Polypeptide fragment for preparing DC (Dendritic Cell) vaccine and DC vaccine
A polypeptide fragment and vaccine technology, applied in the field of biological vaccines, can solve the problems of high production cost and low safety of DC vaccines, and achieve the effects of low production cost, high safety and high product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation process of the DC vaccine of the present invention is as follows:
[0035] 1. Isolation and induction of DC cells:
[0036] Blood samples from breast cancer patients or leukocytes collected by apheresis machine are used to separate peripheral blood mononuclear cells with lymphocyte separation medium, and after the mononuclear cells are separated by adherent culture, they are treated with GM-CSF, IL-4, autologous plasma Lymphocyte culture medium to induce monocytes to differentiate into DC cells. The lymphocyte separation medium and lymphocyte culture medium used were directly purchased from the market.
[0037] 2. Peptide load:
[0038] After 5 days of induction culture, a polypeptide fragment derived from human lactoglobin was added to the culture system. The sequence is shown in Table 1. A polypeptide fragment of one sequence can be added alone, or a mixture of polypeptide fragments of several sequences can be added. The preferred concentration is 1...
Embodiment 1
[0044] Example 1 Design and Synthesis of Antigen Polypeptide Fragments
[0045] Obtain the amino acid sequence of human mammaglobin (also known as mammaglobin-A protein) from the NCBI database, and compare different polypeptide fragments with HLA-A0201 online (https: / / www-bimas.cit.nih.gov / molbio / hla_bind / ) Calculate the affinity and other data and score. The scoring results are shown in Table 2. The initial positions of the five predicted polypeptide fragments are concentrated in 2-4, and the scores are all high. Therefore, the polypeptide fragments of 2-10 and 4-12 are selected. Tested as a representative; in addition, the initial positions of 4 polypeptide fragments were concentrated at 80-84, and the scores were quite different, so the polypeptide fragments with higher scores of 83-92 and 80-89 were selected for detection; according to Table 2 A total of 9 polypeptides were selected for synthesis based on the above reasons, and their sequences are shown in Table 3. The po...
