Lactobacillus plantarum and microecological preparation, preparation method and application thereof
A technology of Lactobacillus plantarum and microecological preparations, applied in the direction of microorganism-based methods, biochemical equipment and methods, Lactobacillus, etc., can solve the problems of unsatisfactory effect and stability of lactic acid bacteria, and achieve the prevention and treatment of Mycoplasma gallisepticum disease, improve the body's immunity, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Initial screening of immunocompetent lactic acid bacteria
[0043] 1Materials and methods
[0044] 1.1 Material
[0045] MRS medium: calculated by mass percentage, glucose 2.0%, sodium citrate 0.2%, sodium acetate 0.5%, dipotassium hydrogen phosphate 0.5%, manganese sulfate 0.02%, magnesium sulfate 0.05%, peptone 1.0%, beef extract 1.0%, Yeast extract 0.5%, Tween-80 0.1%, pH 6.0.
[0046] IEC-6 cells (small intestinal epithelial cells) were purchased from the cell bank of the Type Culture Collection Committee of the Chinese Academy of Sciences.
[0047] 1.2 method
[0048] 1.2.1 Preparation of bacterial suspension
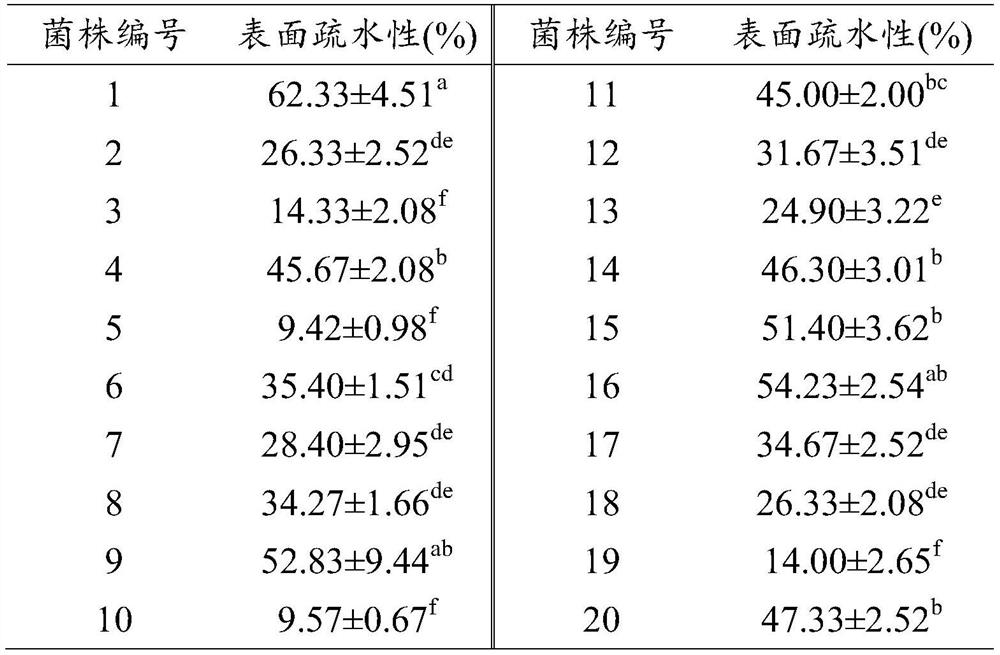
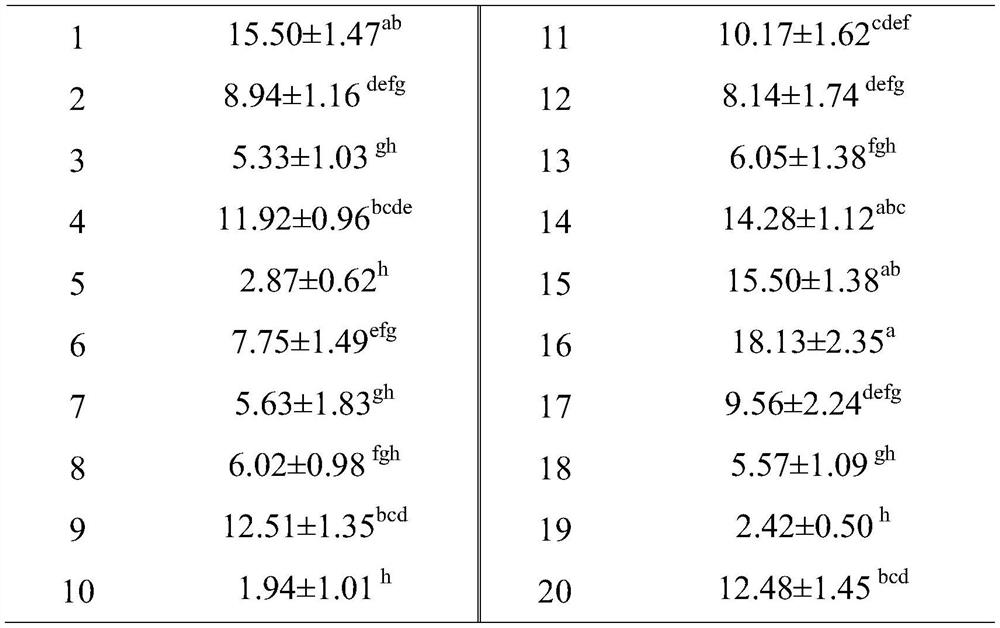
[0049] Twenty strains of lactic acid bacteria provided by the Culture Collection Center of Bioengineering Research Institute of Shandong Baolai Lilai Bioengineering Co., Ltd. were respectively inoculated into MRS liquid medium, cultured at 37°C for 16-18h, and centrifuged at 4°C 3000rpm for 15min to collect the bacteria. The cells were suspended in PBS solut...
Embodiment 2
[0075] Example 2 Rescreening of immunocompetent lactic acid bacteria
[0076] 1Materials and methods
[0077] 1.1 Material
