Novel gene engineering subunit vaccine for mycoplasma gallisepticum
A technology of mycoplasma gallisepticum and amino acids, which is applied in the direction of vaccines, veterinary vaccines, bacterial antigen components, etc., can solve the problems of lack, weak immune protection of vaccines, and easy mutation of antigenic proteins, so as to achieve sufficient supply and protein immunogen Good sex and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Example 1 Construction and Identification of Transfer Vector pF-MGC1
[0144] 1. Amplification and purification of the MGC1 gene The codon-optimized MGC1 gene (SEQ ID NO: 1) was synthesized in Nanjing GenScript and cloned into the pUC17 vector to obtain the pUC-MGC1 plasmid vector. The pUC-MGC1 plasmid was used as a template, and MGC1-F and MGC1-R were used as upstream and downstream primers for PCR amplification (the gene sequences of MGC1-F and MGC1-R are shown in SEQ ID NO.11 and 12). For the amplification system, see Table 1.
[0145] Table 1 MGC1 gene amplification system
[0146]
[0147] The reaction conditions were: 95°C pre-denaturation for 5 minutes; 94°C denaturation for 45 seconds, 54°C annealing for 45 seconds, 72°C extension for 1 minute, 35 cycles; 72°C extension for 10 minutes.
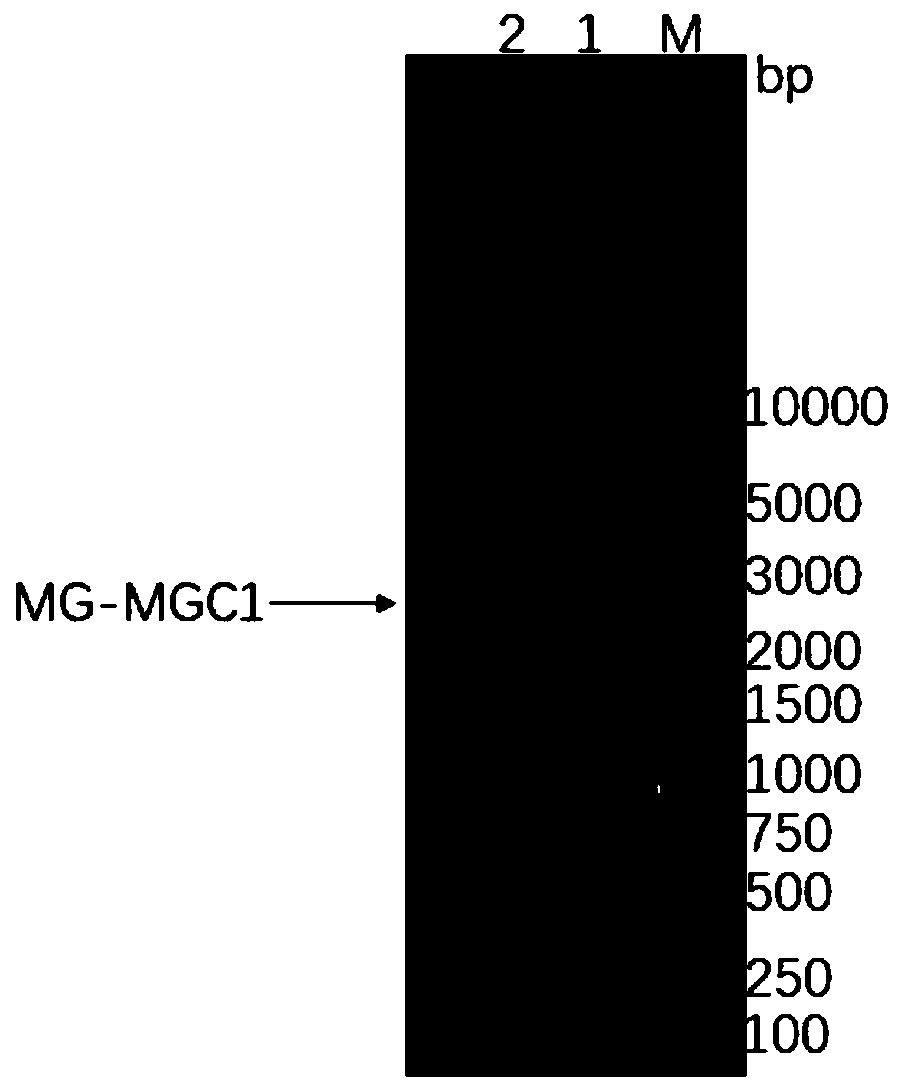
[0148] Perform gel electrophoresis on the PCR product to verify the size of the target gene, such as figure 1 As shown, the target band appeared at the position of 2.9kbp,...
Embodiment 2
[0161] Example 2 Construction and Identification of Transfer Vector pF-MGC2
[0162] 1. Amplification and purification of the MGC2 gene The codon-optimized MGC2 gene (SEQ ID NO: 3) was synthesized in Nanjing GenScript and cloned into the pUC17 vector to obtain the pUC-MGC2 plasmid vector. The pUC-MGC2 plasmid was used as a template, and MGC2-F and MGC2-R were used as upstream and downstream primers for PCR amplification (the gene sequences of MGC2-F and MGC2-R are shown in SEQ ID NO.13 and 14). For the amplification system, see table 5.
[0163] Table 5 MGC2 gene amplification system
[0164]
[0165]
[0166] The reaction conditions were: 95°C pre-denaturation for 5 minutes; 94°C denaturation for 45 seconds, 54°C annealing for 45 seconds, 72°C extension for 1 minute, 35 cycles; 72°C extension for 10 minutes.
[0167] Perform gel electrophoresis on the PCR product to verify the size of the target gene, such as Figure 4 As shown, the target band appeared at the positi...
Embodiment 3
[0179] Example 3 Construction and Identification of Transfer Vector pF-MGC3
[0180] 1. Amplification and purification of the MGC3 gene The codon-optimized MGC3 gene (SEQ ID NO: 5) was synthesized in Nanjing GenScript and cloned into the pUC17 vector to obtain the pUC-MGC3 plasmid vector. The pUC-MGC3 plasmid was used as a template, and MGC3-F and MGC3-R were used as upstream and downstream primers for PCR amplification (the gene sequences of MGC3-F and MGC3-R are shown in SEQ ID NO.15 and 16). For the amplification system, see Table 9.
[0181] Table 9 MGC3 gene amplification system
[0182]
[0183] The reaction conditions were: 95°C pre-denaturation for 5 minutes; 94°C denaturation for 45 seconds, 54°C annealing for 45 seconds, 72°C extension for 1 minute, 35 cycles; 72°C extension for 10 minutes.
[0184] Perform gel electrophoresis on the PCR product to verify the size of the target gene, such as Figure 7 As shown, the target band appeared at the position of 2.7kbp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com