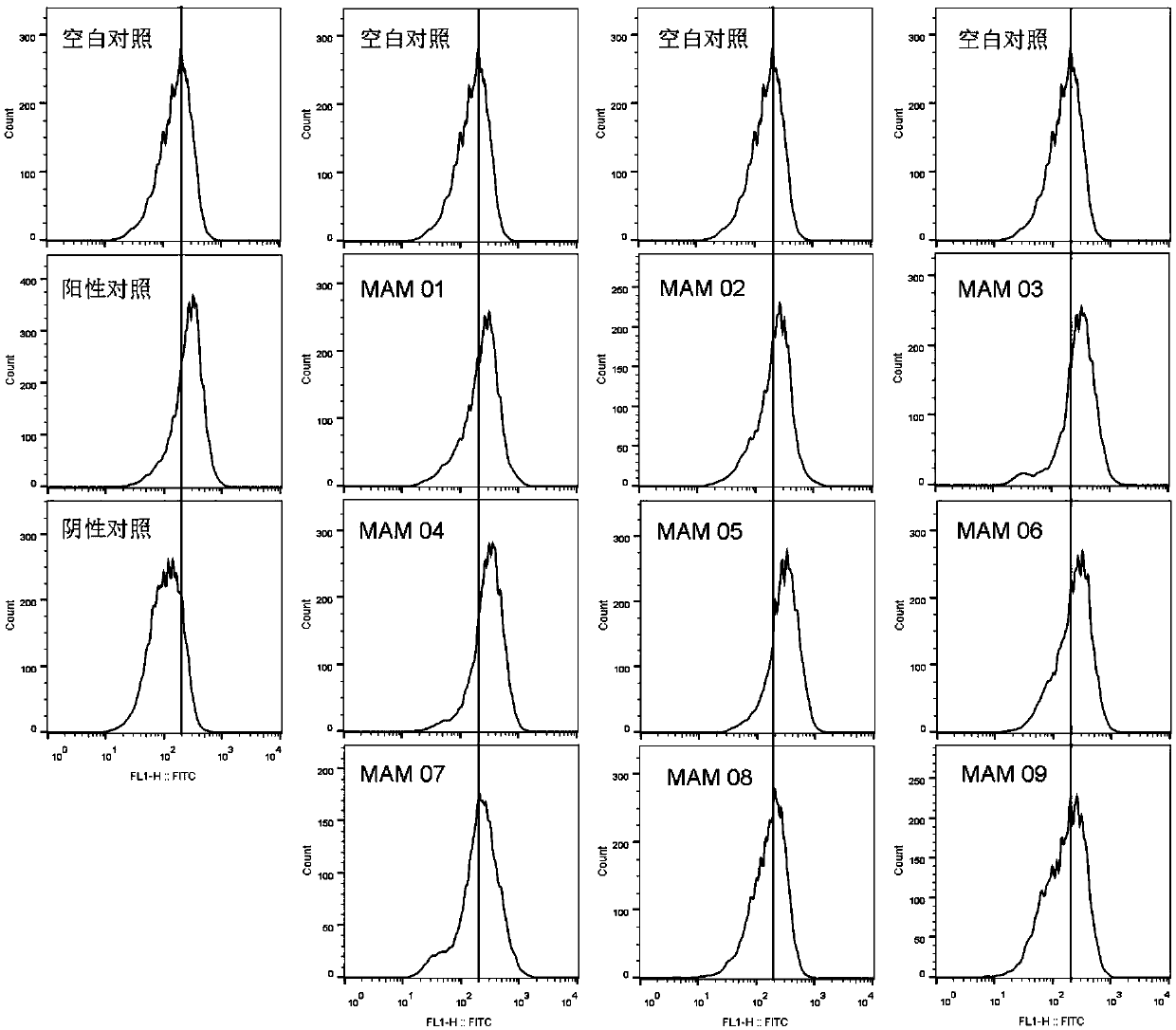
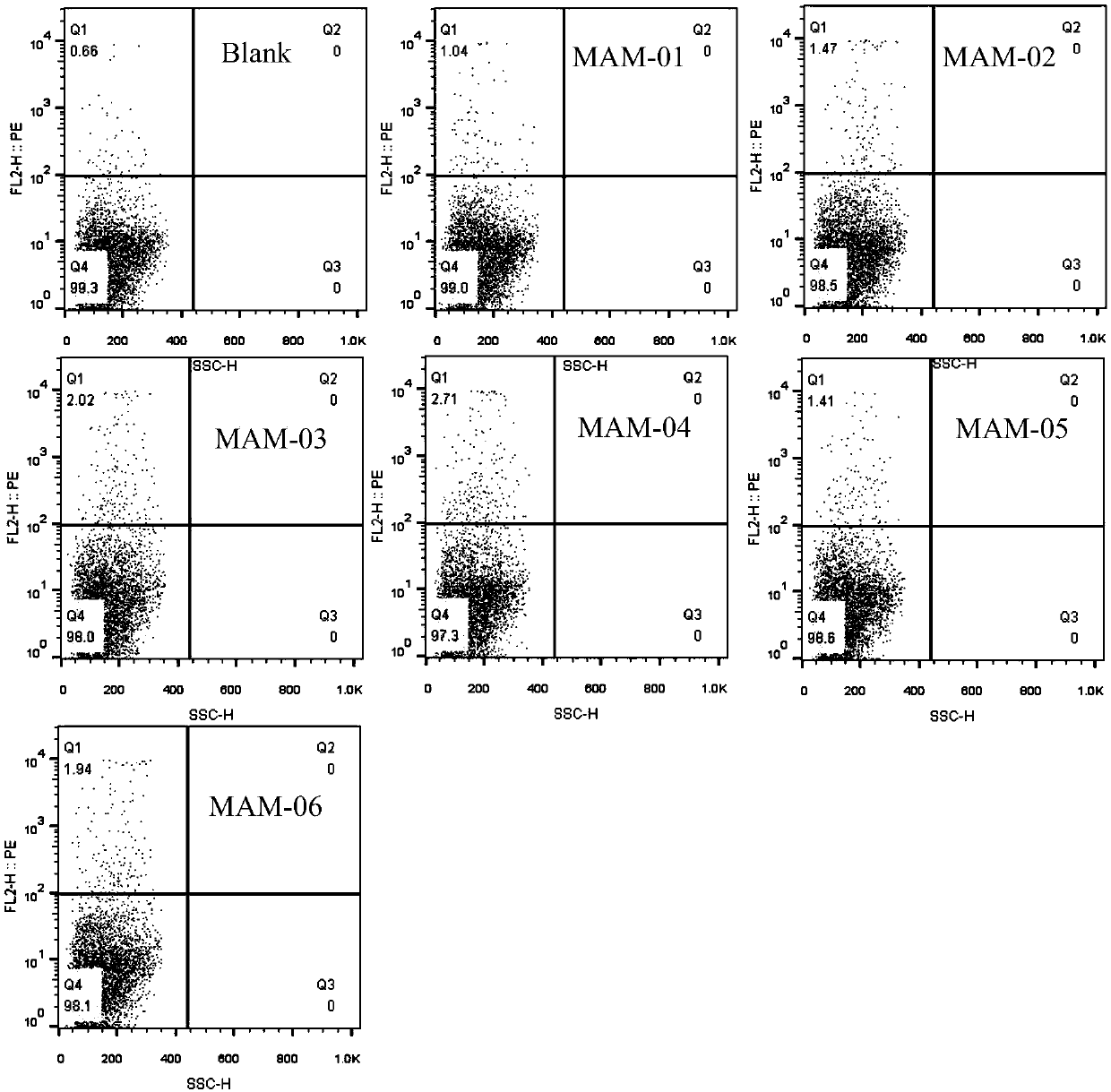
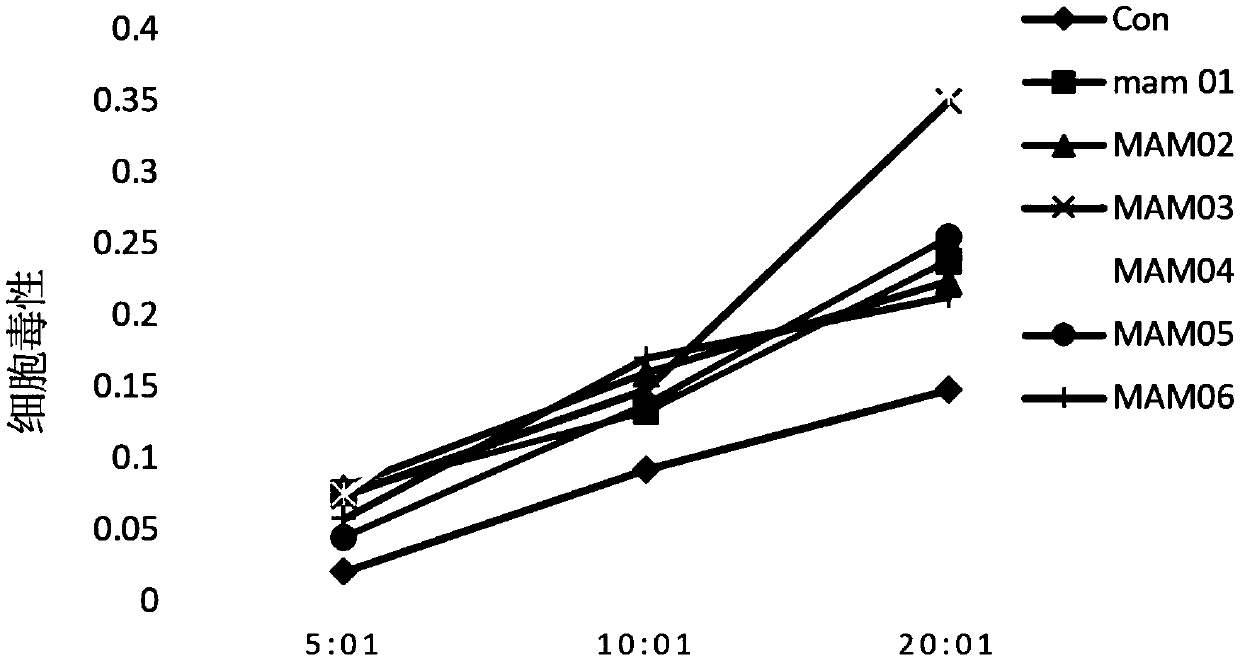
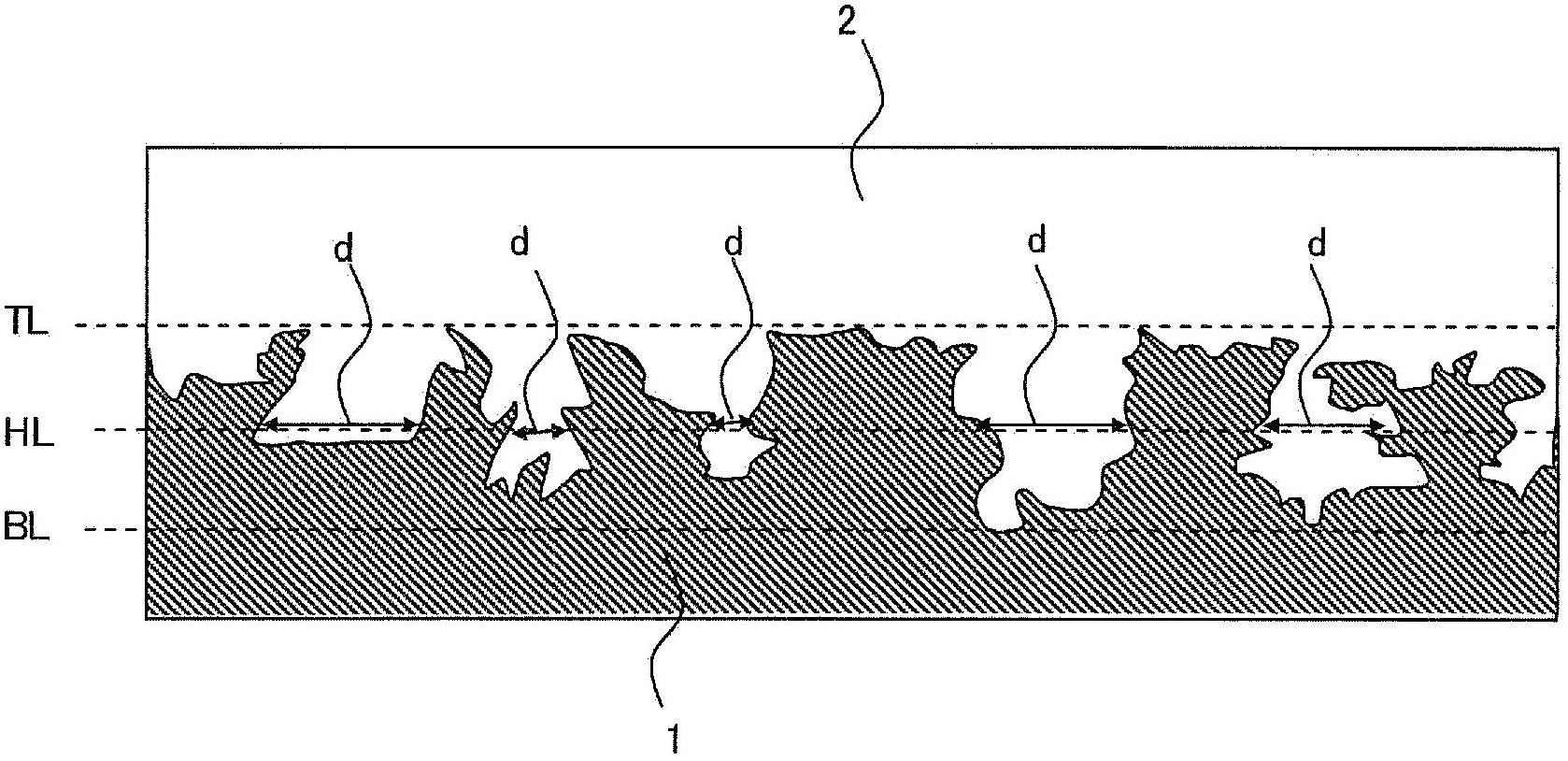
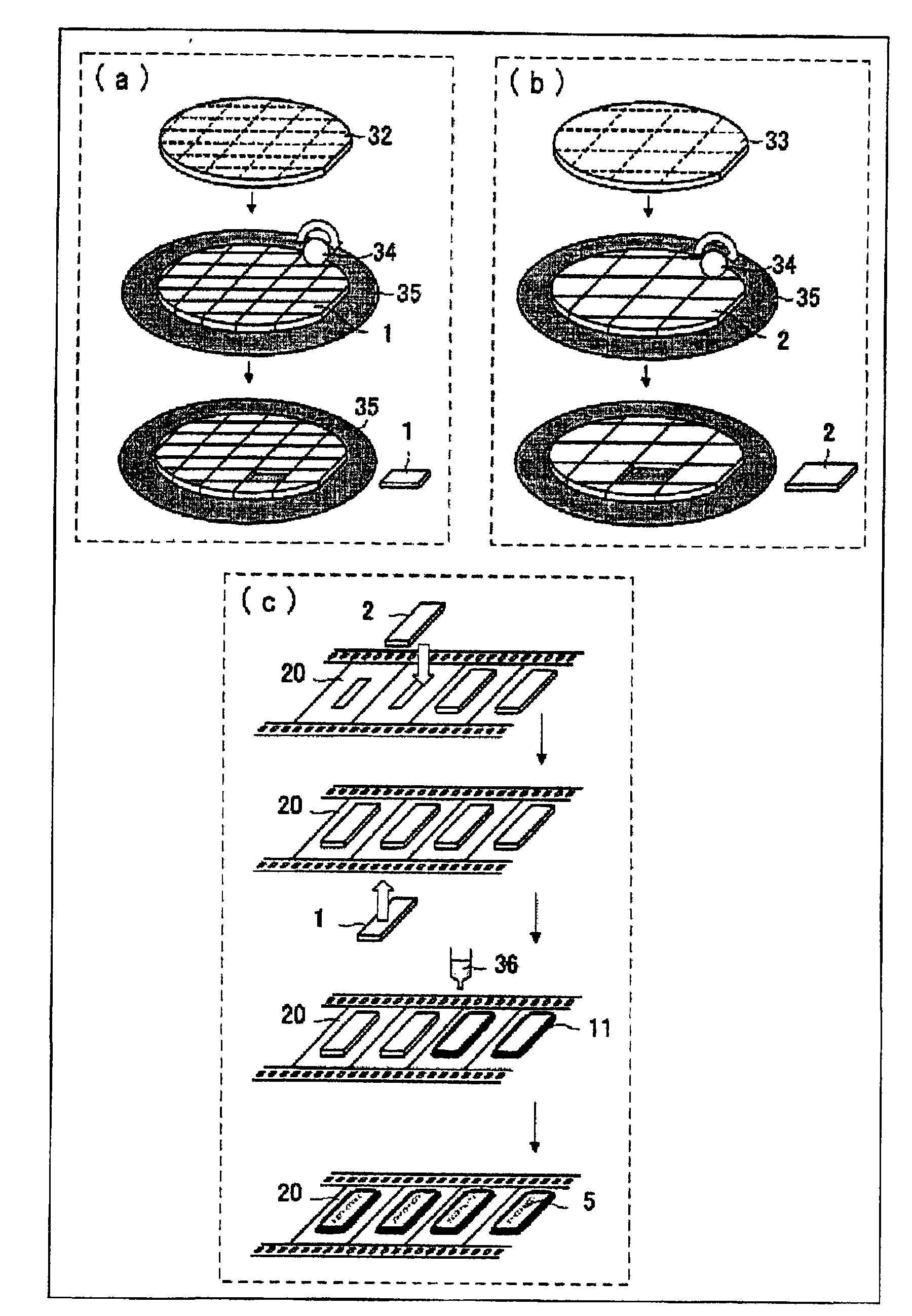
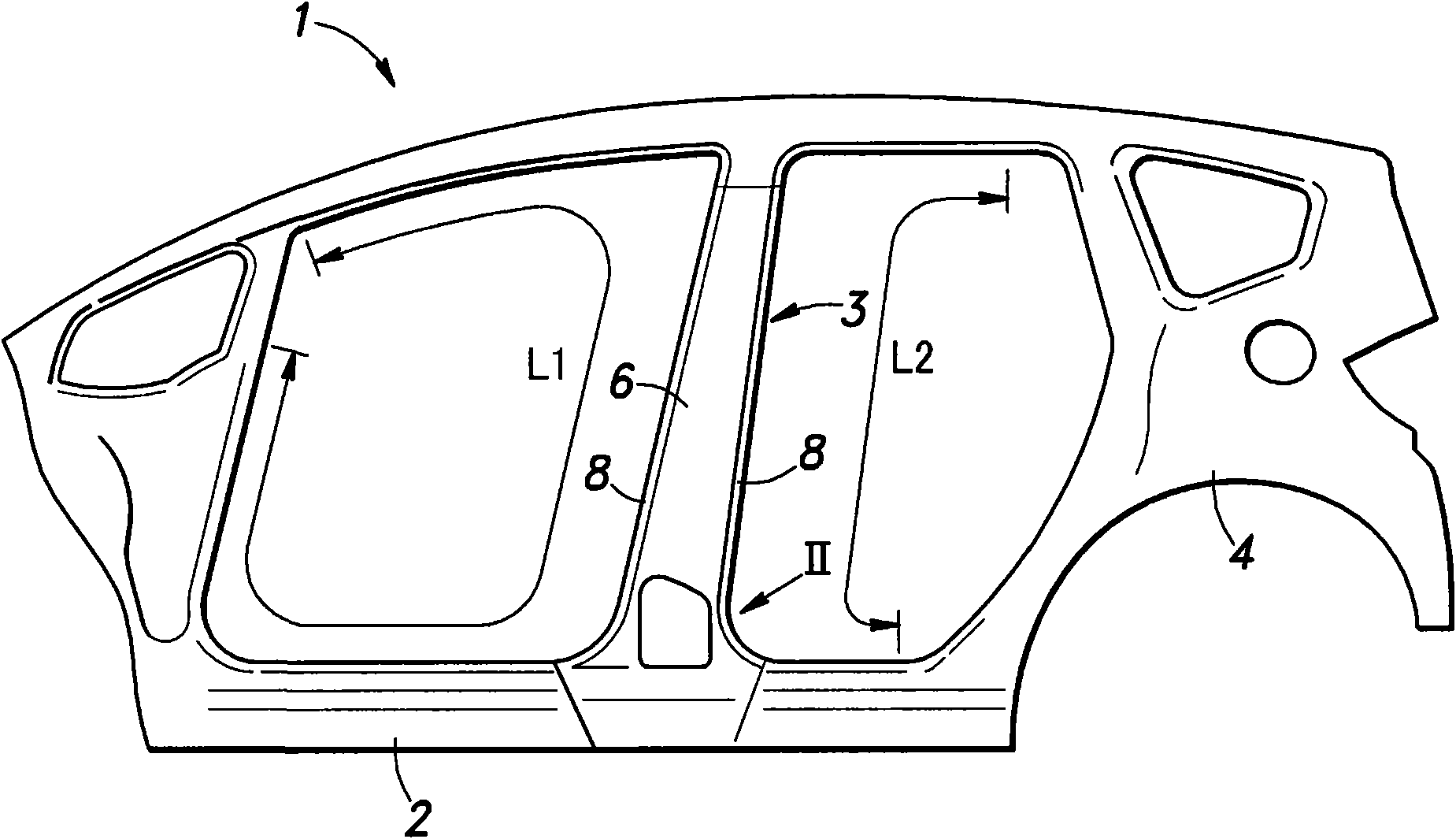
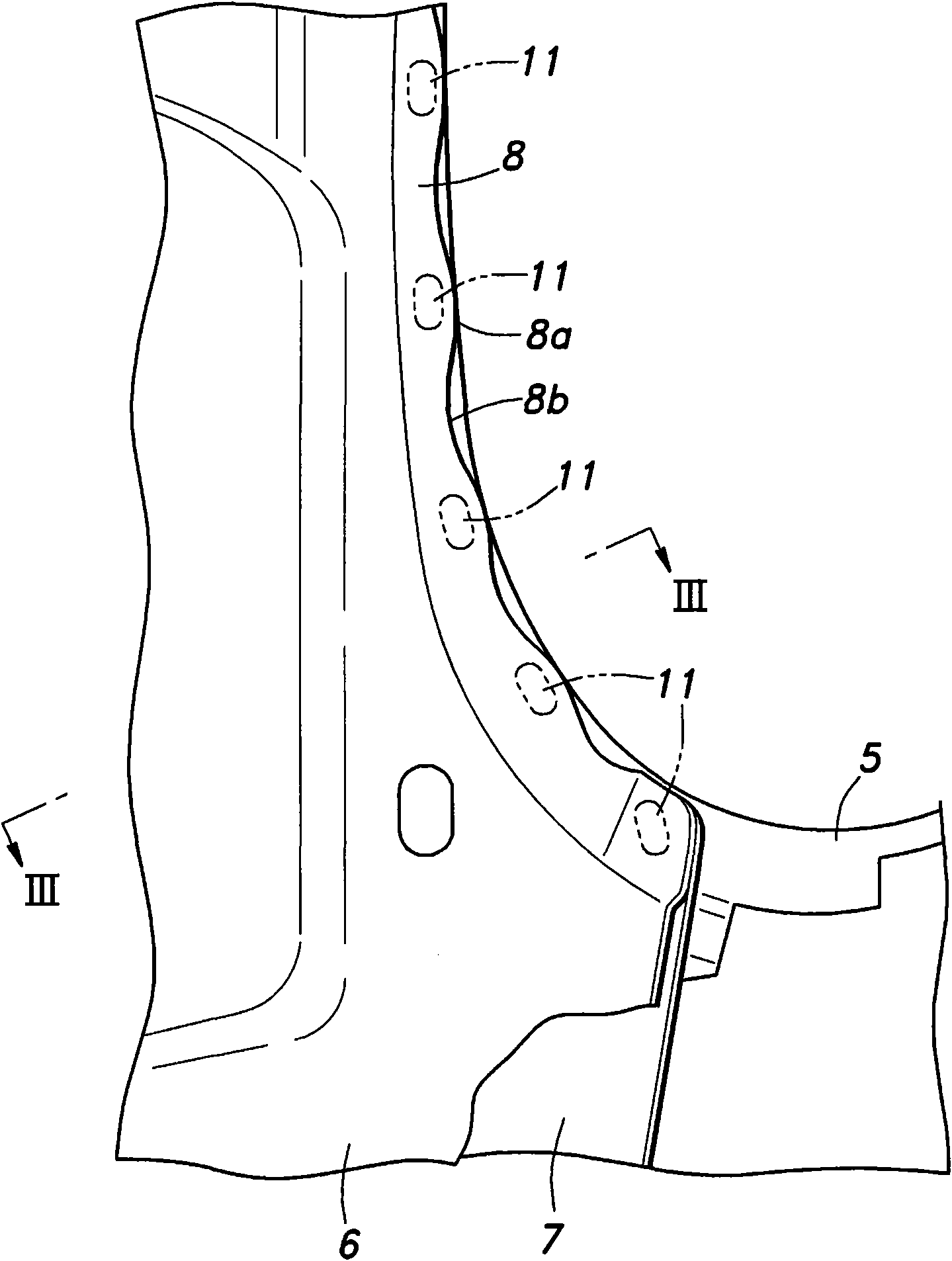
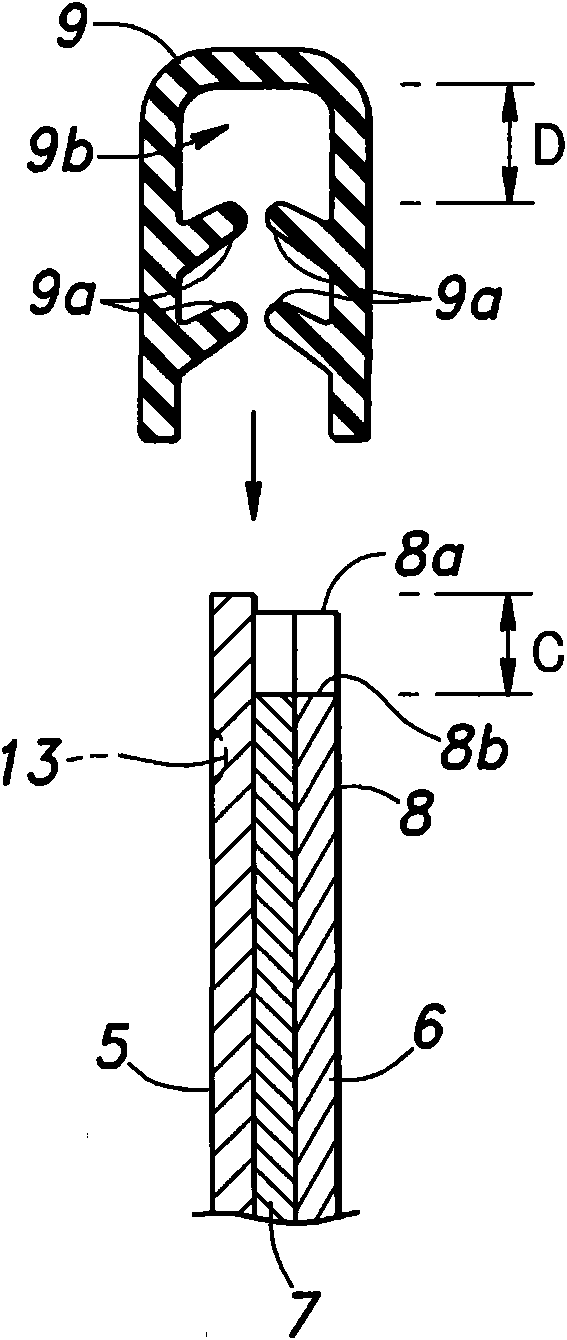
Patents
Literature
107results about How to "Easy Quality Control" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mesenchymal stem cell injection, preparation method and application thereof in preparing medicine for treating diabetes
InactiveCN102920735AIncrease productionEasy quality controlCell dissociation methodsPeptide/protein ingredientsVitamin CSide effect
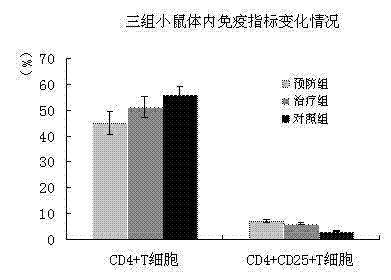
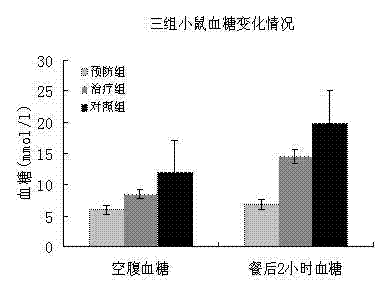
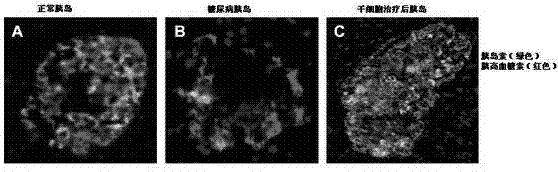
The invention discloses a mesenchymal stem cell injection, a preparation method and application of the mesenchymal stem cell injection in preparing a medicine for treating diabetes; the mesenchymal stem cells are derived from human umbilical cord and placenta; the mesenchymal stem cell injection consists of components: human mesenchymal stem cells, human albumin, low molecular heparin calcium, compound amino acid, vitamin C of 0.5% and dissolving medium; and the dissolving medium can be a compound electrolyte solution or glucose or normal saline. According to the mesenchymal stem cell injection, the preparation method and the application of the mesenchymal stem cell injection in preparing the medicine for treating the diabetes, the mesenchymal stem cell injection is used for repairing injured islet beta cells, and the blood sugar is reduced by secretion of the endogenous insulin, therefore, a purpose of foundational treating the diabetes is achieved. A disease course of the diabetes can be reversed, patients are helped in escaping out of inconvenience and toxic and side effects of taking the endogenous drug and injecting the insulin as well as serious complications caused by poor control of the blood sugar; and the 1-type and 2-type diabetes are treated thoroughly.