[0078] MRS medium: calculated by mass percentage, glucose 2.0%, sodium citrate 0.2%, sodium acetate 0.5%, dipotassium hydrogen phosphate 0.5%, manganese sulfate 0.02%, magnesium sulfate 0.05%, peptone 1.0%, beef extract 1.0%, Yeast extract 0.5%, Tween-80 0.1%, pH 6.0. The solid medium needs to add 1.5% agar powder.
[0079] Improved MRS medium: Use sucrose instead of glucose, and the others are the same as MRS medium.
[0080] 1.2 method
[0081] 1.2.1 Primary screening of extracellular polysaccharide-producing lactic acid bacteria (colony drawing method)
[0082] The 8 strains of lactic acid bacteria (see Table 4) initially screened in Example 1 were inoculated into the MRS medium for activation, and the activated lactic acid bacteria were inoculated into the modified MRS solid medium by the plate streaking method, and cultured at 37°C for 24 hours. Observe the...
Embodiment 3
[0097] Example 3 Lactic acid bacteria immune function test
[0098] 1Materials and methods
[0099] 1.1 Material
[0100] Healthy Kunming mice, purchased from Shandong Taibang Biological Products Co., Ltd.; Mouse Interleukin 2 (IL-2) ELISA Test Kit, Mouse Interferon-γ (IFN-γ) ELISA Kit, Mouse Immunoglobulin G (IgG) ELISA kits were purchased from Beijing Equation Biotechnology Co., Ltd.
[0101] 1.2 method
[0102] 1.2.1 Lactic acid bacteria immune function test
[0103] Choose 210 healthy Kunming mice, half male and half, weighing 18-20g, and rearing them in a quiet, warm, and avoiding bright environment for 3 days. After adapting for 3 days, they are randomly divided into 6 groups, each with 35 mice, which are the blank group and the model. Group, positive control group, No. 1 strain group, No. 15 strain group, No. 16 strain group. Before the start of the experiment, the other groups except the blank group were injected intraperitoneally with 80 mg / kg cyclophosphamide for 3 consecut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com