously described in Example 1 above. One week after the last immunization, animals were sacrificed. After sacrifice, the livers were separated and comminuted individually using a Stomacher machine. The resulting mixture was filtered and treated with 10 ml ACK lysis buffer (Bioscience) for 5 minutes to remove RBCs. The mixture was pelleted and hepatocytes were separated from lymphocytes by using a 35% Percoll gradient. The pelleted lymphocytes were resuspended in complete medium. Experiments were performed with or without hepatic perfusion and no differences were observed.
[0103] T cells were isolated from each liver and stimulated with overlapping peptides corresponding to each individual construct. Immunization-induced HCV-specific T cells were identified by IFN-γ expression detected by intracellular cytokine stai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com