Owner:青岛奥克生物开发有限公司
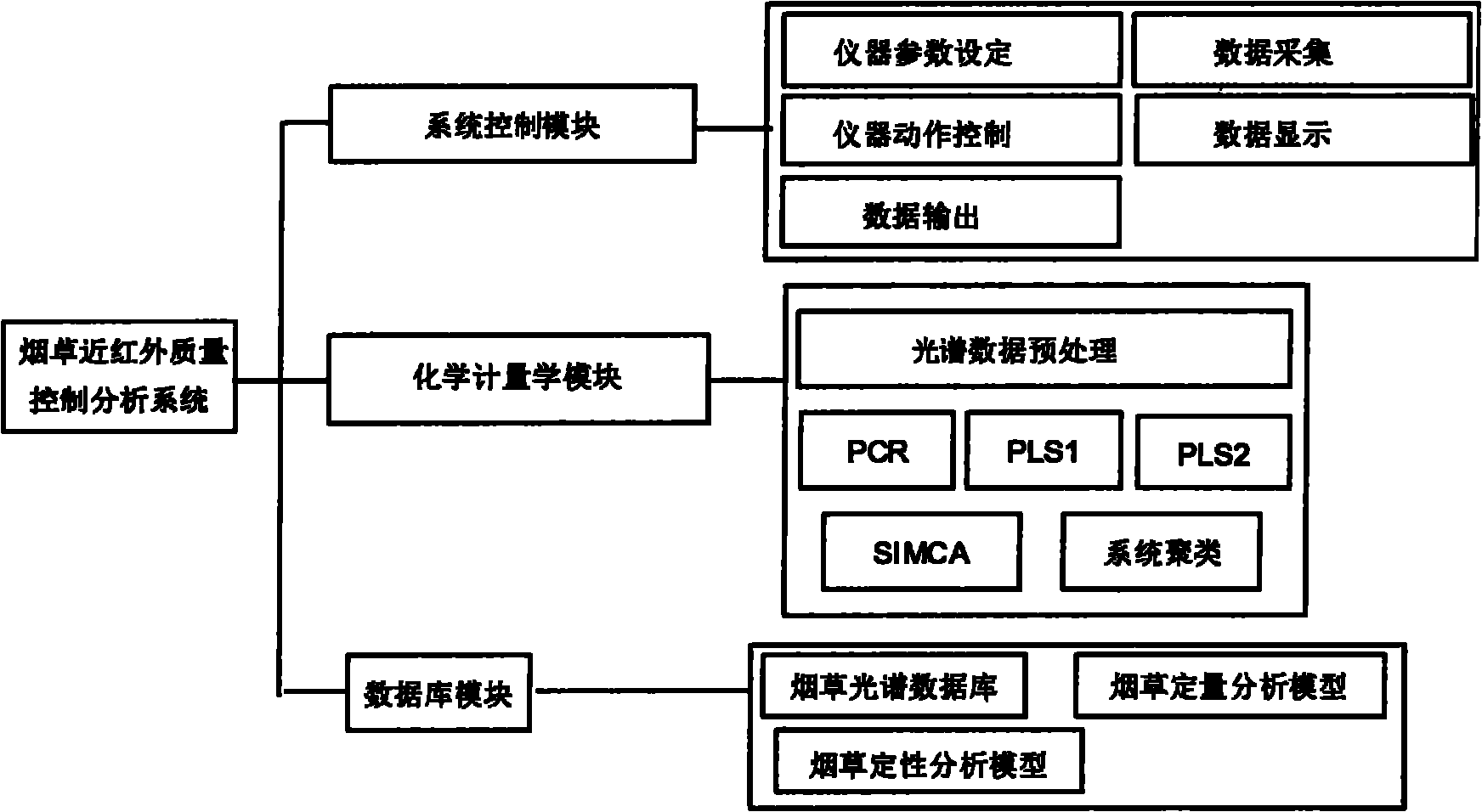
Near infrared quality control analysis method and system of tobacco
InactiveCN101995388AAchieve consistencyRealize evaluationColor/spectral properties measurementsQuality controlEngineering
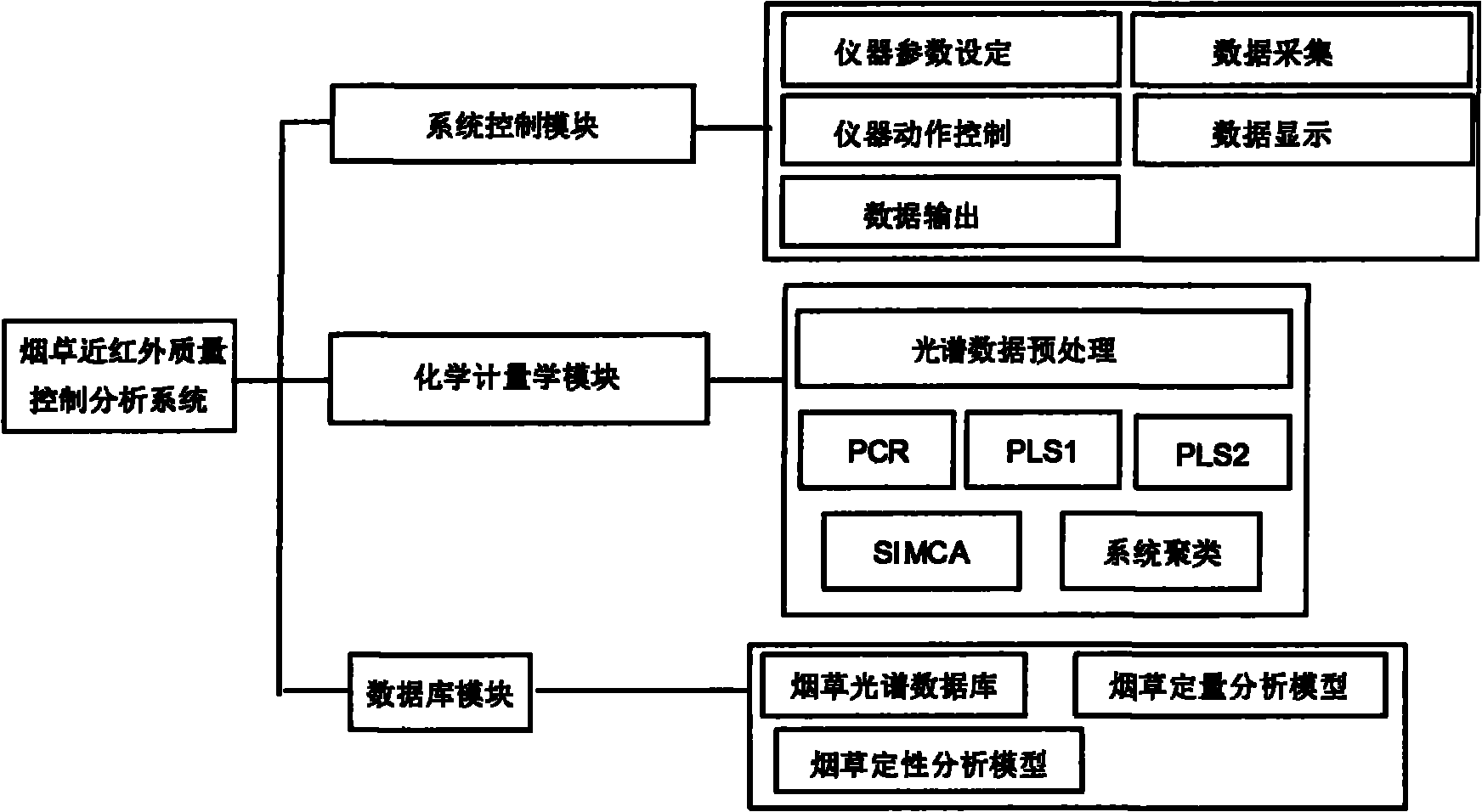
The invention discloses a near infrared quality control analysis method of tobacco. The method comprises the following steps of: collecting representative tobacco samples and grinding into powder; opening a tobacco sample acquisition instrument and preheating; setting parameters of the instrument; scanning the representative tobacco samples one by one; selecting an unknown sample, grinding into powder and forming a scanner spectrogram; opening a chemometric module of an analysis system, selecting an algorithm, preprocessing spectral data and establishing a correction model: setting a wavelength range of between 700 and 1,100nm, standardizing a spectrum value and chemical value, saving the established model and starting to correct the model; and predicting the sample and starting to calculate after selecting the unknown sample so as to obtain a quantitative and qualitative sample analysis result. Through the method and the system, the quality parameter of the tobacco can be quickly measured, and the quality of the tobacco can be quickly measured, controlled and analyzed on site. The method and the system are also suitable for quality monitoring of medicaments, milk, milk powder and the like, raw material comparison, analysis of product consistency and the like.
