Chimeric HCV (hepatitis C virus) vaccine taking influenza virus as carrier and preparation method thereof
A technology of influenza virus and chimeric vaccine, applied in the field of HCV chimeric vaccine and its preparation, can solve the problems of weakened side effects, DNA vaccine safety, comprehensive response and limited protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
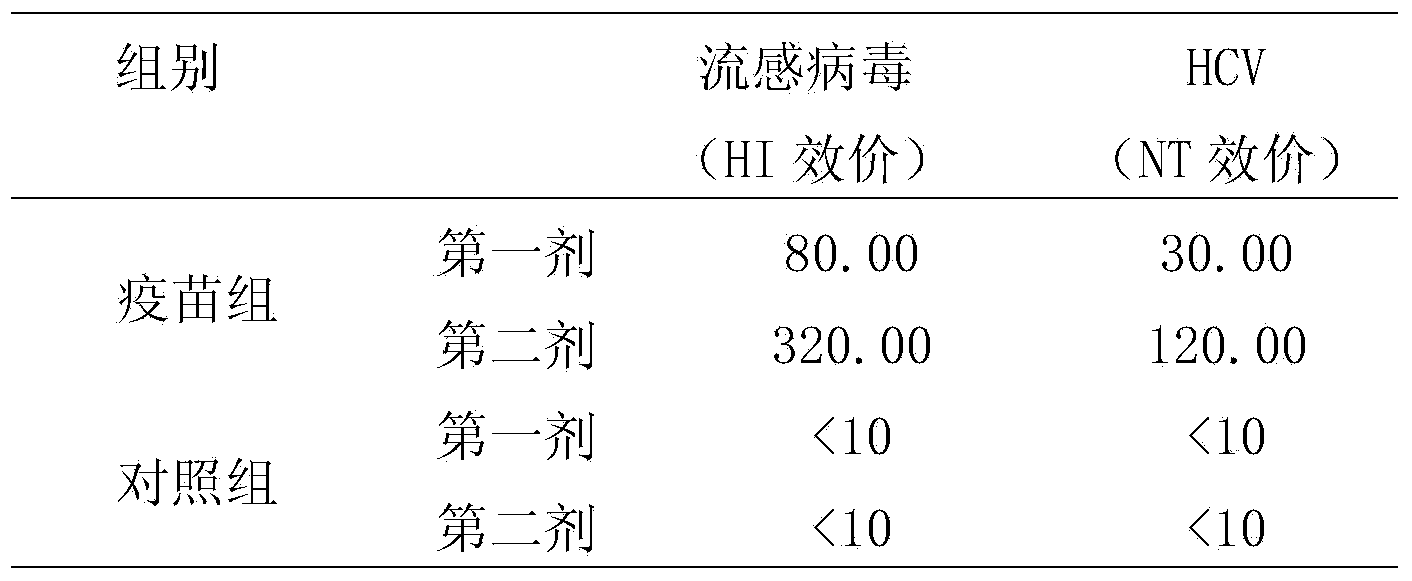
[0064] Experimental example 1, the preparation of the HCV chimeric vaccine rFLU-HCV / NS1 of the chimeric HCV-C / E1 / E2 dominant epitope with influenza virus as the carrier
[0065] 1. The dominant epitope sequence of HCV-C / E1 / E2 in subtype 1b HCV is shown in SEQ ID No.1, which has been verified by animal experiments and serum of clinical patients, and meets the requirements of the next experiment.
