Preparation method and application of tumor tissue complete antigen
A tumor tissue and pan-antigen technology, applied in the field of tumor tissue pan-antigen preparation, can solve the problems of insufficient vaccine immunogenicity, inability to obtain a sufficient number of tumor cells, and single activation of immune targets.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] The preparation method of the tumor vaccine provided by the present invention is applicable to the preparation of DC vaccines for various solid tumors, and the tumor is selected from lymphoma, myeloma, kidney cancer, prostate cancer, malignant melanoma, breast cancer, or colon cancer, etc. Solid tumors; preferably kidney cancer, prostate cancer, malignant melanoma, lymphoma, myeloma.
[0072] The present invention establishes a new DC vaccine preparation technology. In a preferred example, the specific operation steps are as follows:
[0073] (1) Extraction of tumor antigens
[0074] 1. Formaldehyde-fixed and paraffin-embedded patient tumor tissue, cut into 5-10 μm thick slices;
[0075] 2. Mix the flakes with pure xylene to dissolve the paraffin;
[0076] 3. Centrifuge to remove xylene, add absolute ethanol, and centrifuge several times to remove toxic organic solvents to obtain precipitates; then wash away the organic solvents with ethanol;
[0077] 4. Add an aqueo...
Embodiment 1
[0099] Preparation of Lymphoma DC Vaccine
[0100] (1) Extraction of tumor antigens
[0101] 1. For the lymphoma (diffuse large B-cell lymphoma) tumor tissue of the patient who was fixed in formaldehyde and embedded in paraffin, cut 0.2g of tumor tissue into 5-10μm thick slices and put them into a 15ml centrifuge tube;
[0102] 2. Add 10ml of 100% pure xylene, mix immediately, and incubate at 50°C for 5 minutes to dissolve the paraffin;
[0103] 3. 400g, centrifuge for 1 minute, discard xylene. Add 10ml of absolute ethanol, shake and mix;
[0104] 4. 400g, centrifuge for 1 minute, discard the ethanol, then add 10ml of absolute ethanol, shake and mix;
[0105] 5. 400g, centrifuge for 1 minute, remove residual ethanol as much as possible without destroying the precipitate;
[0106] 6. Add 20% Na 2 CO 3 Solution 10ml, put in 37℃ water bath for 1-6 hours;
[0107] 7. Centrifuge at 400g for 1 minute, discard all the liquid, then add 10ml of water for injection, vortex and mi...
Embodiment 2
[0126] Induction of tumor-specific CTL by DC vaccine in vitro
[0127] 1. Prepare the tumor antigen according to the method of Example 1.
[0128] 2. Prepare DC according to the method steps of Example 1.
[0129] 3. DC antigen challenge: Prepare according to the relevant steps of Example 1 to obtain DC vaccine.
[0130] 4. Grouping and in vitro cytotoxic T lymphocyte (CTL) culture:
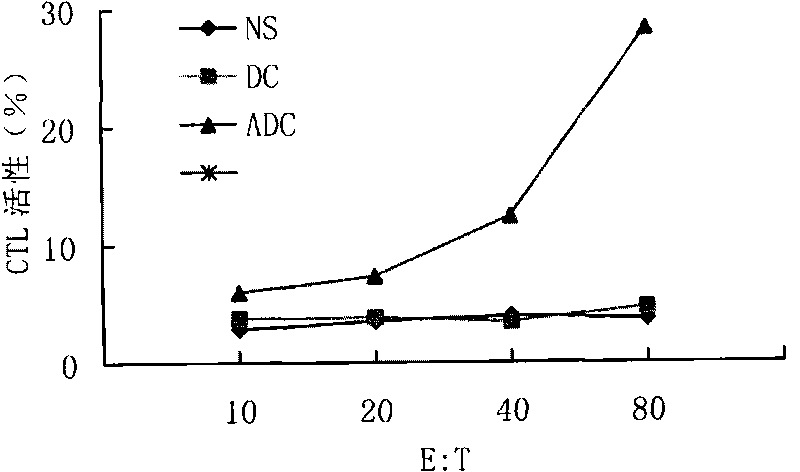
[0131] The peripheral blood mononuclear cells of patients were prepared, and 5×10 5 / ml of cell suspension, seeded into 4 culture flasks, 20ml in each flask, and then added 1×10 5 DC vaccine (ADC);
[0132] DC (DC) and normal saline (NS) were added to the control group, fresh complete medium and DC vaccine were supplemented on the 7th day of culture, and the cells were cultured until the 14th day.
[0133] 5. CTL detection: the patient's tumor cells were used as target cells, and the killing activity of CTL in vitro was detected by LDH method.
[0134] Results: The DC vaccine can induce the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com