Owner:BEIJING KAIYUAN SHENGSHI SCI & TECH DEV
Epoxy Resin, Curable Resin Composition and Cured Product Thereof
InactiveUS20090117388A1Prevent decrease in performanceEasy quality controlPlastic/resin/waxes insulatorsSynthetic resin layered productsPhenolsPolyresin
Disclosed is a phenol aralkyl epoxy resin having a structure wherein at least a phenol or a naphthol is bound by using an aralkyl group as a linking group and a structure represented by formula (1) below, while satisfying the condition 1 below. This epoxy resin is excellent in workability during production of a composition and is easy to control quality. Condition 1: The following relation (α) is satisfied with A being the hydroxyl equivalent (as measured in accordance with JIS K 0070) of a phenol-modified epoxy resin obtained by adding an equivalent molar amount of phenol relative to the epoxy equivalent of the epoxy resin, and B being the epoxy equivalent of the epoxy resin. 50≦1000×(A−B) / B≦250 (α)
Owner:NIPPON KAYAKU CO LTD
Preparation method and application of classical swine fever virus recombinant subunit vaccine
InactiveCN104826100ANo risk of contaminationImprove securityAntiviralsAntibody medical ingredientsProtein targetVaccine Production
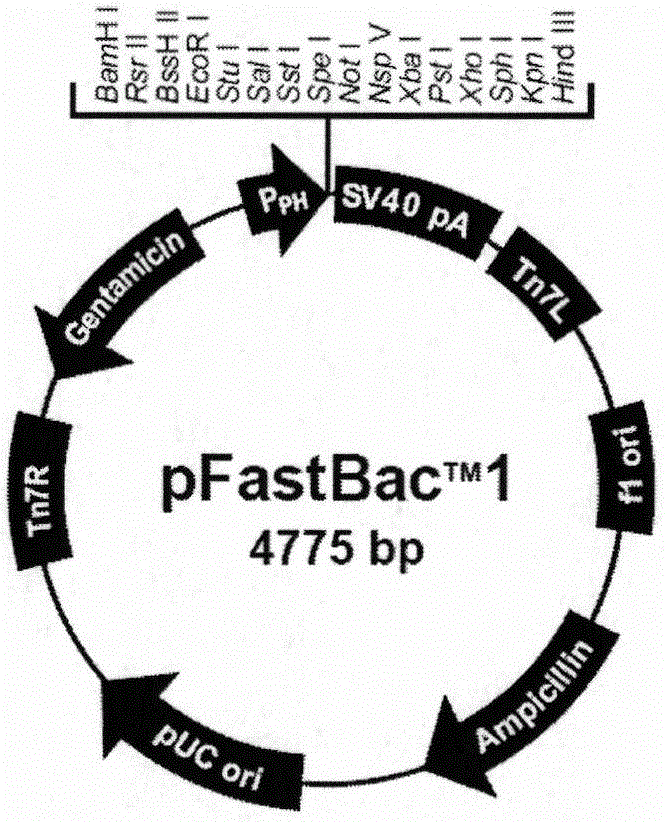
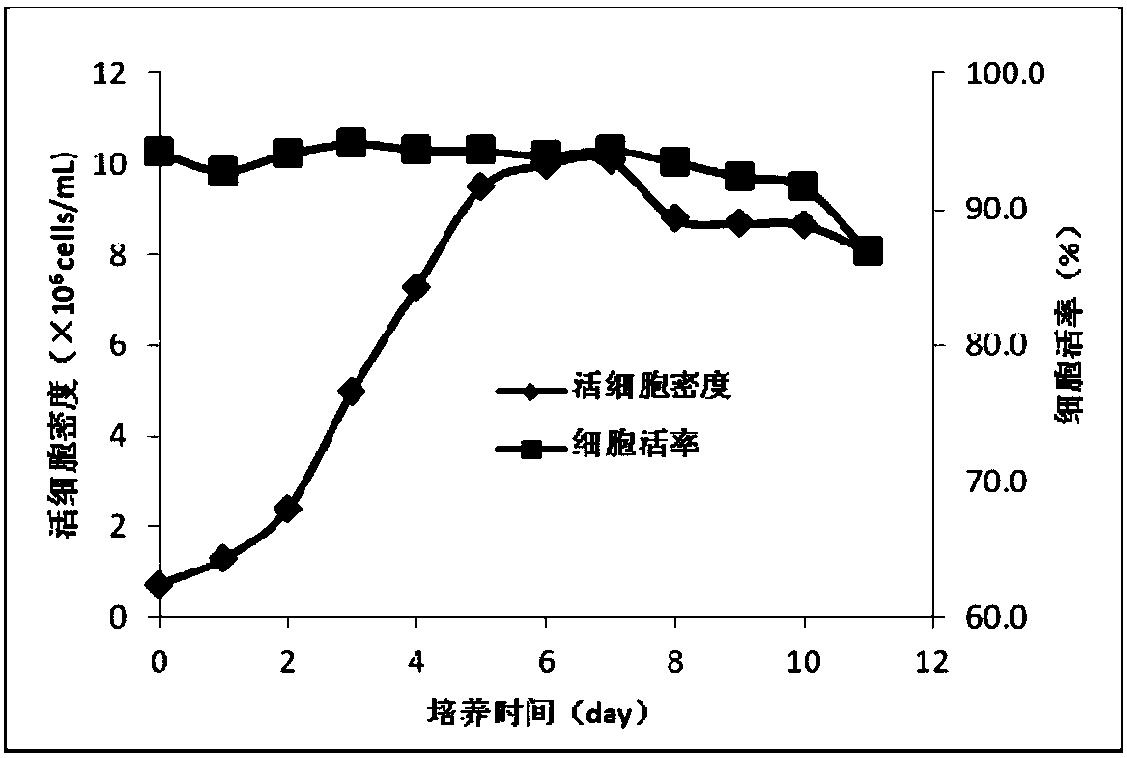
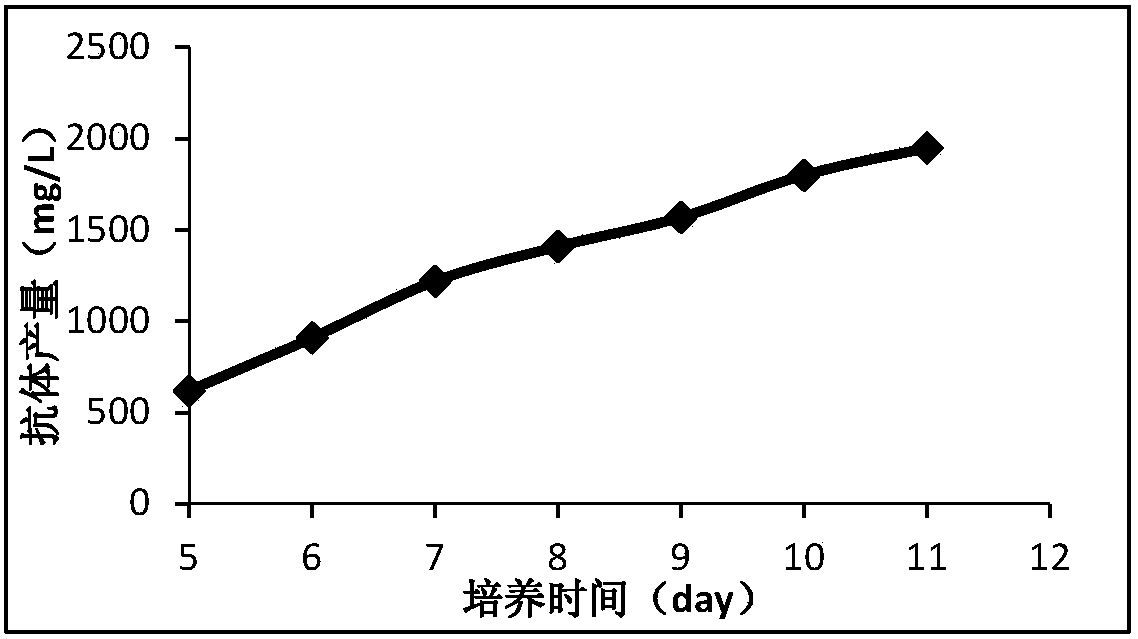
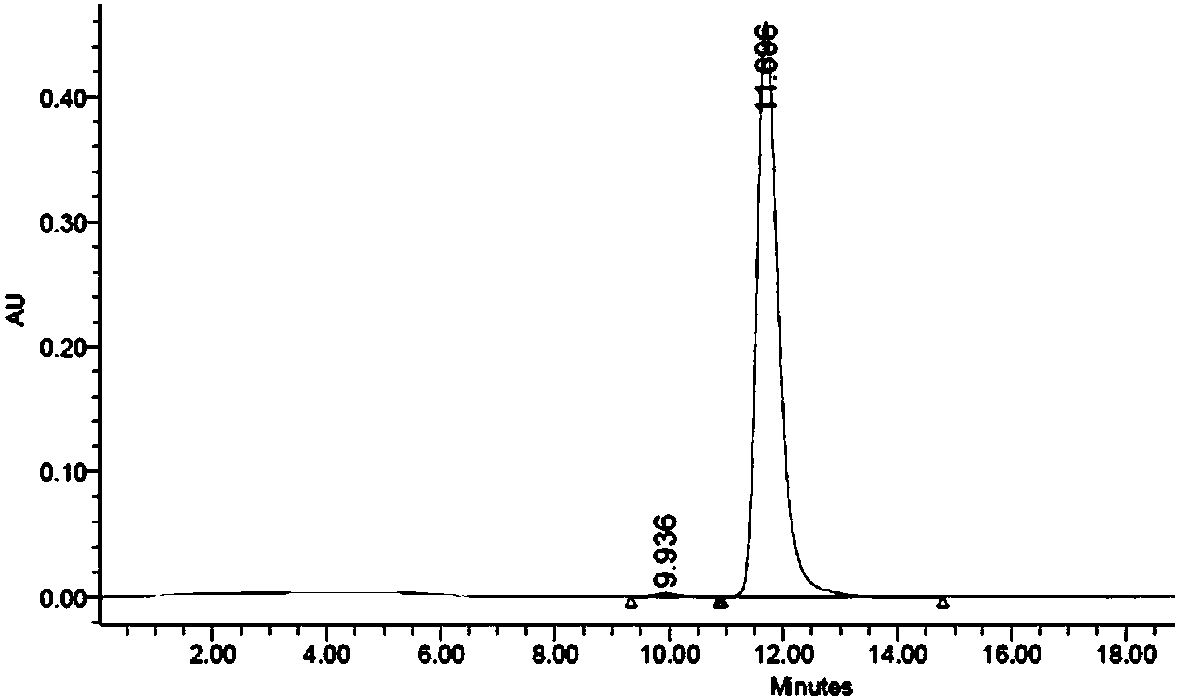
The invention discloses a preparation method and application of a classical swine fever virus recombinant subunit vaccine with the amino acid sequence shown as SEQ ID No.1. The preparation method of the classical swine fever virus recombinant subunit vaccine typically includes the following steps: classical swine fever E2 truncated protein (TE2) coding gene is cloned into baculovirus vector pFastBacTM1, and is then transfected into Sf9 insect cells to obtain recombinant baculovirus capable of expressing protein TE2. The high five insect cells in logarithmic growth phase are infected by the recombinant baculovirus, so that a large amount of the protein TE2 can be expressed in a cell culture supernatant. Finally, the cell culture supernatant is recovered and purified to obtain a large amount of the recombinant protein TE2 with the purity more than 90%. According to the method, the target protein can be harvested from the cell culture supernatant, the time of protein purification is reduced, consumption of a large amount of time can be avoided, and the vaccine production process can be simplified. Under the premise of simplification of the vaccine production process, the recombinant protein TE2 has the advantages of strong immunogenicity and high safety, and the animal experiments prove that the recombinant protein can effectively stimulate the body to produce a highly effective humoral immune response.
Owner:NOVO BIOTECH CORP
Efficient energy-saving kervit microbead insulated sand slurry
The invention is a high-efficiency, energy-saving, vitrified microball thermal insulation mortar thermal insulation system, comprising: cement, vitrified microballs, re-dispersible latex powder, composite fibers, methyl cellulose ether and air-entraining agent, where the composite fibers include tensile fiber, PP fiber and wood fiber. The invention has antiaging and fireproofing properties, not hollow swelling and cracking, and having high intensity and good binding property, and having heat conductivity coefficient of 0.05W / m.k-0.06W / m.k, saving energy consumption of buildings, applied to new buildings and existing buildings. As constructing, the invention is simple to operate, and the quality is easy to control, able to largely raise construction efficiency and convenient to large-scale spreading and application.
Owner:李珠
Cell culture medium and method for producing protein
InactiveCN107760651AImprove protein expression abilityEasy Quality ControlGenetically modified cellsCulture processChemical compositionCulture cell
The invention belongs to the field of biological pharmacy, and provides a cell culture medium and a method for producing protein by using the culture medium to culture cells. According to the cell culture medium, amino acid optimization is carried out on the basis of a culture medium limited by chemical components, so that the protein expression capacity of a cell is improved. The method providedby the invention is simple in process control and is beneficial to large-scale production.
Owner:SICHUAN KELUN BIOTECH BIOPHARMACEUTICAL CO LTD
Polypeptide fragment for preparing DC (Dendritic Cell) vaccine and DC vaccine
InactiveCN109575118AImprove securityReduce manufacturing costCulture processCancer antigen ingredientsHuman serum albuminAmino acid
The invention discloses a polypeptide fragment for preparing DC (Dendritic Cell) vaccine and the DC vaccine. The polypeptide fragment is selected from at least one of amino acid sequences as shown inSEQ ID NO. 1 to SEQ ID NO. 6. The DC vaccine contains polypeptide fragment loaded DC. Specifically, the DC vaccine is prepared from the polypeptide fragment loaded DC, human serum albumin and normal saline, wherein the mass concentration of the human serum albumin in the normal saline is 5 to 20 percent, and the density of the polypeptide fragment loaded DC is (0.5-2)*10<6> per mL. According to the polypeptide fragment for preparing the DC vaccine and the DC vaccine, disclosed by the invention, human mammaglobin is selected as an attack target spot, an effective DC polypeptide vaccine preparedfrom the polypeptide fragment obtained through computer design and experimental screening can be used for treating patients suffering from HER2 (Human Epidermal Growth Factor Receptor-2) positive / negative breast cancer, and preventing recurrence and metastasis. The DC vaccine is capable of reducing the production cost while increasing the vaccine safety.