[0066] 2. Construction of recombinant plasmid pC / E1 / E2-NS1
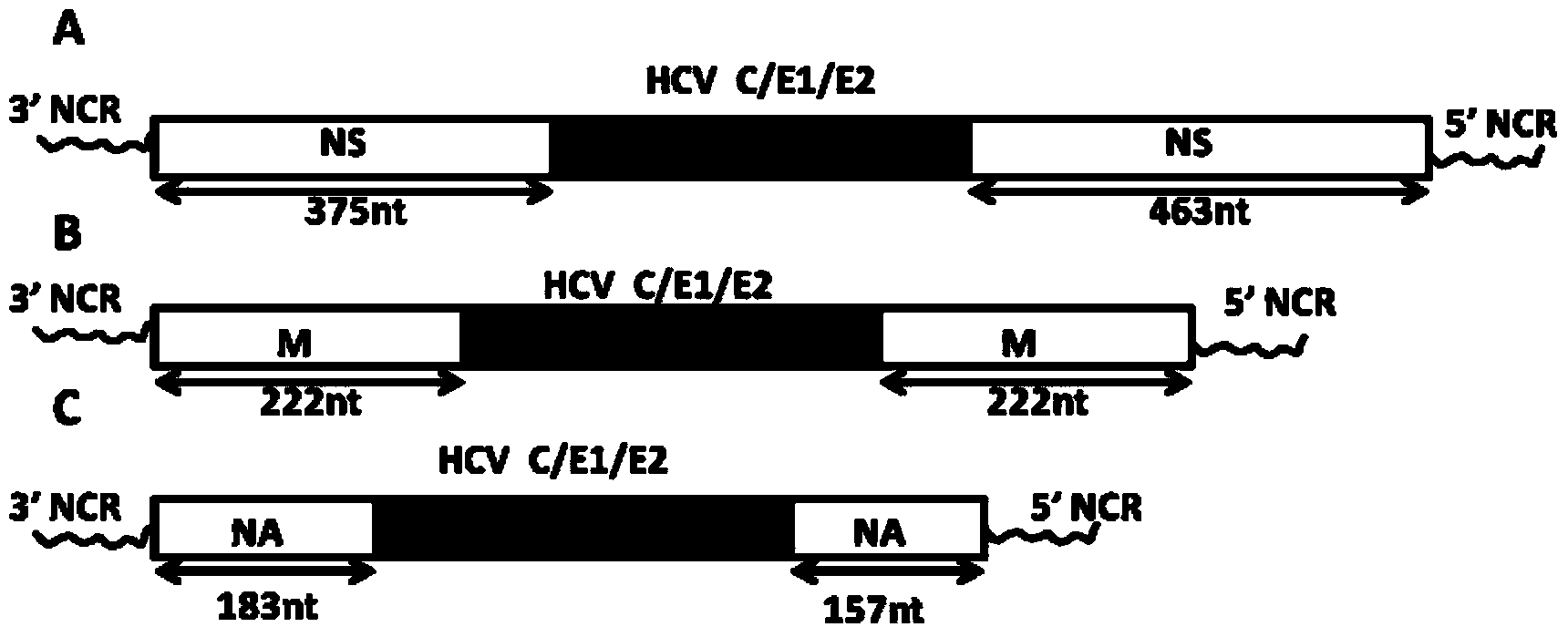
[0067] The recombinant plasmid pC / E1 / E2-NS1, the specific strategy is as follows figure 1 As shown in A.
[0068] Specifically, insert the HCV-C / E1 / E2 dominant epitope gene obtained above into the 5' end of the NS gene open reading frame (ORF) of the cold-adapted and attenuated influenza virus strain A / AA / 6 / 60 After the 375th nucleotide, a linker (5'-UAAUG-3') is added in the middle. The linker functions as both a terminator and a promoter, and finally the 376th from the 5' end of the open reading frame of the NS gene F...
experiment example 2
[0088] Experimental example 2, the preparation of the HCV chimeric vaccine rFLU-HCV / M of the chimeric HCV-C / E1 / E2 dominant epitope with influenza virus as the carrier
[0089] 1. The dominant epitope sequence of HCV-C / E1 / E2 in subtype 1b HCV is shown in SEQ ID No.1, which was verified by immunizing animals according to the method in step 1 of Example 1, and met the requirements of the next experiment.
[0090] 2. Construction of recombinant plasmid pC / E1 / E2-M
[0091] Using the M gene fragment of the cold-adapted and attenuated influenza virus strain A / AA / 6 / 60 as the target for inserting the dominant epitope gene of HCV-C / E1 / E2, the recombinant plasmid pC / E1 / E2-M, specific strategies such as figure 1 Shown in B.
[0092] Specifically, insert the HCV-C / E1 / E2 dominant epitope gene obtained above into the first 222 nucleosides of the open reading frame (ORF) of the M gene of the cold-adapted and attenuated influenza virus strain A / AA / 6 / 60 Between the acid and the last 222 nuc...
Embodiment 3
[0110] Embodiment 3, the preparation of the HCV chimeric vaccine rFLU-HCV / NA of the chimeric HCV-C / E1 / E2 dominant epitope with influenza virus as the carrier
[0111] 1. The dominant epitope sequence of HCV-C / E1 / E2 in subtype 1b HCV is shown in SEQ ID No.1, which was verified by immunizing animals according to the method in step 1 of Example 1, and met the requirements of the next experiment.
[0112] 2. Construction of recombinant plasmid pC / E1 / E2-NA
[0113] Using the NA gene fragment of A / California / 07 / 2009 (H1N1) as the target for inserting the dominant epitope gene of HCV-C / E1 / E2, the recombinant plasmid pC / E1 / E2-NA was constructed by molecular biology methods, specifically Strategies such as figure 1 C shown.
[0114] Specifically, the HCV-C / E1 / E2 dominant antigen epitope gene obtained above is inserted into the first 183 nucleosides of the NA gene open reading frame (ORF) of the influenza virus strain H1N1 subtype A / California / 07 / 2009 Between the acid and the last 15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com