Embodiment 2
[0051] Example 2 Detecting the Binding Ability of the Polypeptide to HLA-A0201
[0052] 1) Reagents: IMDM medium, fetal bovine serum, cells expressing HLA-A0201T2, HLA-A02 antibody.
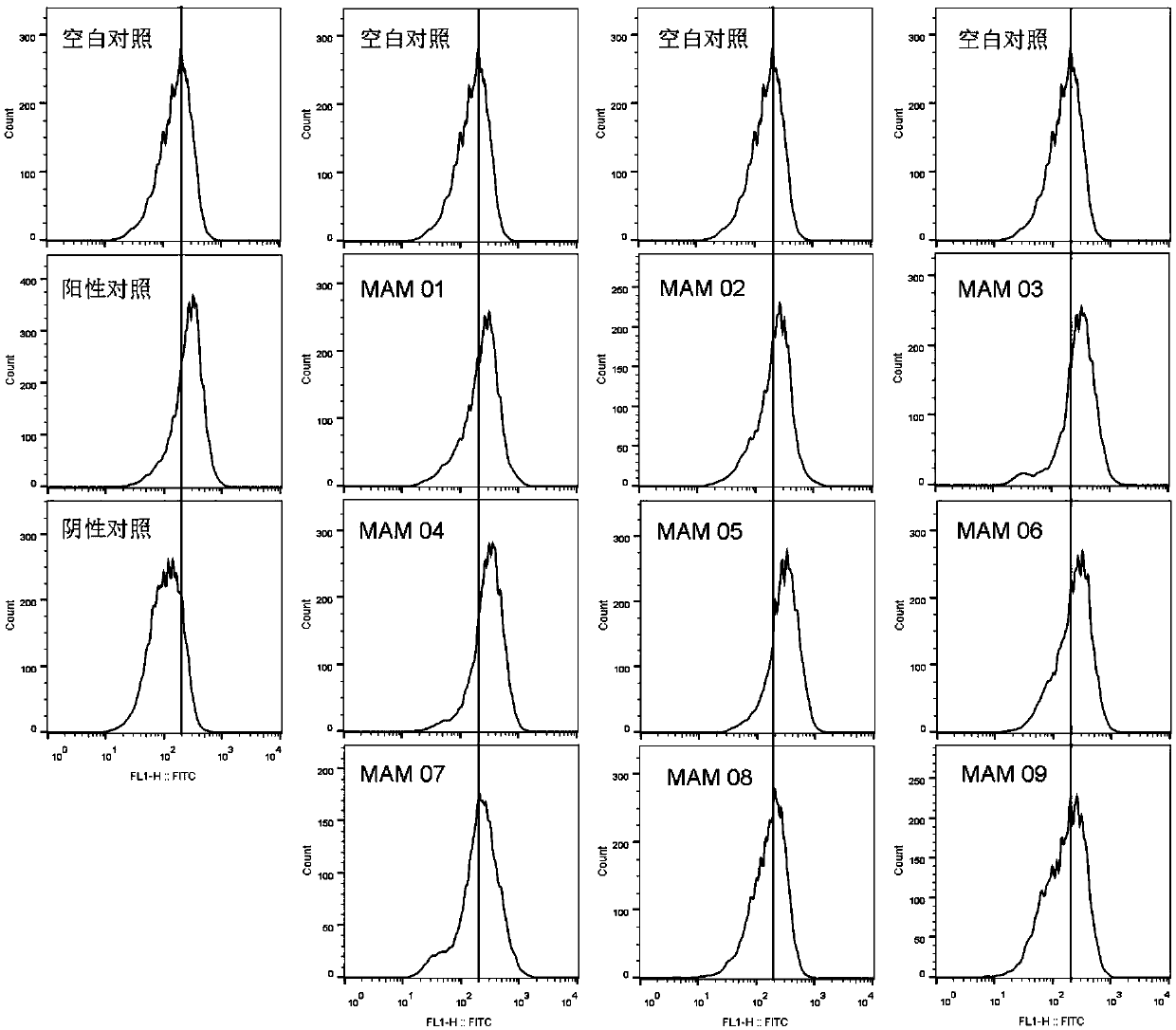
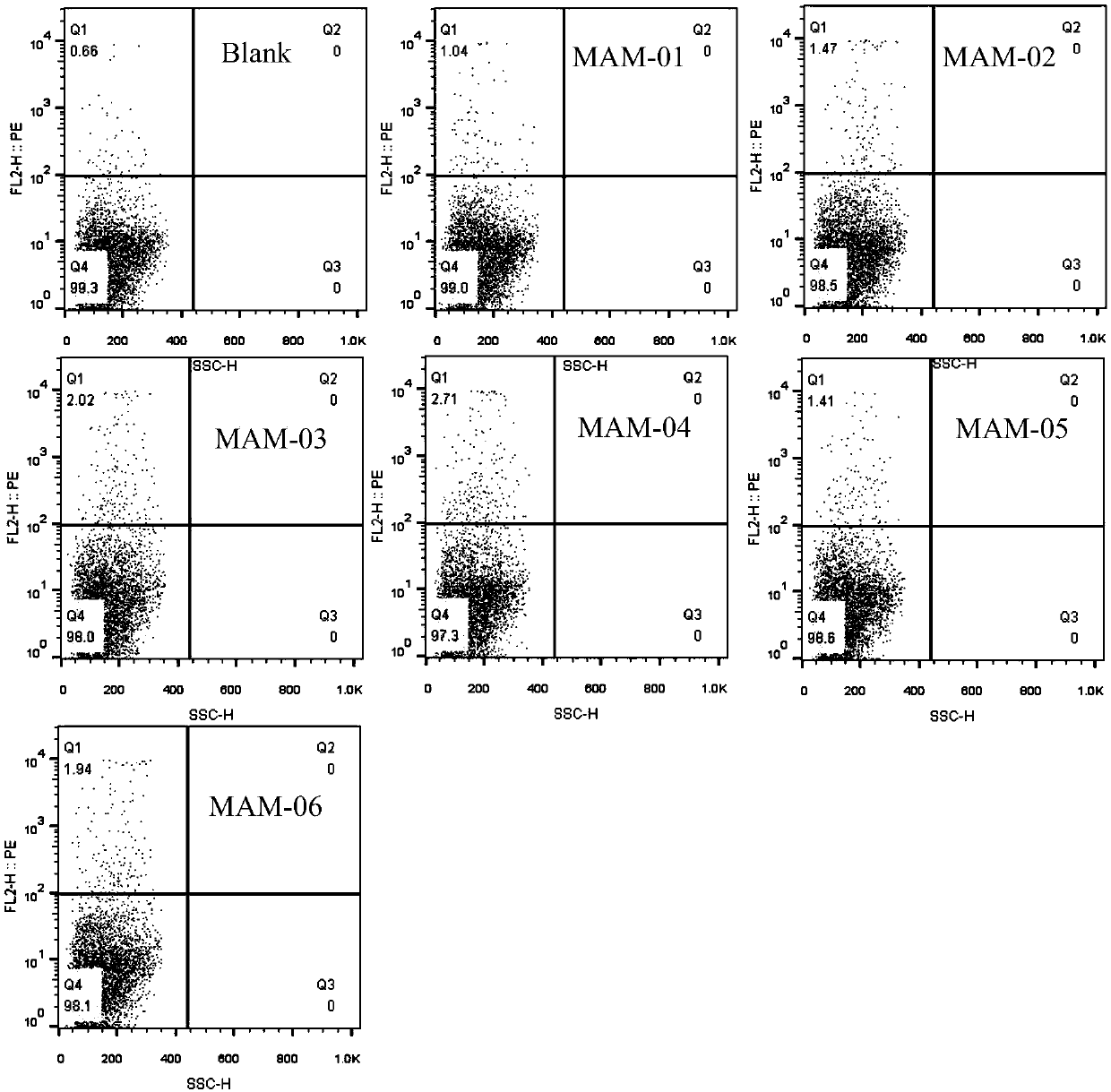
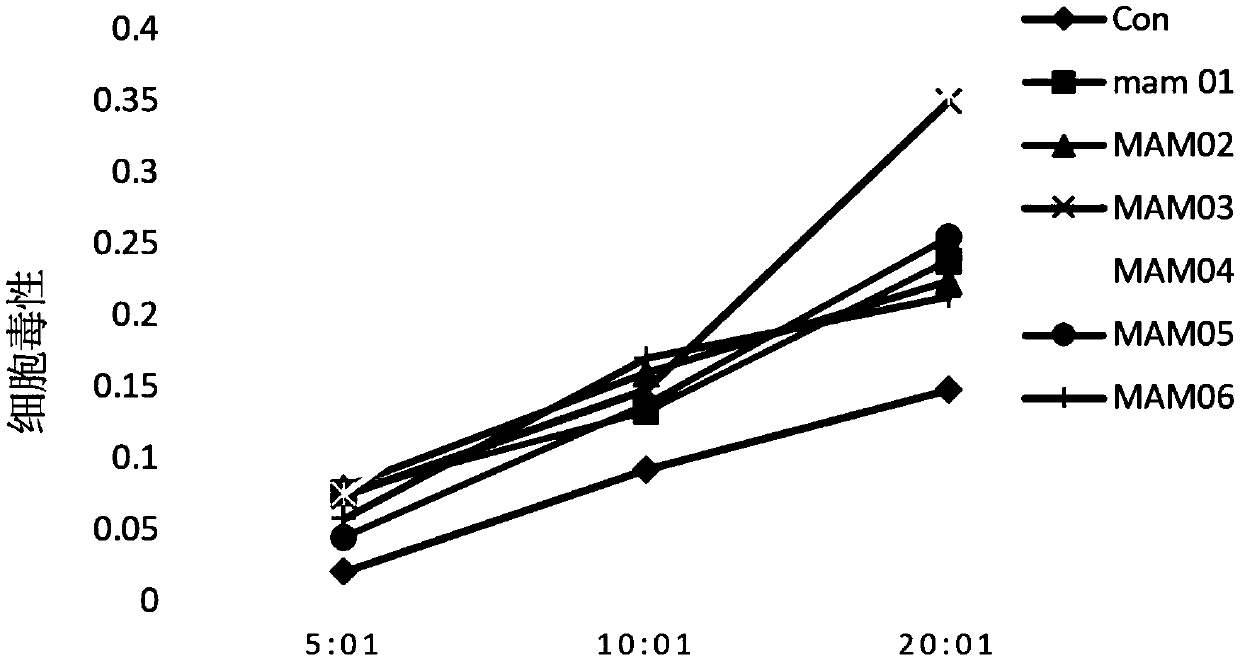
[0053] Polypeptide: positive control PI-9 (sequence is PYVSRLLGI, shown in SEQ ID NO.7), dissolved in DMSO, final concentration 20mg / mL; negative control GL-9 (sequence is GILGFVFTL, shown in SEQ ID NO.8) , dissolved in DMSO, with a final concentration of 20 mg / mL; the target polypeptide MAM01-06, whose sequence is shown in Table 2, was dissolved in DMSO, with a final concentration of 20 mg / mL. The dissolved peptides need to be subpackaged and stored at -80°C, and repeated freezing and thawing should be avoided during use.
[0054] 2) Method:
[0055] Cell culture: T2 cells (HLA-A0201), suspended in complete medium (IMDM+20% FBS), at 37°C, 5% CO 2 cultured under conditions.
[0056] Peptide loading: adjust the concentration of T2 cells to 1*10 with complete medium 6 / mL density, add differen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com