Owner:英普乐孚生物技术(上海)有限公司
Aluminium/resin composite exhibiting excellent weather resistance and manufacturing method for the same
ActiveCN102652058AHigh strengthImprove air tightnessMetal layered productsCorrosion resistantWeather resistance
Disclosed is an aluminium / resin composite which: exhibits extremely high interfacial adhesion strength and air tightness between an integrally-bonded, shaped aluminium article made from an aluminium alloy, and a molded resin article; retains the excellent adhesion strength and air tightness of the aluminium / resin bond surface in harsh environments such as those caused by temperature, humidity, dust, and corrosive materials; and is corrosion resistant, heat resistant, weather resistant, durable, and exhibits excellent peel strength on the aluminium / resin interfacial surface. Also disclosed is a manufacturing method for the aluminium / resin composite. The aluminium / resin composite contains a shaped aluminium article having undulations on the surface, and a molded resin article which is bonded to the shaped aluminium article when the resin is butted thereto. A plurality of concave sections are formed due to the undulations, and an aluminium coating film is formed on the outermost surface of the undulations. Fitting sections are formed on the molded resin article where the resin enters the concave sections and hardens. The components of the aluminium / resin composite which exhibits excellent weather resistance are engaged by the concave sections and the fitting sections.
Owner:NIPPON LIGHT METAL CO LTD
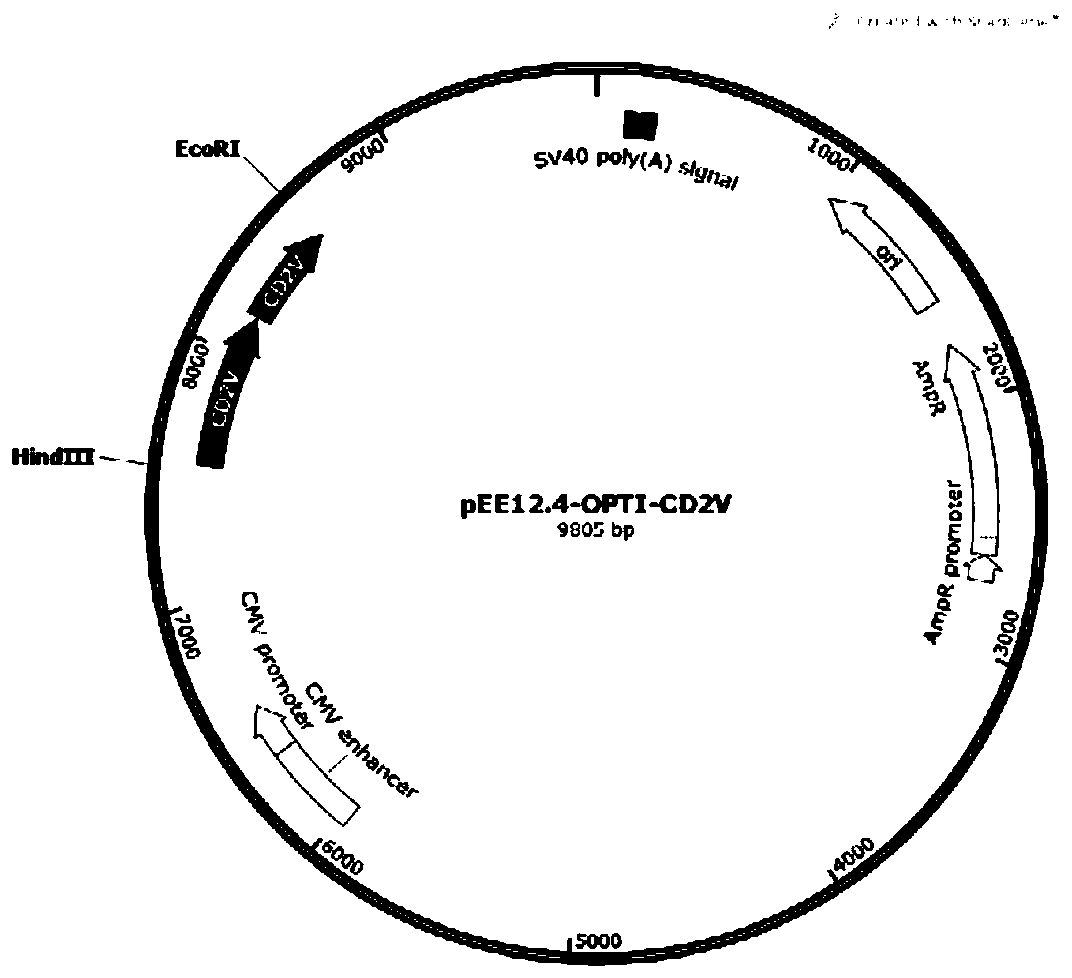
Recombinant African swine fever virus CD2V subunit protein as well as preparation method and application thereof
PendingCN111471089ABatch-to-batch stabilityImprove controllabilityViral antigen ingredientsVirus peptidesAdjuvantAfrican swine fever
The invention discloses a recombinant African swine fever virus CD2V subunit protein as well as a preparation method and application thereof. The protein comprises an extracellular region and an intracellular region of African swine fever virus surface envelope protein, and the amino acid sequence of the protein is shown as SEQ ID NO.3. The preparation method comprises the following steps: 1) cloning a codon-optimized gene sequence shown as SEQ ID NO.1 into an eukaryotic expression vector; 2) transfecting a recombinant expression vector containing the African swine fever virus subunit proteincoding gene into CHO cells; 3) culturing, screening and domesticating a CHO cell strain in the step 2) to obtain a highly-expressed cell strain; 4) fermenting and culturing the cell strain in the step3), and performing purifying to obtain the African swine fever virus CD2V subunit protein; and 5) mixing the CD2V protein with a pharmaceutically acceptable adjuvant to obtain a subunit vaccine. Theinvention can provide the African swine fever surface CD2V subunit protein which can be industrially produced on a large scale, the preparation method is simple and low in cost, and the prepared vaccine can reach the existing national standard.
Owner:NOVO BIOTECH CORP
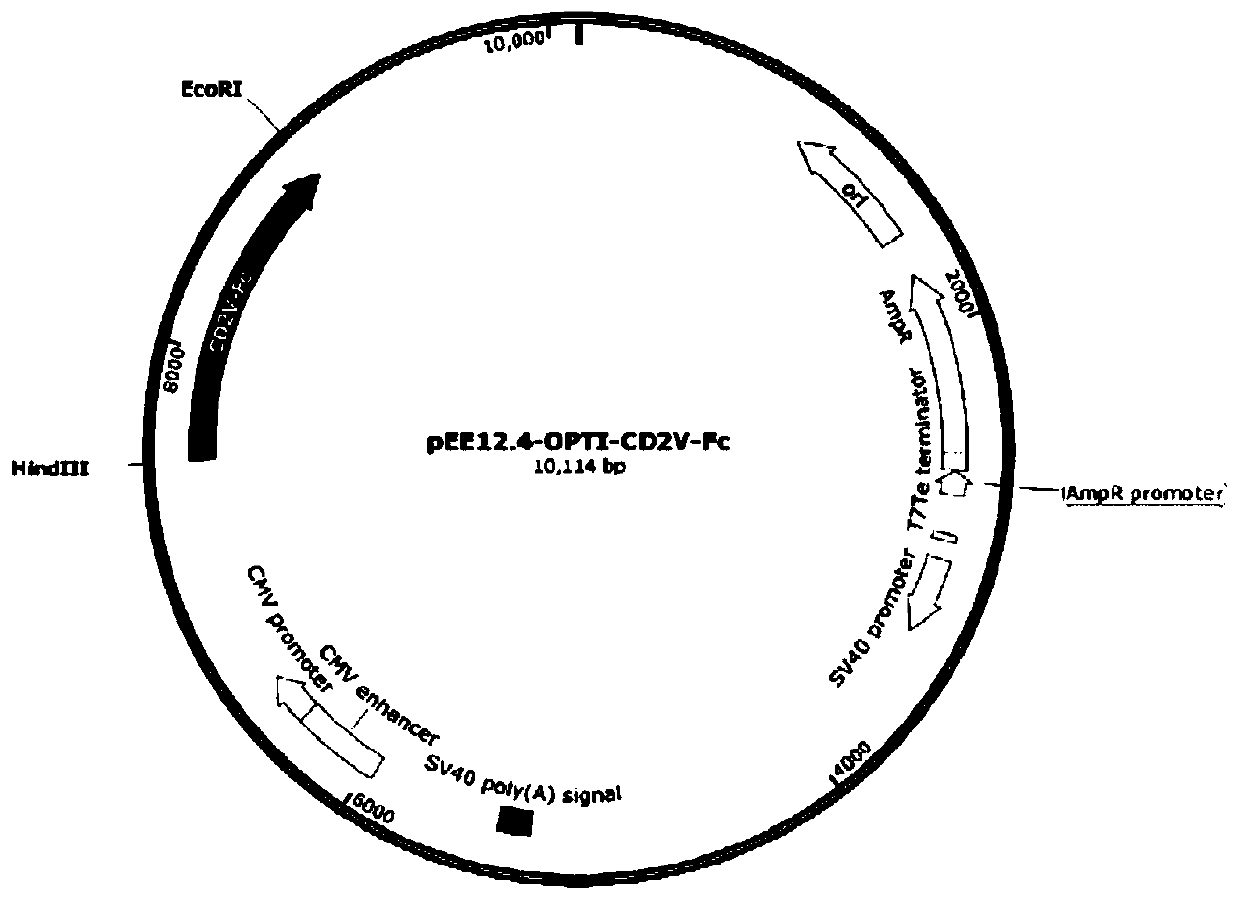
Subunit fusion protein CD2V-Fc, preparation method and application thereof
ActiveCN111393531AIncrease productionImprove controllabilityAntibody mimetics/scaffoldsViral antigen ingredientsAfrican swine fever virusAmino acid
The invention provides a subunit fusion protein CD2V-Fc, a preparation method and application thereof. The subunit fusion protein CD2V-Fc contains an extracellular region of an African swine fever virus surface envelope protein CD2V and an antibody Fc protein of a pig, and the amino acid sequence of the subunit fusion protein CD2V-Fc is as shown in SEQ ID NO. 1. The CD2V-Fc can be subjected to soluble expression in a large amount, the protein is stable, a plurality of problems in the prior art are overcome, and the preparation method is simple and low in cost.
Owner:NOVO BIOTECH CORP
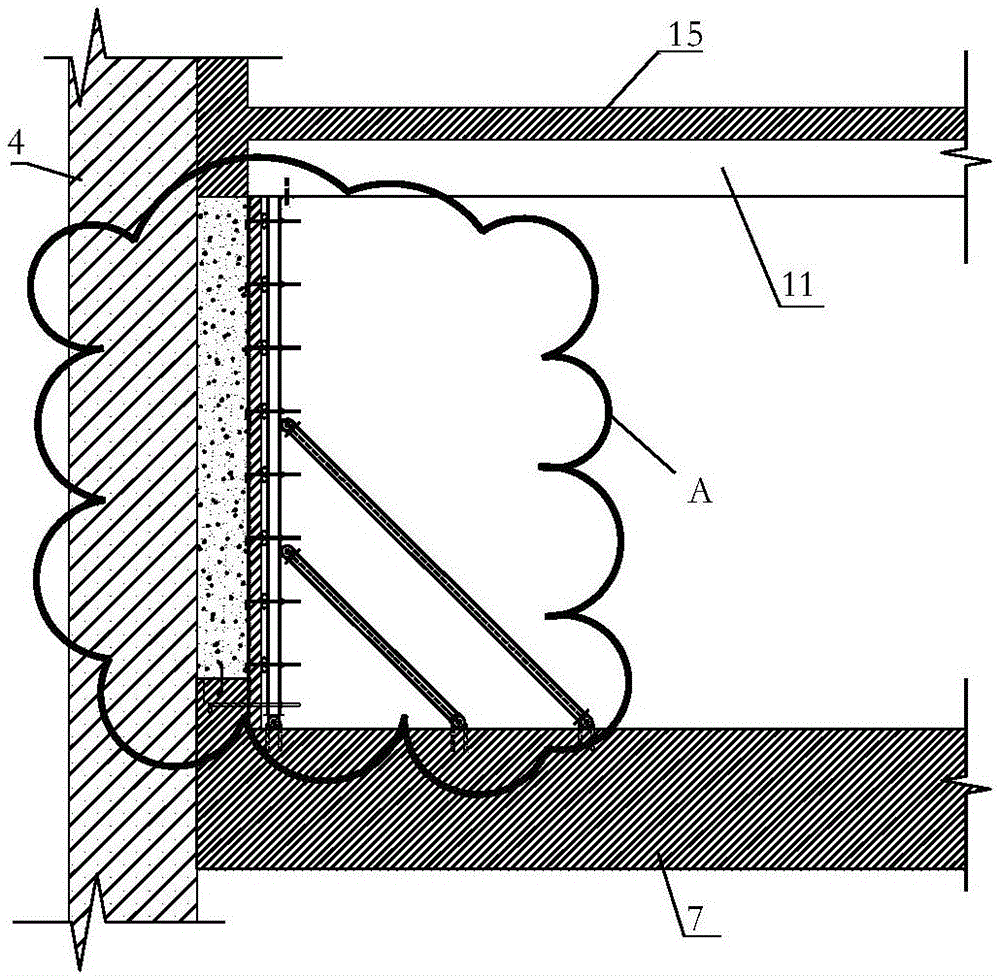
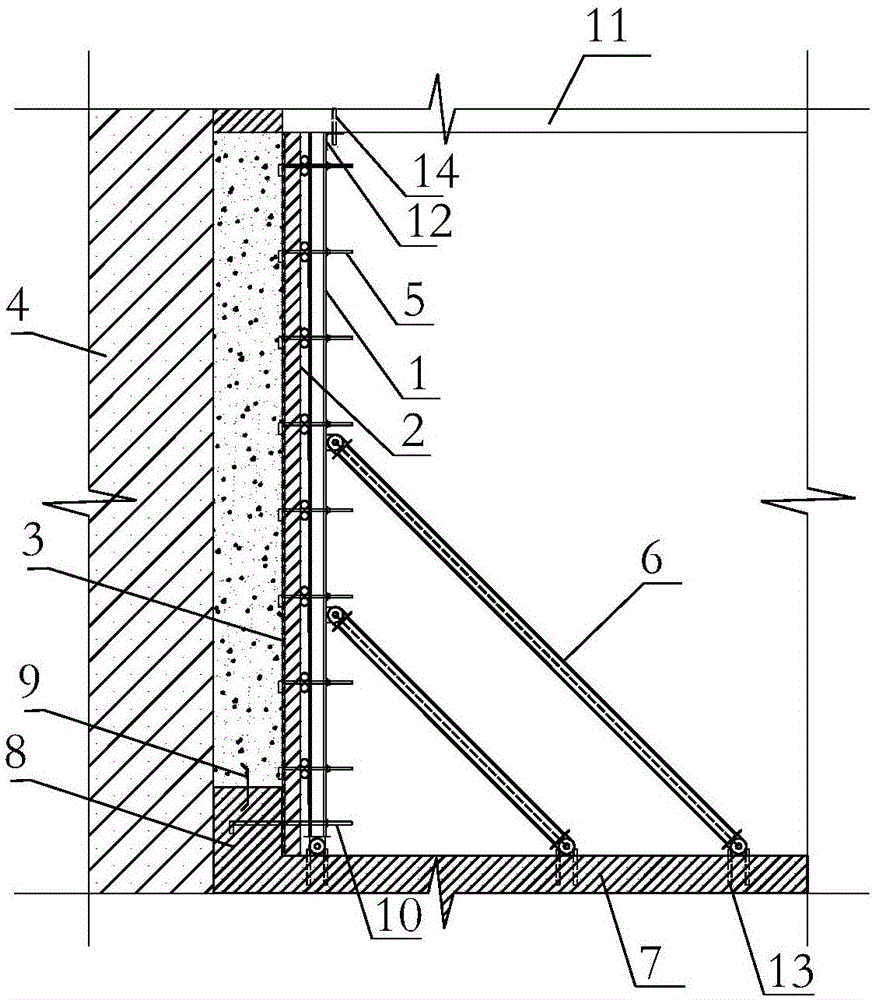
Basement exterior wall single-side formwork supporting system and method
InactiveCN106677533AEfficient and convenient supportEasy quality controlAuxillary members of forms/shuttering/falseworksBuilding material handlingSteel platesConcrete beams
The invention discloses a basement exterior wall single-side formwork supporting system and method and relates to the technical field of building construction. The basement exterior wall single-side formwork supporting system and method are disclosed to solve the problems that a traditional basement exterior wall single-side formwork supporting system is low in supporting efficiency and the supporting quality is hard to guarantee. According to the formwork supporting system, a reinforcing rod, a batten, a formwork and a fender post are fixedly connected in sequence along a basement exterior wall from inside to outside through split bolts, and inclined struts are arranged between the side, away from the batten, of the reinforcing rod and a foundation slab. The formwork supporting method comprises the steps that firstly, a waterproof steel plate is arranged; secondly, the reinforcing rod, the batten and the formwork are fixedly connected to form the formwork supporting system through the split bolts, the lower end of the formwork supporting system is fixedly connected with steel plates pre-buried in the foundation slab, the upper end of the formwork supporting system is fixedly connected with a concrete beam through angle steel and anchor bars, and the inclined struts are installed; and thirdly, joints and the formwork supporting system are fixedly connected through waterproof split bolts.
