Subunit fusion protein CD2V-Fc, preparation method and application thereof
A fusion protein and subunit technology, applied in biochemical equipment and methods, fusion polypeptides, chemical instruments and methods, etc., can solve the problems of difficult application and poor stability of the extracellular region of CD2V, and achieve batch-to-batch stability and ease of use. Mass production, highly controllable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]Example 1 Expression and preparation of CD2V-Fc protein
[0033] 1.1 Selection of African swine fever CD2V-Fc protein
[0034] The African swine fever structural protein CD2V is a polypeptide encoded by the EP402R gene. According to prediction analysis, there is a transmembrane region at 207-229aa. Studies have shown that the CD2V protein can interact with red blood cells and play a role in the spread of the virus and the damage of lymphocytes. important role in the process. Therefore, the use of CD2V protein as an antigen has good prevention and control of African swine fever infection. There is no report that the protein can be expressed and purified on a large scale in a eukaryotic expression system. This may be caused by the instability of the CD2V protein. The present invention In order to solve this important technical problem, a porcine antibody Fc fragment was introduced at the carboxy-terminus of CD2V to ensure the structure and protein stability of CD2V.
[0...
Embodiment 2
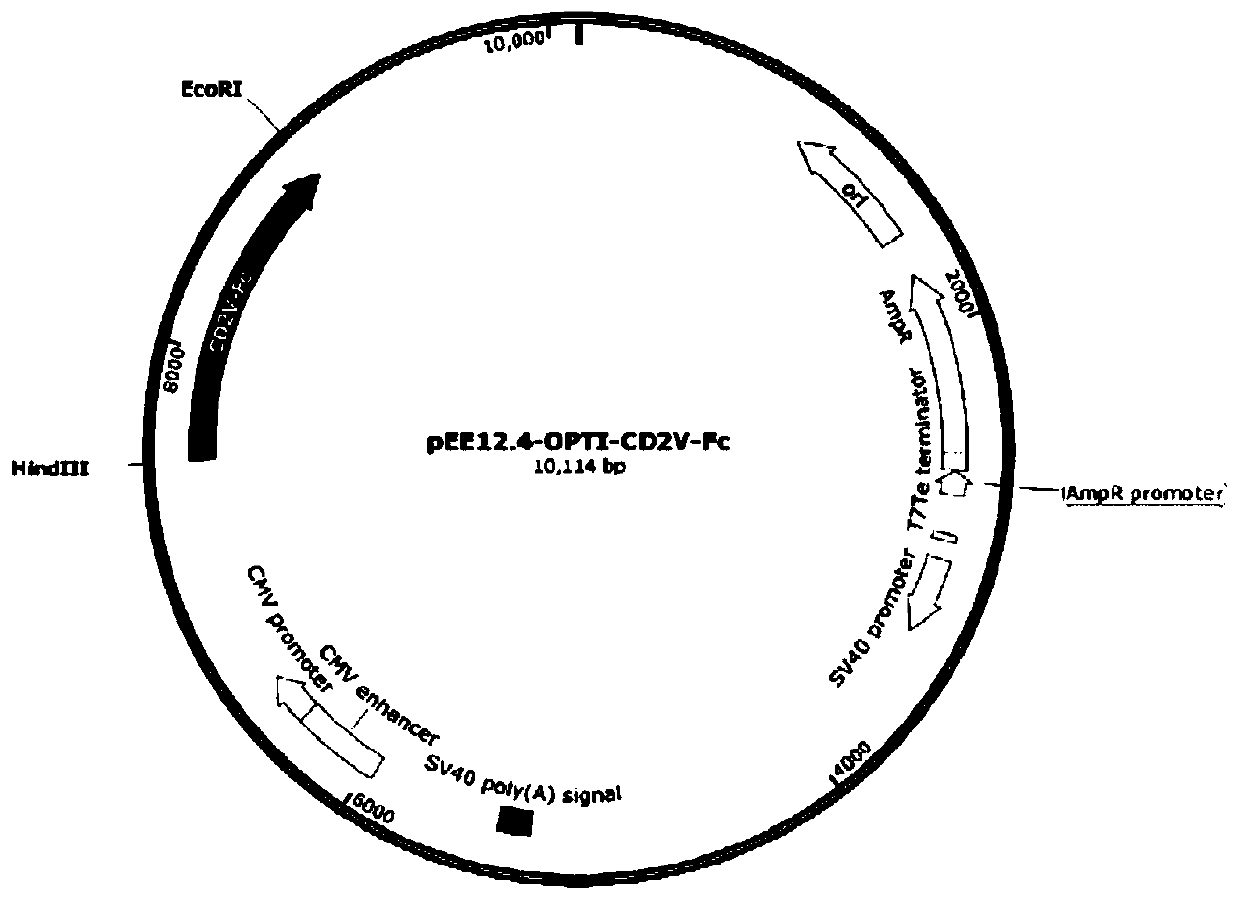
[0041] Example 2: Construction of pEE12.4-OPTI-CD2V-Fc recombinant plasmid
[0042] 2.1 PCR amplification of the target fragment OPTI-CD2V-Fc
[0043] 2.1.1 PCR reaction
[0044] (1) Primer design and synthesis
[0045] Upstream primer: 5'-acgaAGCTTGCCGCCACCATGATCAT-3'
[0046] Downstream primer: 5'-GCGGAATTGAATTCTTAATGGTGATG-3'
[0047] (2) Add 50 μL of the sample system, as shown in the table below:
[0048]
[0049] PCR amplification program:
[0050]
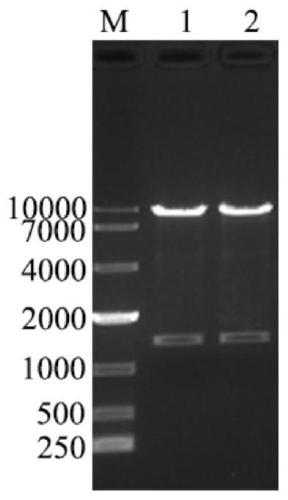
[0051] 2.1.2 Gel recovery of PCR products
[0052] (1) Mark the sample collection EP tube, adsorption column and collection tube;
[0053] (2) Take the weight of the marked empty EP tube, and record the value;
[0054] (3) Carefully cut out a single target DNA band from the agarose gel with a scalpel on a gel cutter and put it into a clean 1.5mL centrifuge tube;
[0055] (4) Add 600 μL PC buffer to the 1.5mL centrifuge tube in step (3), place in a 50°C water bath for about 5 minutes, and gently turn the centrif...
Embodiment 3
[0119] Example 3: Establishment of transfection of pEE12.4-OPTI-CD2V-Fc recombinant plasmid into CHO-K1 cells and monoclonal screening
[0120] 3.1 CHO-K1 cell transfection
[0121] (1) Preparation: UV sterilization in a biological safety cabinet for 30 minutes; DMEM / F12 (containing 10% serum, 1% double antibody), DMEM / F12 and PBS were placed in a 37°C water bath and preheated to 37°C.
[0122] (2) Take out the cells (10 cm cell culture dish) from the incubator at 37° C., discard the supernatant medium, wash the cells once with pre-warmed 8 mL PBS, and discard the PBS.
[0123] (3) Add 1-2mL 0.25% trypsin-EDTA to each 10cm cell culture dish, digest at room temperature for about 2 minutes, observe under the microscope that the cells shrink and become round, and appear as single cells.
[0124] (4) Add 4 mL of DMEM / F12 (containing 10% serum, 1% double antibody) to terminate the digestion reaction, and blow the cells away with a pipette.
[0125] (5) Transfer the digested cells...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com