Preparation method and application of tumor tissue complete antigen
A tumor tissue and pan-antigen technology, applied in the field of tumor tissue pan-antigen preparation, can solve the problems of DC vaccine containing a lot of cell debris, affecting DC activity, unable to inactivate protease, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] The preparation method of the present invention undergoes water vapor treatment to soften the tissue, grinds and breaks the cells through a homogenizer, and better obtains uniform and tiny antigenic substances. On the other hand, high-pressure steam treatment inactivates proteases to avoid degradation of tumors after cell homogenate is broken. antigen. Steam treatment can be performed using methods well known in the art, such as but not limited to high-pressure steam inactivation, atmospheric pressure steam treatment; preferably at a pressure of 0.05-0.3 mPa, more preferably at a pressure of 0.1-0.2 mPa.
[0059] The preparation method of the tumor vaccine provided by the present invention is applicable to the preparation of DC vaccines for various solid tumors, and the tumor is selected from lymphoma, myeloma, kidney cancer, prostate cancer, malignant melanoma, breast cancer, or colon cancer, etc. solid tumors. ; Lymphoma is preferred.
[0060] In a preferred example...
Embodiment 1
[0085] Preparation of Lymphoma DC Vaccine
[0086] (1) Extraction of whole antigen from lymphoma tissue
[0087] 1. Obtain fresh lymphoma biopsy tissue, cut 0.2g of tumor tissue, and cut into 1-3mm thick slices;
[0088] 2. Put it in a high-pressure steam sterilizer for 15 pounds and 15 minutes;
[0089] 3. Homogenize the treated tissue slices in a sterile glass homogenizer until all of them are homogeneous chylus liquid;
[0090] 4. Add 2ml of sterile normal saline, pipette gently several times to dissolve the whole antigen extracted from sterile lymphoma tissue;
[0091] 5. After aliquoting, store at -20°C for later use.
[0092] (2) Culture of immature DC in vitro
[0093] 1. The above-mentioned lymphoma patients were separated by a blood cell separator for 4 cycles, and 1-5×10 9 Mononuclear cell liquid (100-200ml);
[0094] 2. Slowly add the collected mononuclear cell fluid (20ml) to several 50ml Falcon centrifuge tubes pre-added with 20ml of lymphocyte separation me...
Embodiment 2
[0105] DC Vaccine Treats B-cell Lymphoma in Mice
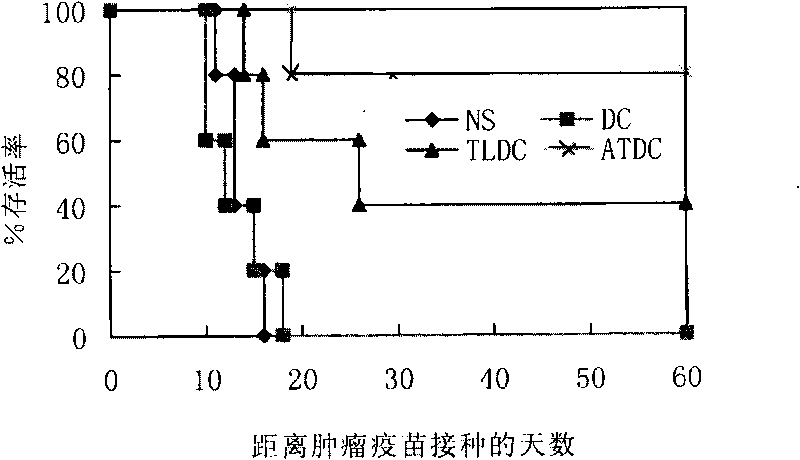
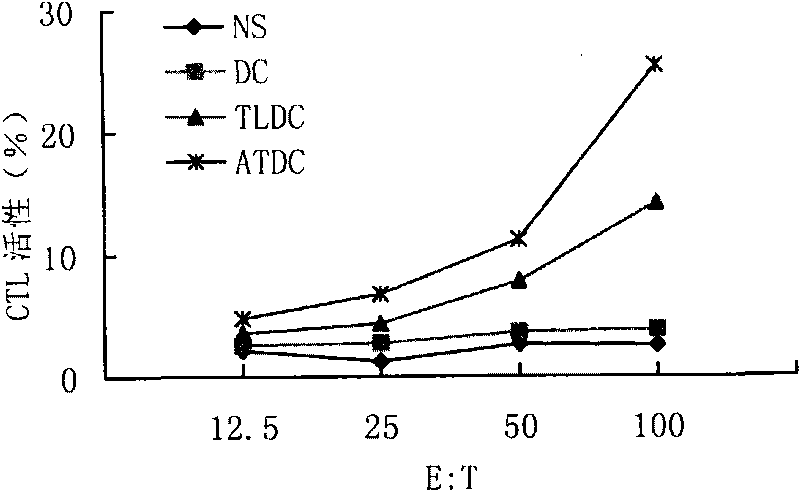
[0106] 1. Preparation of tumor antigen: select mouse lymphoma cell line A20 cells to resuspend with normal saline (1×107 / ml), inject 100 μl subcutaneously into the mouse, cut out 0.2 g of tumor tissue after 3 weeks, follow the steps of Example 1 Prepare tumor whole antigen.
[0107] The control is tumor freeze-thaw antigen, and the preparation method is to cut out 0.2 g of tumor tissue, grind it through a 100-mesh steel mesh, collect 2 ml of the entire grinding liquid, put it into a 5 ml sterile cryopreservation tube, and quickly immerse the cryopreservation tube in liquid nitrogen for quick freezing, 10 After 10 minutes, take it out and put it in room temperature to thaw; freeze and thaw repeatedly 5 times, centrifuge at 3000×g for 30 minutes, collect the supernatant, and filter and sterilize it through a 0.22 μm disposable sterile filter, which is the tumor freeze-thaw antigen.
[0108] 2. Mouse DC culture: take BALB / c mous...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com