Owner:SHANGHAI CONSTR NO 1 GRP
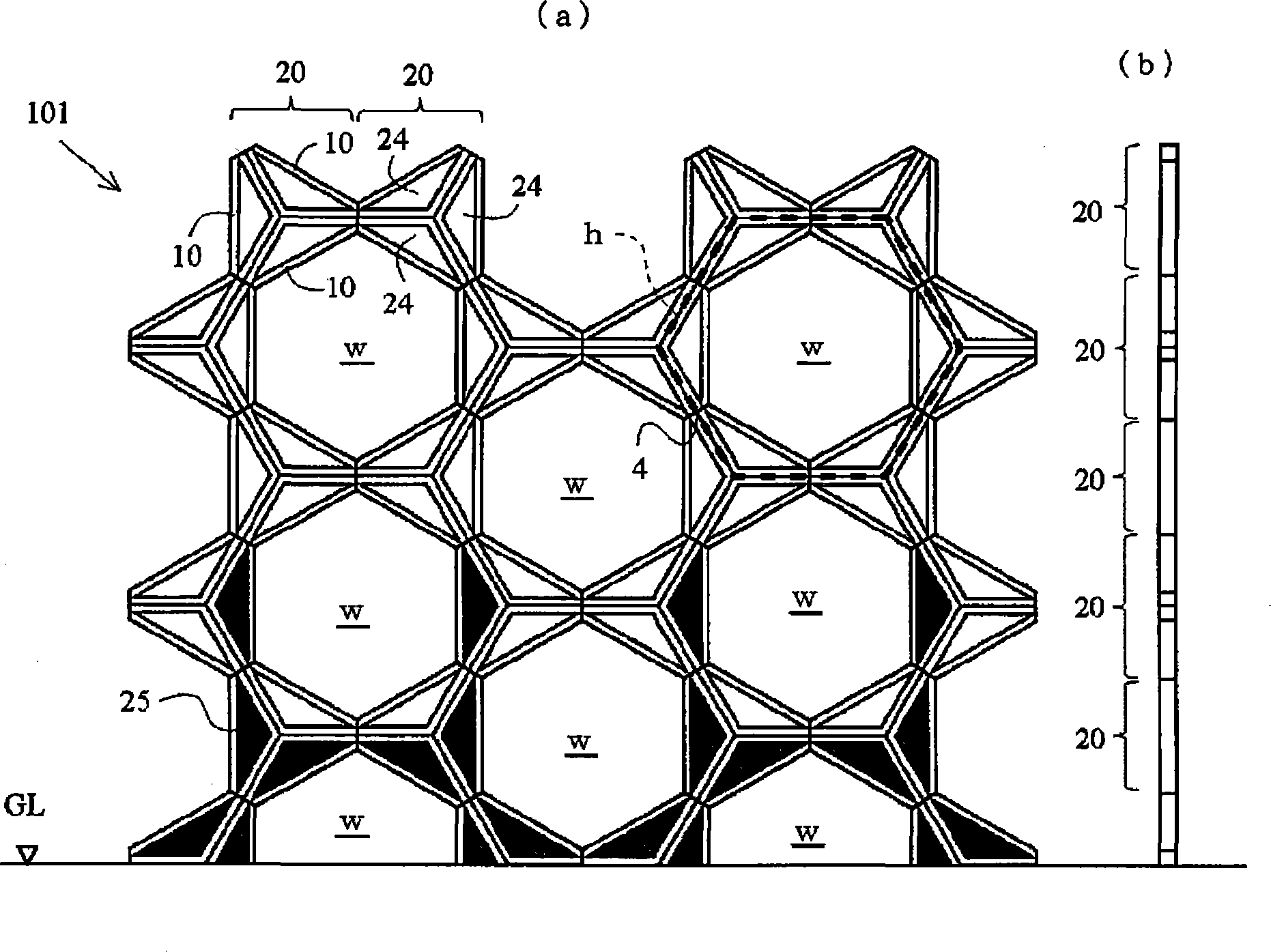
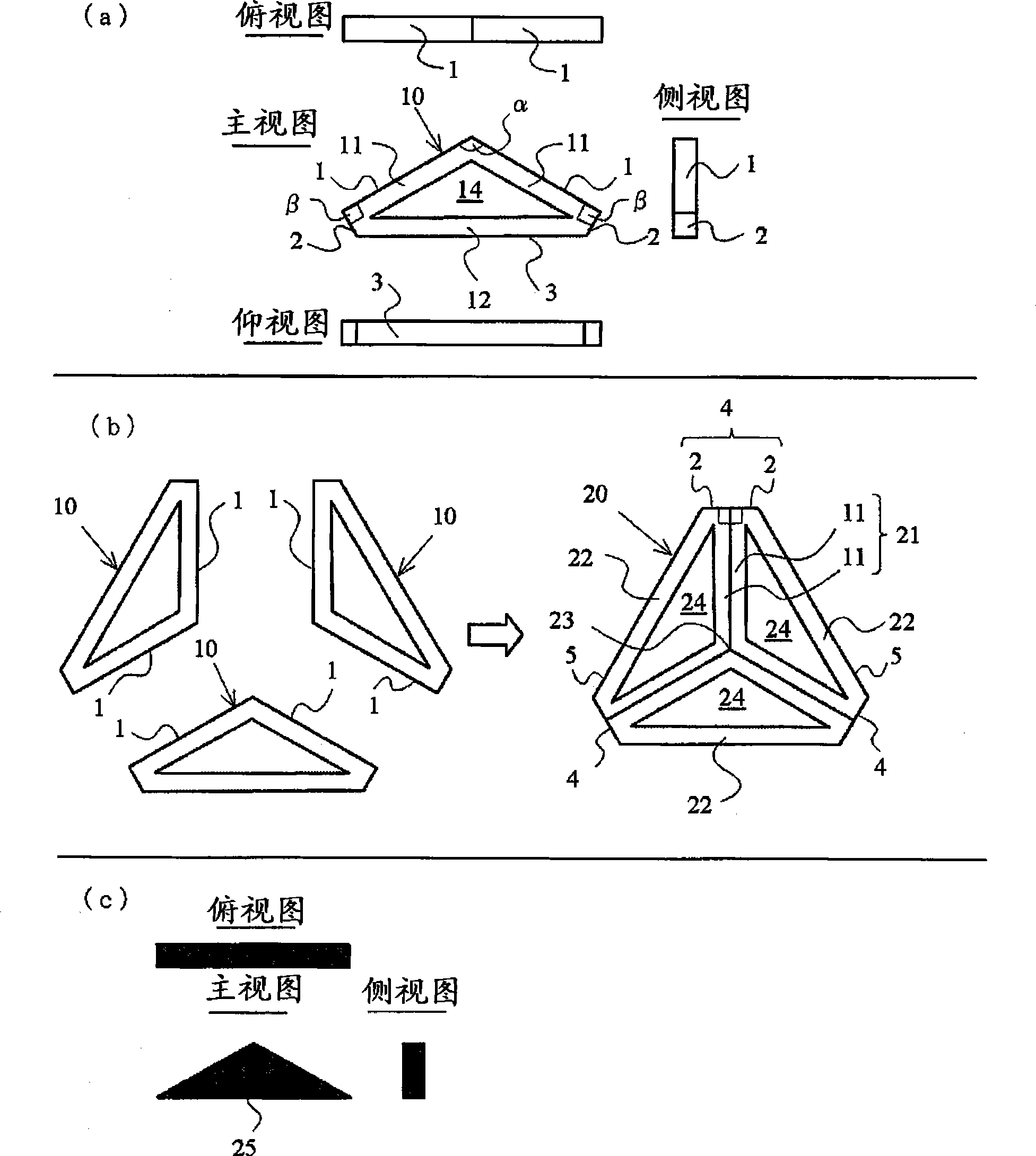
Honeycomb building tectosome
InactiveCN101415891ARigid constructionGuarantee stabilityExtraordinary structuresMarine engineeringEngineering
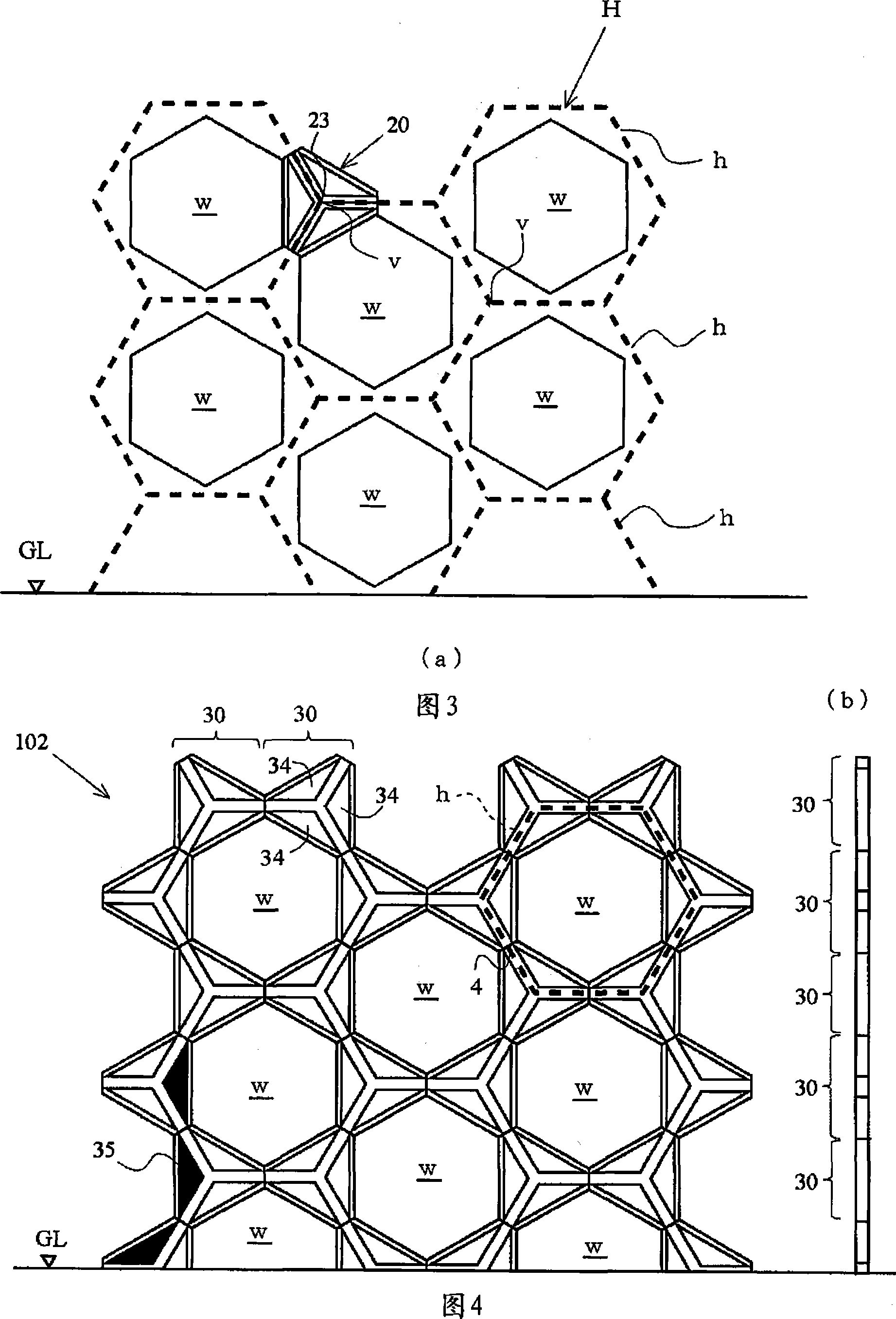
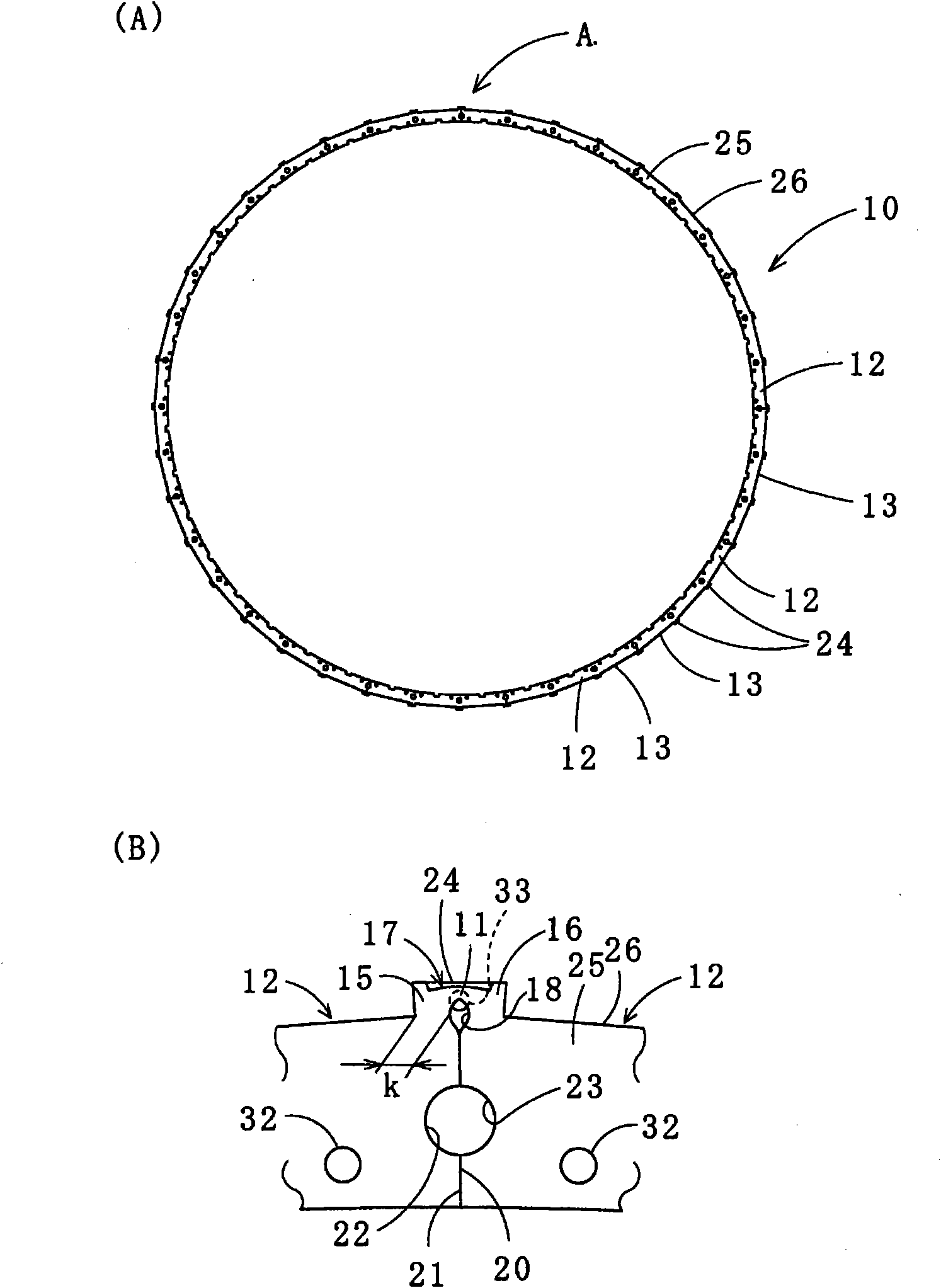
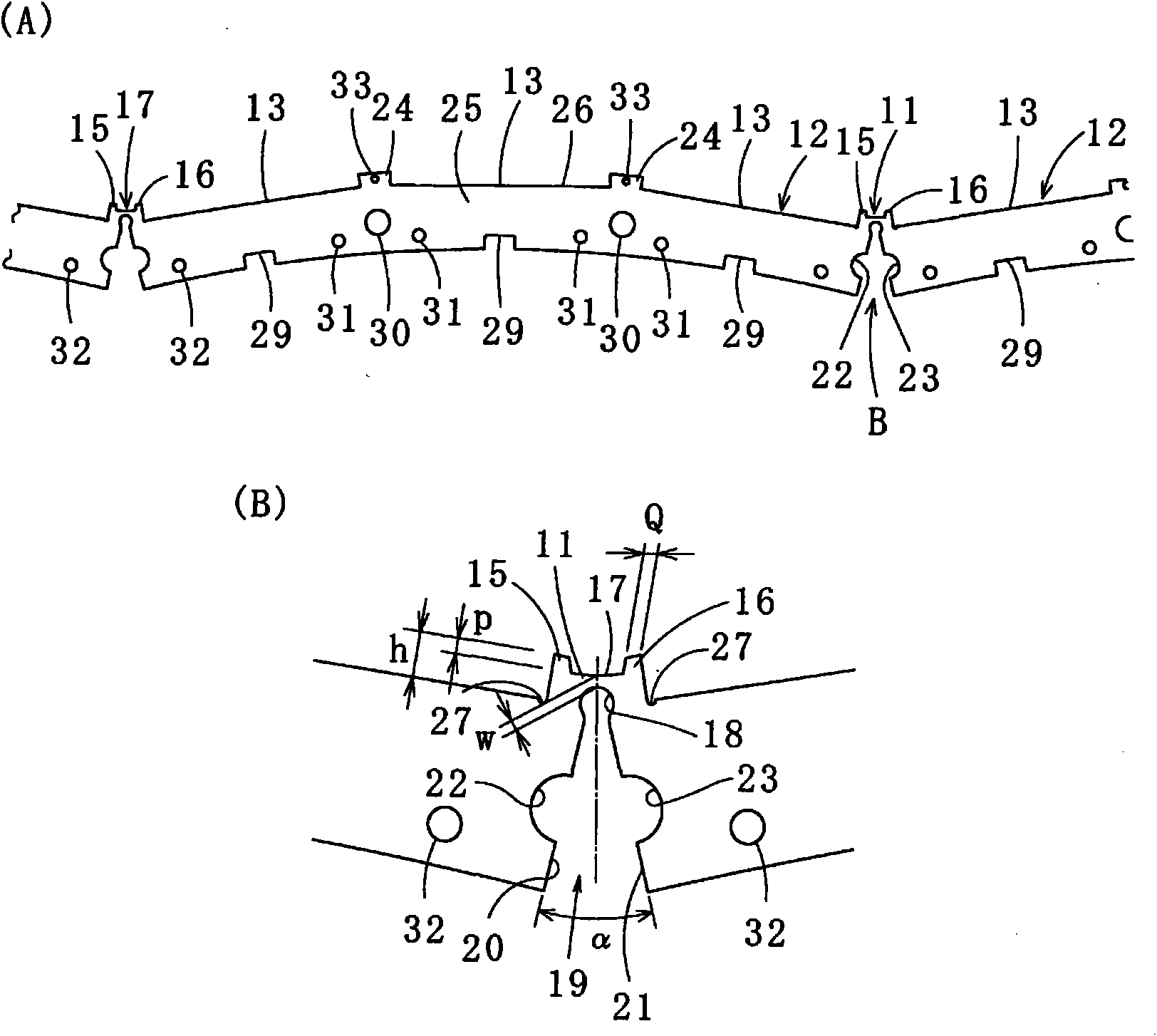
An architectural honeycomb structure exhibiting strong aseismatic resistance and wind pressure resistance and suitable for high-rise and super high-rise buildings,is provided. The architectural honeycomb structure (101, 102) bonding a plurality of frame units in the shape of honeycomb comprises a compound truss unit (20) which is a frame unit arranged to include one vertex of a hexagonal lattice (h) constituting a standing virtual honeycomb shape (H) and formed to have a hexagonal contour, a means for bonding two adjoining compound truss units such that the bonding surface (4), i.e. the outer circumferential surface corresponding to every other side of hexagon in the compound truss units, is located on the side of the hexagonal lattice of virtual honeycomb shape to intersect the side, and a first hexagonal opening (w) surrounded by six composite truss units.
Owner:SEKISUI CHEM CO LTD
Expansion and contraction detecting method for multi-layer plate
InactiveCN109548288AReduce shrinkage test processIncrease productivityPrinted circuit manufactureIndustrial engineering
The invention provides an expansion and contraction detecting method for a multi-layer plate. The method comprises: determining an expansion-contraction coefficient by a production plate after early-stage pressing; modifying an expansion-contraction drilling belt based on the expansion-contraction coefficient; and carrying out drilling processing on the corresponding production plate according tothe expansion-contraction drilling belt. Therefore, the expansion and contraction measuring flow of a pressing plate can be simplified effectively; the drilling production efficiency can be enhanced;quick transferring to a next procedure for production is realized; the expansion and contraction of the plate can be monitored through a peripheral auxiliary hole during drilling; and production is carried out by direct setting the expansion and contraction data by a drilling bench, so that the efficiency is greatly improved.
Owner:HUIZHOU ZHONGJING ELECTRONICS TECH CO LTD
Laminated iron core for rotor
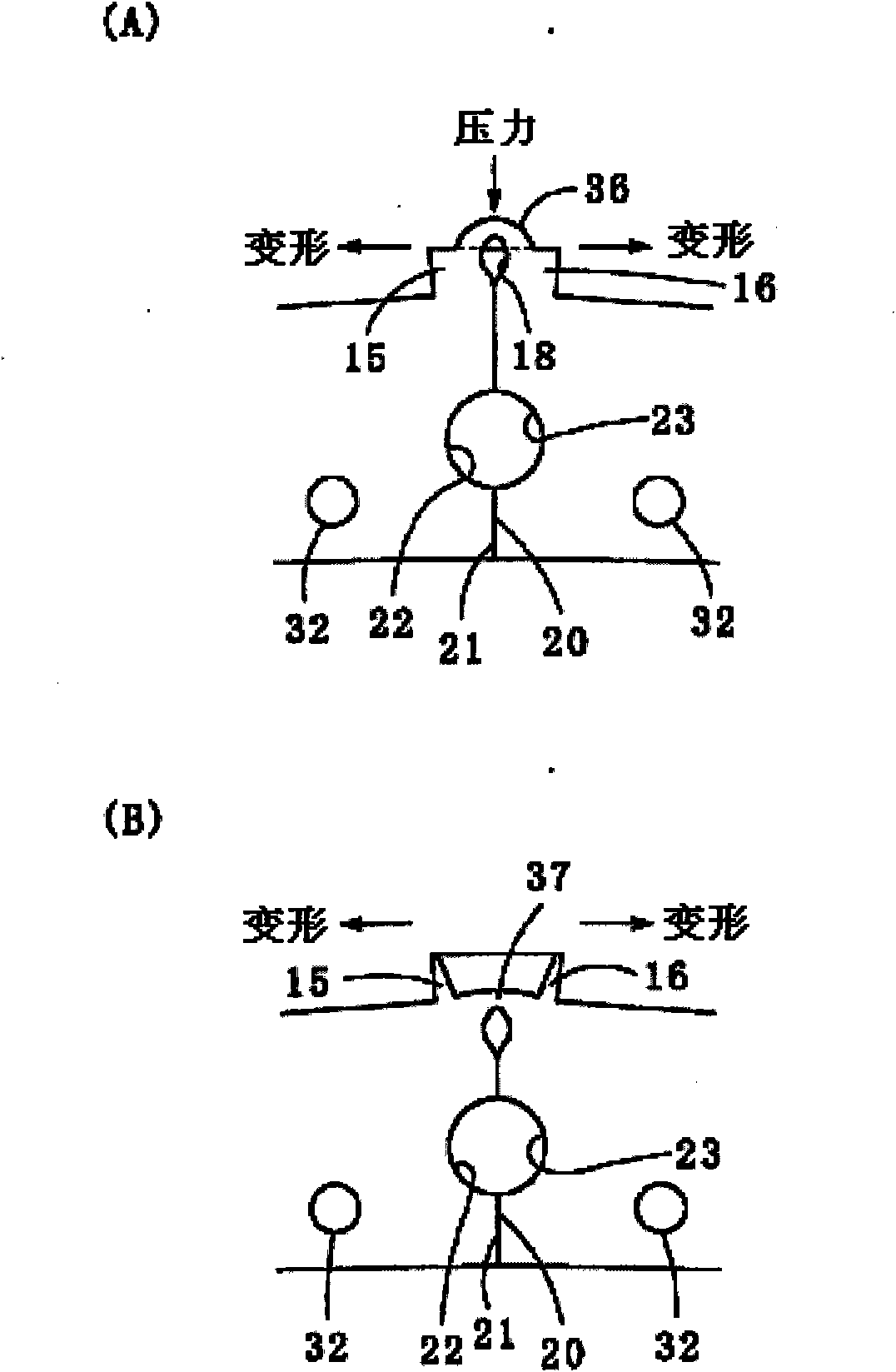
Provided is a laminated iron core for a rotor, in which a permanent magnet holding portion for fixing a permanent magnet on the radially outer side is fixed in shape and can hold the permanent magnet precisely thereon and which is prevented from having such an uneven thickness thereof as might otherwise be caused by a bulge in the thickness direction at an iron core member folding time. Adjoining segment iron core members (12) are equipped at their confronting end faces with permanent magnet holding protrusions (15, 16) having connecting portions (11) at their centers. Permanent magnet holding protrusions (24) of the upper and lower segment iron core members (12) overlapping the permanent magnet holding protrusions (15, 16) to have the connecting portions (11) formed therein are equipped with housings (33), in which the bulging portions formed in the thickness direction at the time of folding the connecting portions (11) are fitted.
Owner:YASKAWA DENKI KK
Stem cell exosome composition for treating knee osteoarthritis
InactiveCN112007049AEasy quality controlEffective treatmentOrganic active ingredientsAntipyreticEngineeringExosome
The invention discloses a stem cell exosome composition for treating knee osteoarthritis. The main component of the composition is exosome derived from stem cells, the exosome is combined hyaluronic acid or PRP to treat knee osteoarthritis, and the composition is more stable and can effectively treat knee osteoarthritis.
Owner:JINAN PANSHENG BIOTECH
GB subunit recombinant protein of porcine pseudorabies virus, and preparation method and application of gB subunit recombinant protein
ActiveCN112142827ABatch-to-batch stabilityImprove controllabilityVirus peptidesDsDNA virusesBaculovirus expressionCell strain
The invention provides a gB subunit recombinant protein of a porcine pseudorabies virus, and a preparation method and application of the gB subunit recombinant protein. The preparation method comprises the following steps of 1) cloning an optimized gB gene sequence into an eukaryotic expression vector to obtain a recombinant plasmid containing a gB subunit protein coding gene of the pseudorabies virus; 2) transfecting the recombinant plasmid containing the gB subunit protein coding gene of the pseudorabies virus into an expression cell; 3) culturing, screening and domesticating the expressioncell in the step 2) to obtain a highly expressed cell strain; and 4) fermenting and culturing the cell strain in the step 3), and performing purification to obtain the gB subunit protein of the pseudorabies virus. The invention provides the gB subunit protein of the porcine pseudorabies virus, and the gB subunit protein can be industrially produced in a large scale; the preparation method is simple; the cost is low; and the yield is much higher than that of an existing baculovirus expression system.
Owner:NOVO BIOTECH CORP
Preparation method and application of tumor tissue complete antigen
InactiveCN101724010AEasy to manufactureEasy quality controlPeptide preparation methodsAntibody medical ingredientsSaline waterDendritic cell
The invention discloses a preparation method and application of a tumor tissue complete antigen. The method comprises the following steps: (1) mixing paraffin embedded samples fixed by formalin (4-10% formaldehyde) of a bioptic tumor tissue with an organic solvent to dissolve the paraffin to obtain solution containing the tumor tissue; (2) centrifuging the solution containing the tumor tissue to obtain precipitate 1; (3) mixing the precipitate 1 with inorganic substance aqueous solution with pH being less than 7 for 0.5-12h and removing the liquid by centrifuging to obtain precipitate 2; (4) placing the precipitate 2 into water steam to inactivate the protease in the cytoplasm for 10-30min to obtain the tumor tissue which inactivates the protease in the cytoplasm; (5) homogenizing the tumor tissue obtained in the step (4) to obtain chyliform liquid; and (6) mixing the chyliform liquid and normal saline (or other solution) to obtain the tumor tissue complete antigen. The invention also discloses a method for obtaining tumor vaccines by using the tumor tissue complete antigen to pulse the dendritic cell.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
Novel gene engineering subunit vaccine for mycoplasma gallisepticum
ActiveCN109999191ANot pathogenicReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsNucleotideVaccine Production
The invention provides an immunological composition and a subunit vaccine. The immunological composition comprises a protein which is selected from one or an arbitrary combination of two or more of mycoplasma gallisepticum-associated proteins encoded with nucleic acid molecules of SEQ ID NO: 1 or 3 or 5 or 7 or 9 or nucleic acid molecules which are 95% or above identical to the nucleotide sequenceof SEQ ID NO: 1 or 3 or 5 or 7 or 9. The vaccine adopts eukaryotic expression, the antigenicity and immunogenicity of the product are similar to those of a natural protein, the expression level is high, the immunogenicity is strong, the protective effect is good, and the vaccine has no pathogenicity to chickens; besides, large-scale serum-free suspension culture preparation of the vaccine can berealized through a bioreactor, and meanwhile the vaccine production cost is greatly reduced.
Owner:苏州沃美生物有限公司
Vegetable tortoise jelly as well as preparation method thereof
The invention discloses vegetable tortoise jelly as well as a preparation method thereof. The vegetable tortoise jelly is prepared from the following raw materials: tortoise jelly powder, onion, tomatoes, carrots, cucumbers, fragrant-flowered garlic, lilies, lotus roots, agaric, pawpaw, mushrooms, corns, a corrigent, a stabilizer, citric acid and water. The preparation method comprises the following steps: (1) selecting the raw materials, and cleaning and drying the raw materials; (2) preparing vegetable mixed powder; (3) preparing initial jelly; (4) sterilizing at high temperature, packaging, and cooling to obtain a tortoise jelly product. The vegetable tortoise jelly disclosed by the invention has the characteristics of being short in preparation period, higher in product stability and easy in quality control. Vegetables are added into the tortoise jelly, so that the taste of the tortoise jelly is improved, bitter taste of conventional tortoise jelly is improved, and the nutritional value of the product is increased.
Owner:陈林均
Modified nanogold-based hydrogen peroxide and peroxidase detection method
ActiveCN107389621AModification technology is matureEasy quality controlTransmissivity measurementsStandard curveOxide
The invention provides a modified nanogold-based hydrogen peroxide and peroxidase detection method. The method comprises the following steps: 1, modifying: adding a p-mercaptophenol or p-aminothiophenol solution into a nanogold solution to modify the nanogold, mixing uniformly, coating with a tin foil, and placing in a dark place to obtain modified nanogold particle sol; 2, establishing a standard curve; 3, detecting a to-be-detected sample: taking an actual hydrogen peroxide sample or a peroxidase-containing sample as a detected object and the modified nanogold particle sol as a detecting object, detecting the optical density of the modified nanogold particle sol containing the actual hydrogen peroxide sample or containing the actual peroxidase sample, and comparing the ratio of the optical density with the standard curve to obtain the concentration or the enzyme activity concentration of the actual hydrogen peroxide sample or the actual peroxidase-containing sample. The method is capable of performing supersensitive detection on the hydrogen peroxide and peroxidase, is low in cost and has the advantage of high detection flux.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Peony astragalus polyglucoside composition for treating liver disease and its preparation method
A Chinese medicine in the form of particle, capsule, tablet, or injection for treating chronic hepatitis, hepatofibrosis and early-phase hepatocirrhosis is prepared from white peony root and astragalus root. Its preparing process is also disclosed.
Owner:魏伟
Preparation method and application of tumor tissue complete antigen
ActiveCN101724011AEasy to manufactureEasy quality controlPeptide preparation methodsAntibody medical ingredientsSaline waterProteinase activity
The invention discloses a preparation method and application of a tumor tissue complete antigen. The method comprises the following steps: (1) placing the lobulated fresh bioptic tumor tissue into water steam to inactivate the protease in the cytoplasm for 10-30min to obtain the tumor tissue which inactivates the protease in the cytoplasm; (2) homogenizing the tumor tissue obtained in the step (1) to obtain chyliform liquid; and (3) mixing the chyliform liquid and normal saline to obtain the tumor tissue complete antigen. The invention also discloses a method for obtaining tumor vaccines by using the tumor tissue complete antigen to pulse the dendritic cell.
Owner:南京海鲸药业股份有限公司
Novel genetic engineering subunit vaccine for avian Newcastle disease viruses
ActiveCN111154778AGood immune effectProcess safetySsRNA viruses negative-senseViral antigen ingredientsAdjuvantF protein
Owner:苏州沃美生物有限公司
Bearing device for wheel
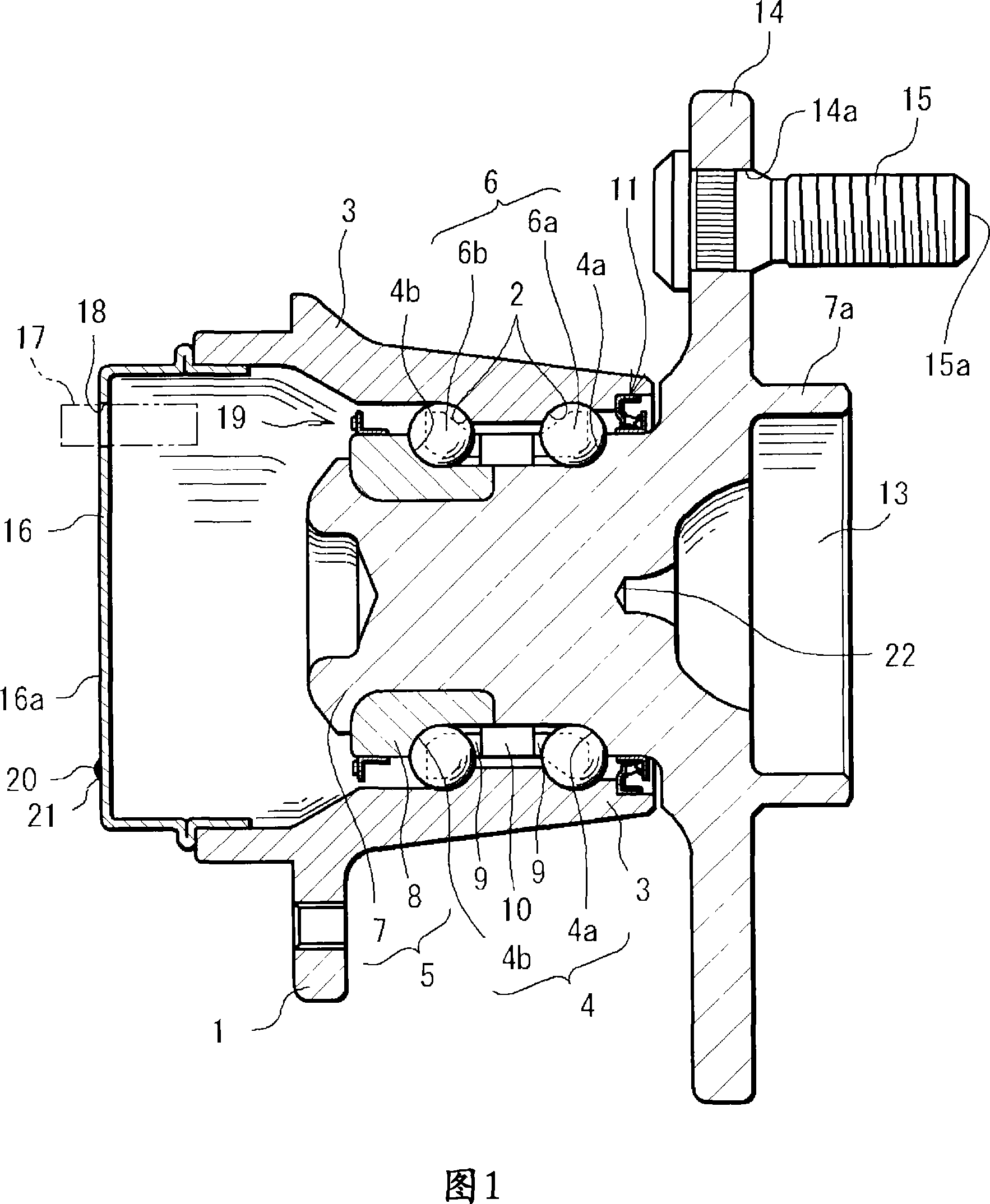
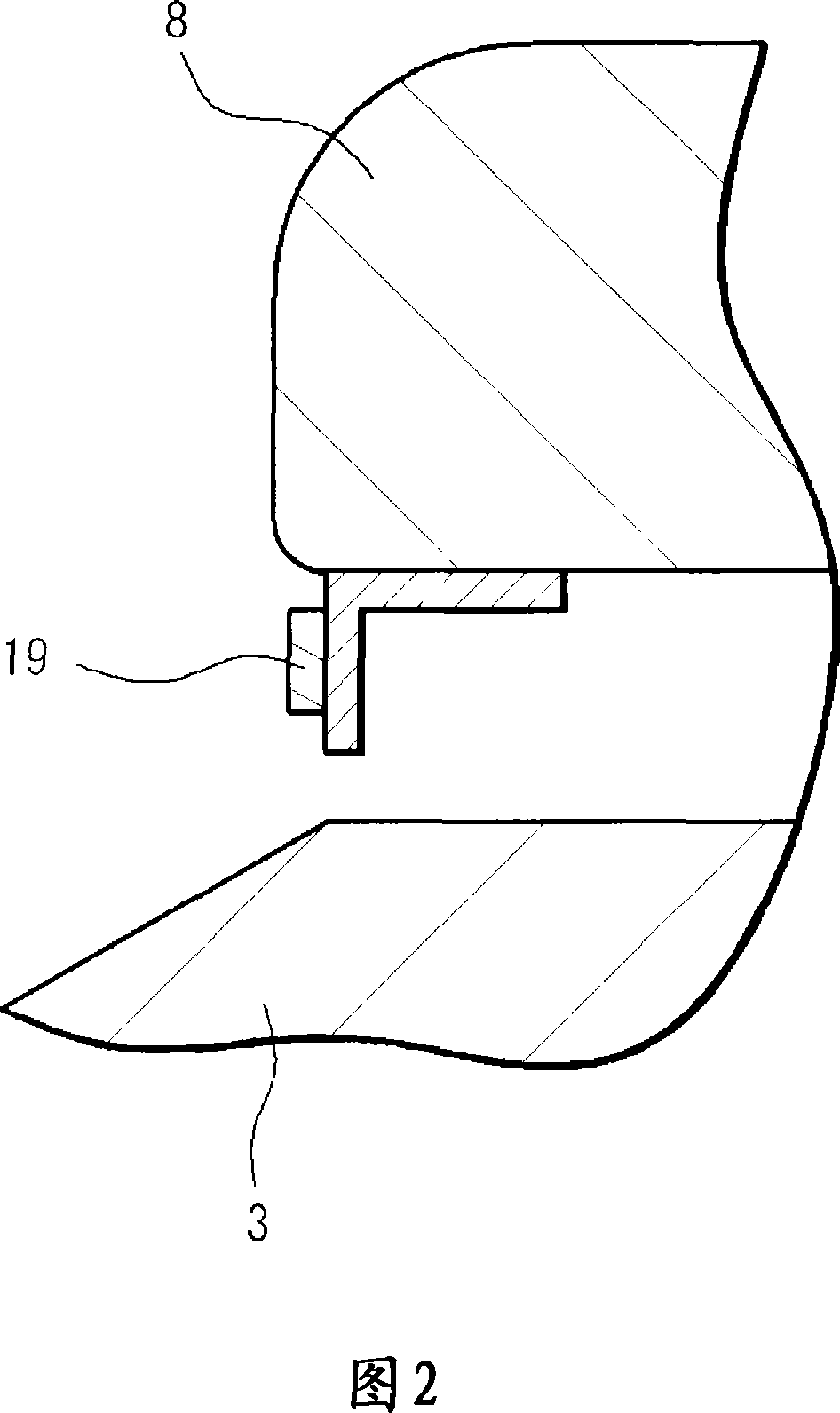
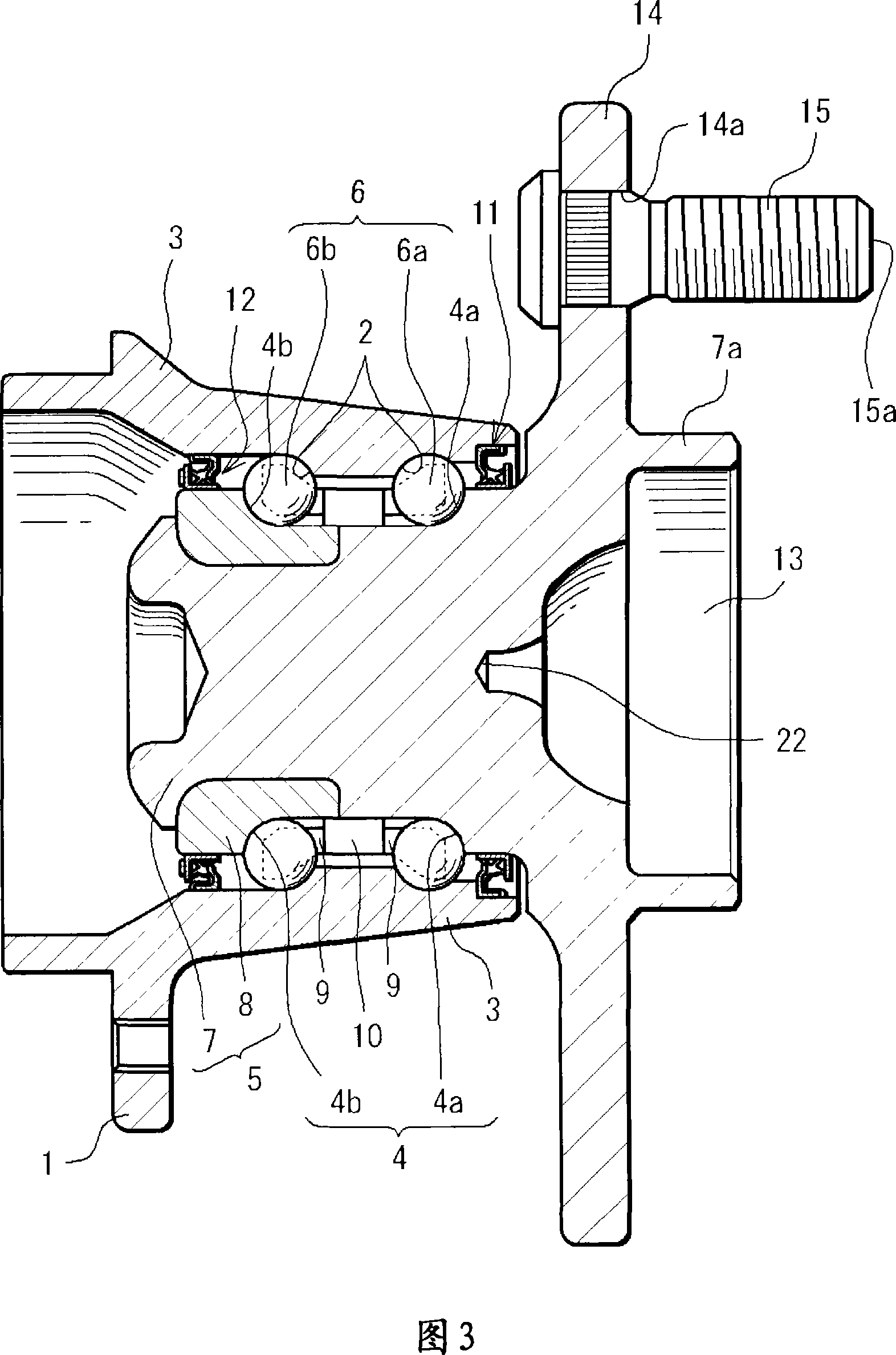
InactiveCN101061325AEasy Quality ControlSave human effortRolling contact bearingsBearing assemblyEngineeringLot number
A bearing device for a wheel, which can be easily traced after it is shipped from a factory, in which a lot number and quantity of a product including the bearing device can be easily specified when inspection or replacement is required after the shipment, and whose quality control can be quickly and easily performed. A bearing device for a wheel, having an outer ring member (3), an inner ring member (5) provided radially inside of and concentrically with the outer ring member (3), and rolling bodies (6) arranged between the outer ring member (3) and the inner ring member (5). An ICchip (20) is provided at the bearing device at a vehicle-outer-side end section or at a vehicle-inner-side end section.
Owner:JTEKT CORP
Semiconductor device, display device and electronic device
InactiveCN101529582AEasy to readRecognizableSemiconductor/solid-state device detailsSolid-state devicesDisplay deviceSemiconductor device
Owner:SHARP KK
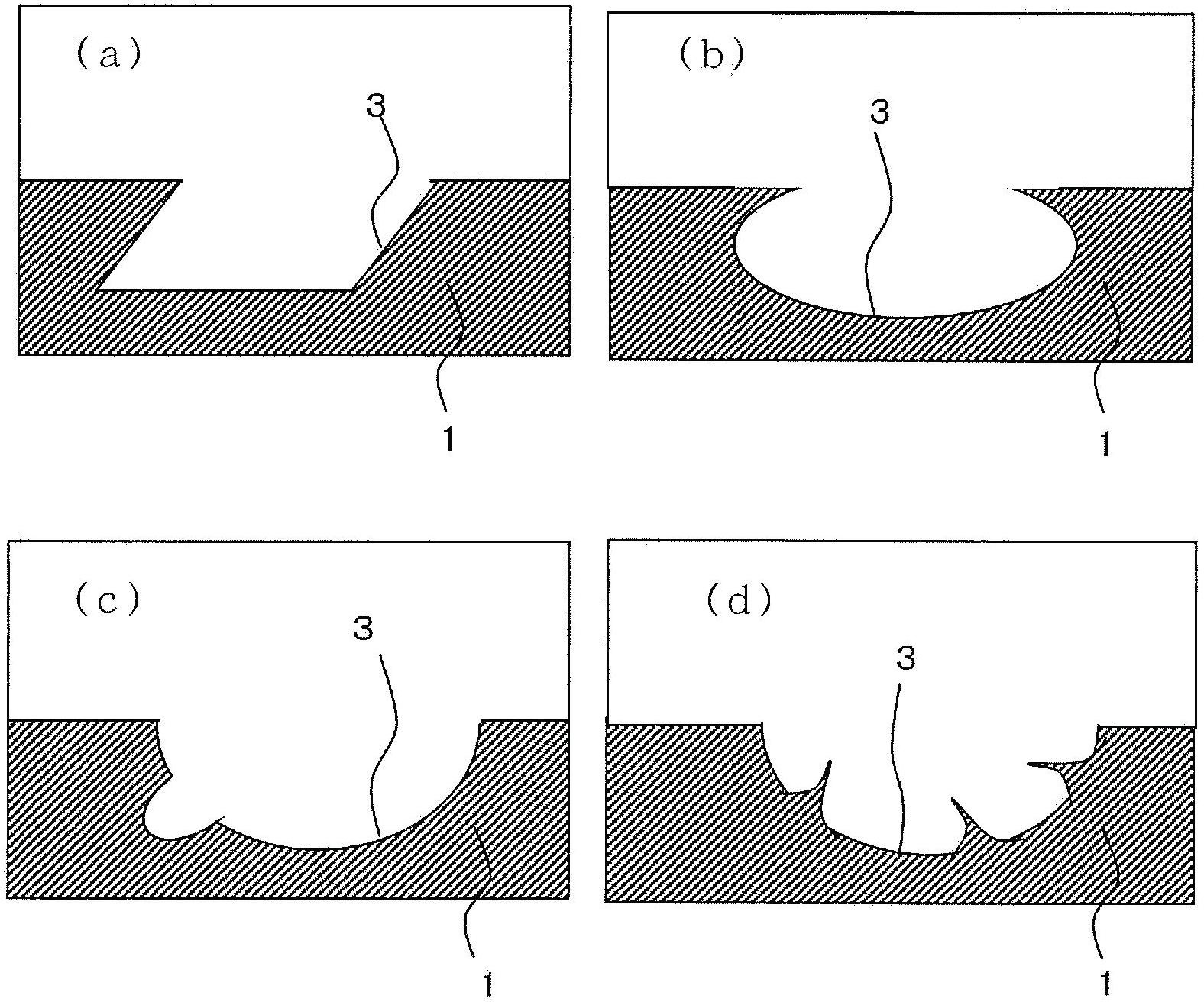
Workpiece to be spot-welded
InactiveCN101626863AEasy to identifyEasy Quality ControlSuperstructure subunitsMetal working apparatusSpot weldingLandform
Work pieces for a center pillar are provided with marks for determining locations for spot welding which are easy to recognize, and this simplifies the task of managing the quality of welding work. The center pillar 3 is formed by a center pillar outer 5 and a center pillar inner 6 in such a manner that a flange 8 formed in the center pillar inner is spot welded to a corresponding part of the center pillar outer. The edge of the flange is given with a wavy shape to indicate the locations for spot welding by the peaks of the wavy shape. As opposed to dents or recesses which cannot be deep enough to be readily recognizable owing to the limit in the thickness of the work piece, the wavy shape may be of such dimensions as to permit required recognizability. This ensures spot welding to be performed on designed locations, and improves the quality of the spot welding work.
Owner:HONDA MOTOR CO LTD
Production technology for carbon fiber products
The invention discloses a production process of a carbon fiber product, comprising the steps of: a. hot pressing and compounding carbon fiber cloth and a thermoplastic material to obtain a composite plate; b. performing molding processing on the composite plate to obtain a molded part, and the carbon fiber cloth Located on the inner side of the molded part; c, impregnating the resin on the carbon fiber cloth on the inner side of the molded part; d, curing the resin to obtain a carbon fiber product. The purpose of the present invention is to provide a production process of carbon fiber products to achieve the purpose of simplifying the production process of carbon fiber products, eliminating the harm to the environment and human body during the production process, and realizing mass production.
Owner:WENZHOU OUHAI KAISHI LUGGAGE & BAG FACTORY
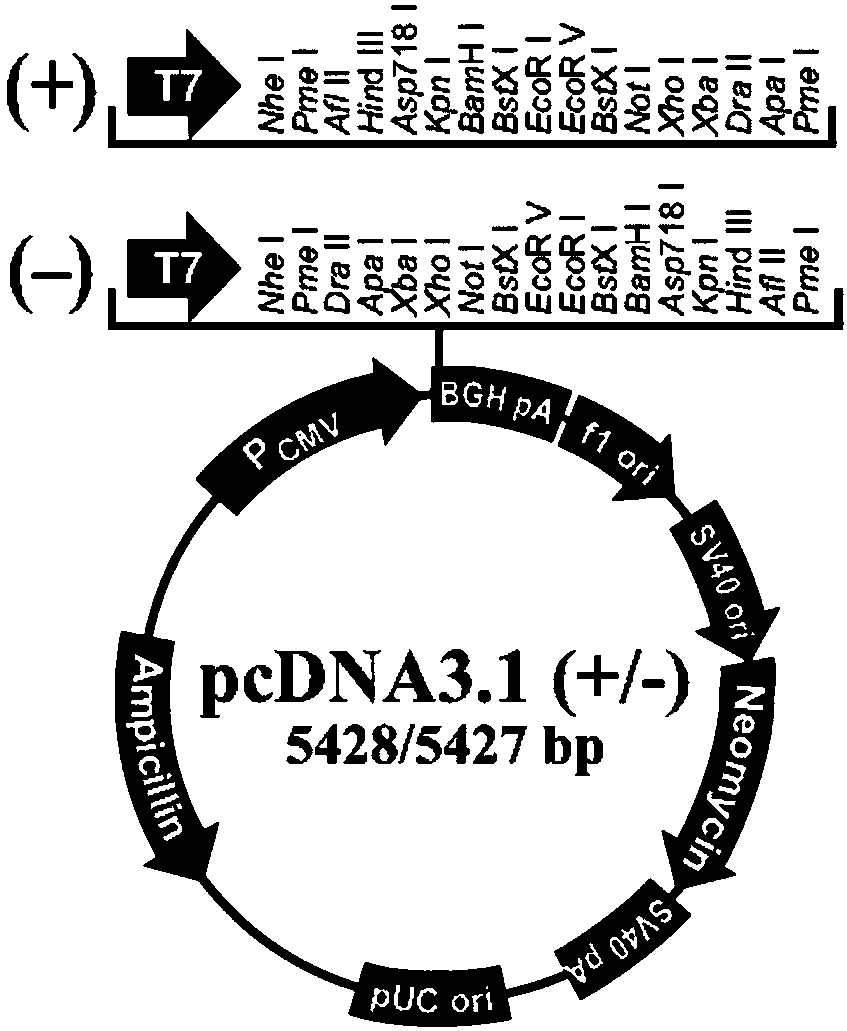
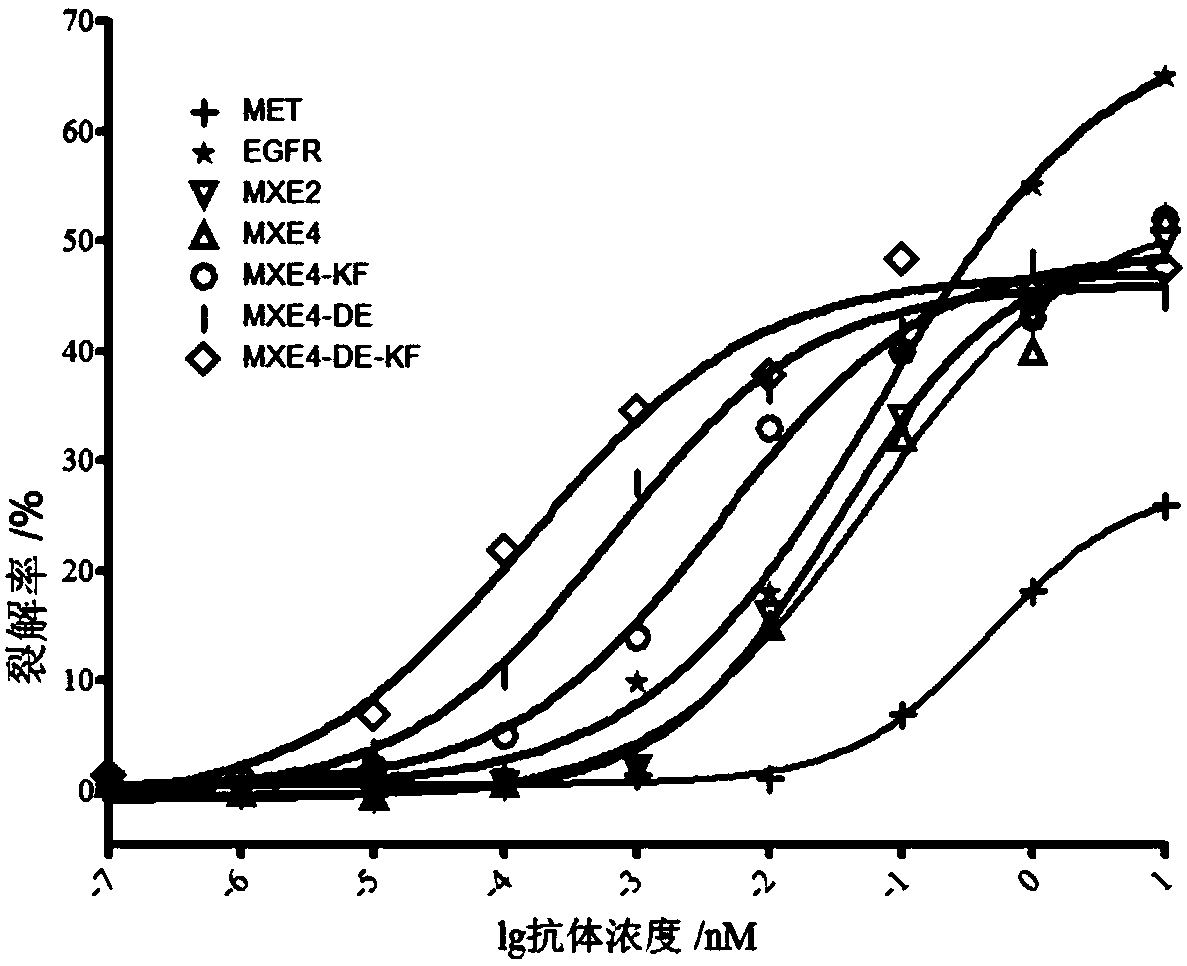
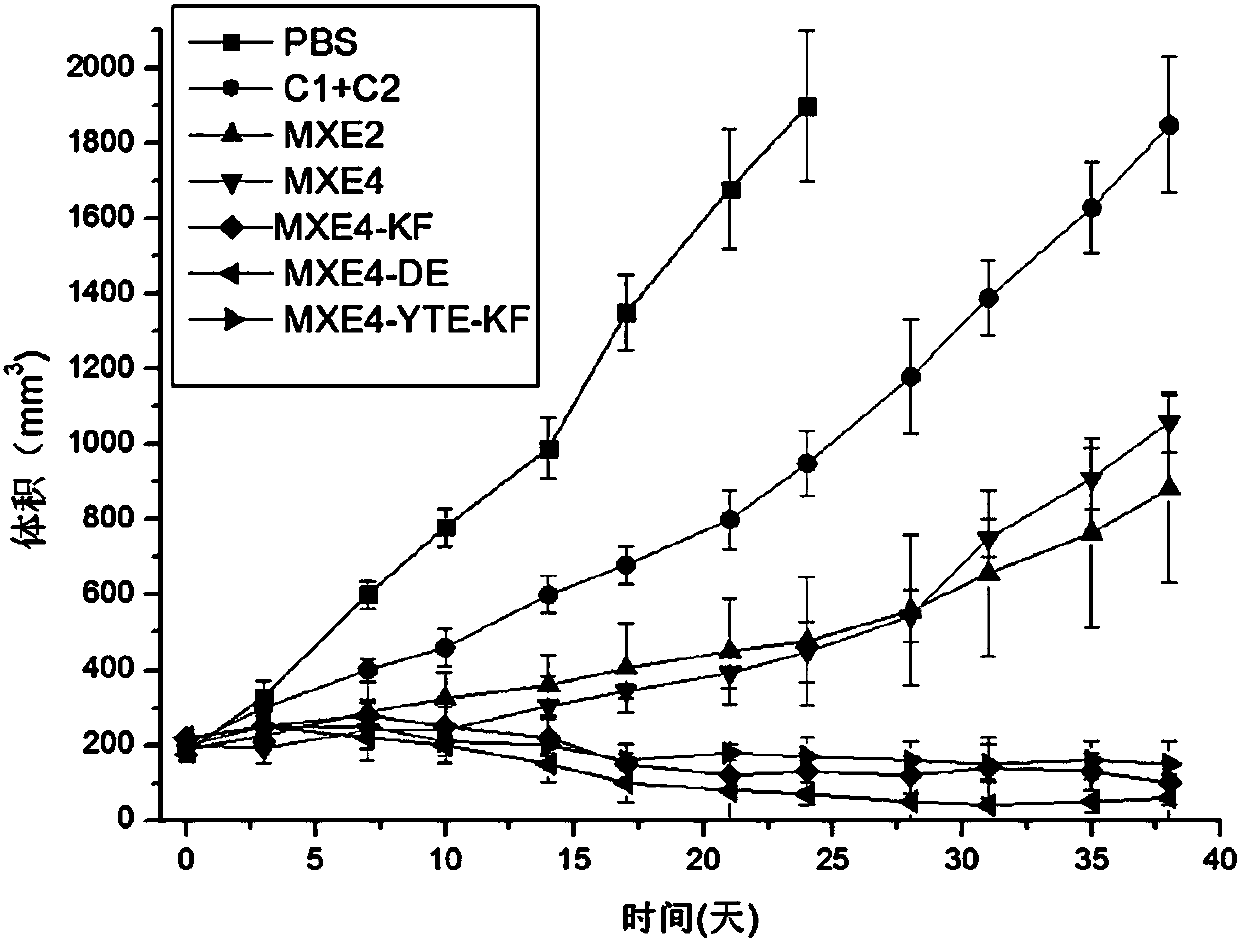
Bispecific antibody against EGFR protein and MET protein
ActiveCN110028584AEasy quality controlGood curative effectAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHalf-lifeBispecific antibody
The invention discloses a bispecific antibody against EGFR protein and MET protein. The glycosylation modification makes the bispecific antibody contain a core fucose glycoform ratio of not more than4.5% to increase ADCC activity; and by modifying an amino acid sequence of a Fc segment, the ADCC activity of the bispecific antibody is enhanced, or the affinity for FcRn is enhanced to extend the half-life. The bispecific antibody prepared by the invention has simple preparation, stable structure and better tumor inhibition effect, and has a good market prospect.
Owner:北京科昕生物科技有限公司
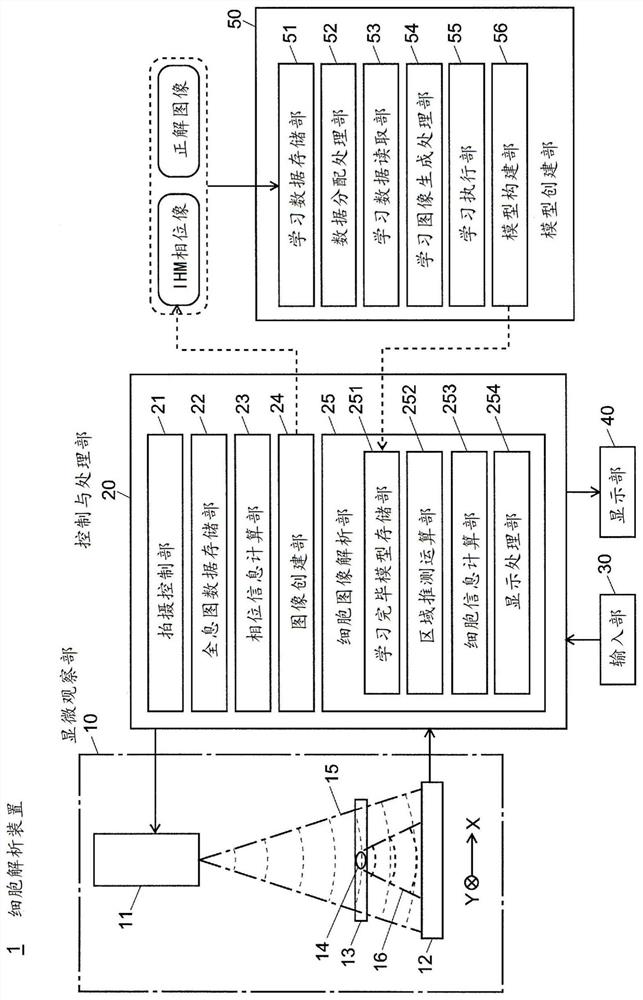
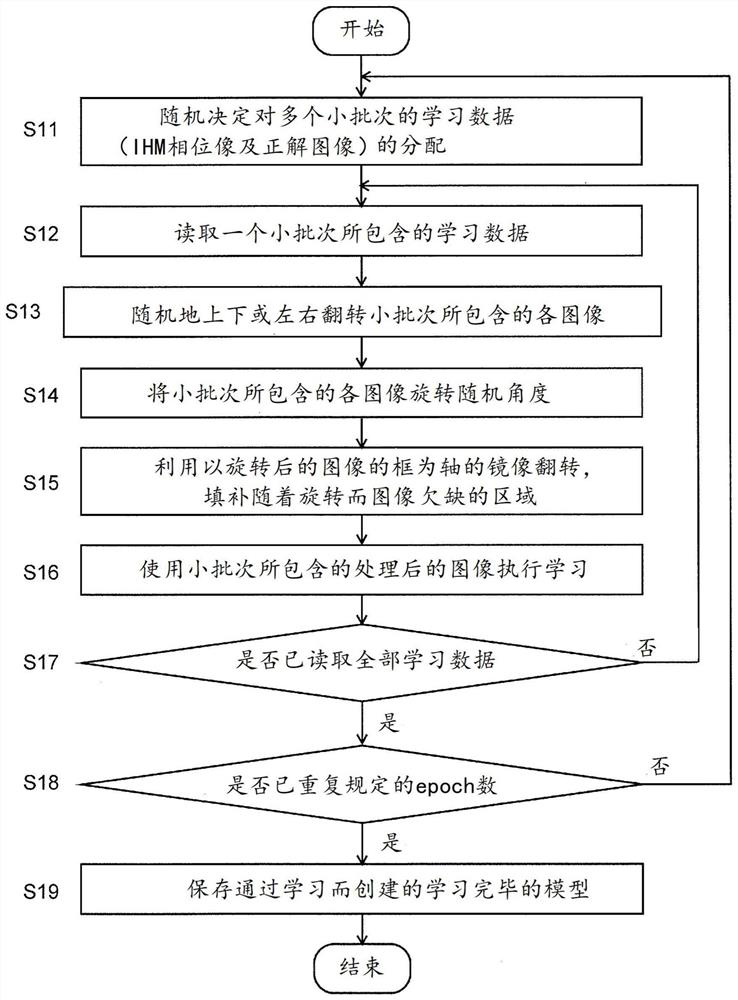
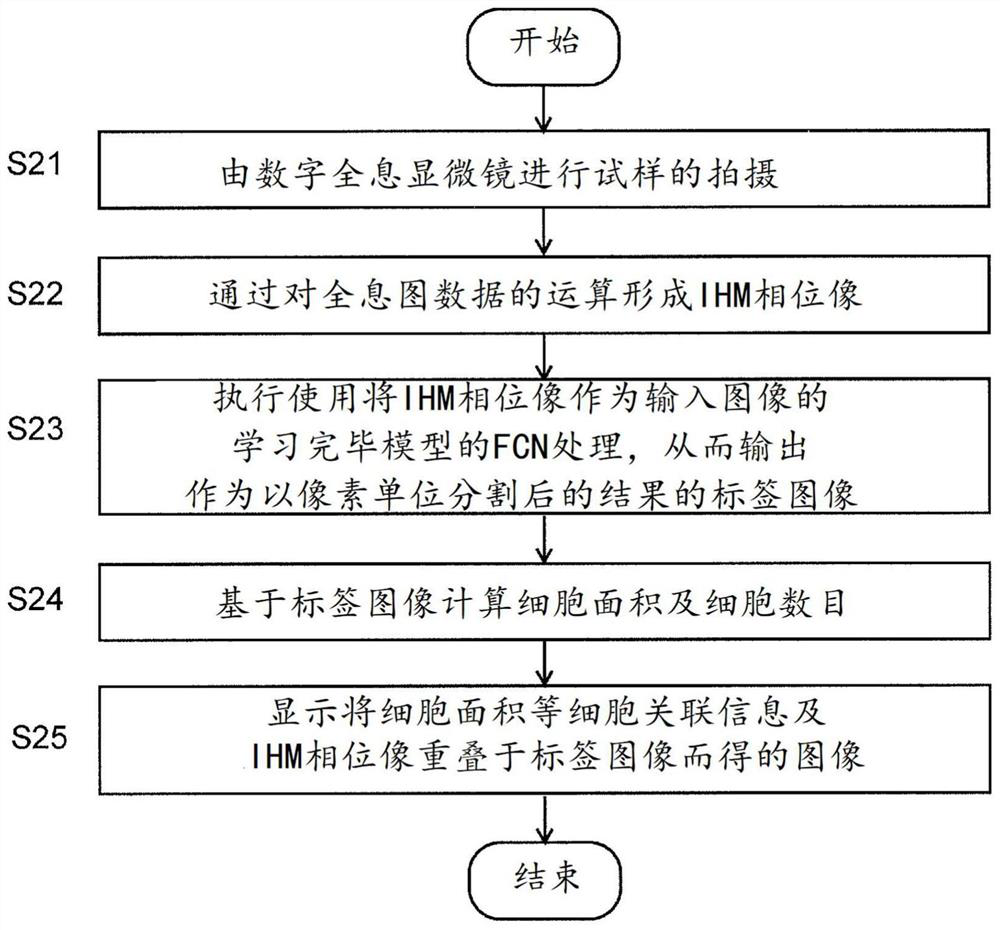
Cellular image analysis method, cellular image analysis device, and learning model creation method
PendingCN111837157AEasy Quality ControlImprove productivityImage enhancementImage analysisCell regionMirror image
In the present invention, a phase image is formed by computation from a holographic image of a cell, a segmentation is carried out for each pixel for the phase image using a fully convolutional neuralnetwork, and an undifferentiated cell region, an undifferentiated deviated cell region, a foreign body region, etc. are identified. During learning, when a learning image included in a mini-batch hasbeen read in (S13), the image is randomly inverted vertically or horizontally (S13) and then is rotated by a random angle (S14). A portion of a pre-rotation image which has been lost within the frameby said rotation is compensated for by a mirror-image inversion with an edge of a post-rotation image as an axis thereof (S15). Learning of a fully convolutional neural network is carried out using the learning image thus generated (S16). The same processes are repeated for all mini-batches, and the learning is repeated for a prescribed number of iterations while learning data allocated to the mini-batch is shuffled. Learning model precision is thus improved. As rotationally invariant characteristics may be learned, it is possible to identify, with good precision, cell colonies being of various shapes.
Owner:SHIMADZU SEISAKUSHO CO LTD
Breast cancer stem cell vaccine as well as preparation method and application thereof
ActiveCN104225592AImprove defenseEnhance immune functionTumor/cancer cellsAntibody medical ingredientsFreeze thawingAdjuvant
The invention discloses a tumor stem cell vaccine as well as a preparation method and application thereof. A breast cancer stem cell vaccine provided by the invention comprises inactivated breast cancer stem cells, mannatide and fat emulsion which are mixed according to the volume ratio of 3:1:1. The preparation method of the breast cancer stem cell vaccine comprises the following steps: obtaining breast cancer stem cells; inactivating the breast cancer stem cells; freeze-thawing the inactivated breast cancer stem cells into normal saline, and mixing the inactivated breast cancer stem cells with adjuvant mannatide and the fat emulsion, so as to obtain the breast cancer stem cell vaccine. The invention further provides the application of the breast cancer stem cell vaccine, prepared according to the preparation method provided by the invention, in preparing medicines for treating breast cancer. The invention provides a novel way for the treatment of breast cancer and the preparation of the vaccine, an active curative effect on a breast cancer patient is achieved, the immune function of the patient is enhanced, the lifetime of the patient is prolonged, and the active effect is achieved.
Owner:广州复大医疗有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com