Bispecific antibody against EGFR protein and MET protein
A bispecific antibody and specific technology, applied in the field of bispecific antibody and its preparation, can solve the problems of poor guiding effect and low affinity of target molecules, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Preparation of bispecific antibodies
[0073] 1. Sequence design
[0074] The amino acid sequence composition and coding nucleic acid sequence of the antibody prepared by the present invention are shown in Table 1.
[0075] Table 1 Amino acid sequence and nucleic acid sequence of bispecific antibody
[0076]
[0077]
[0078]
[0079] 2. The expression of antibodies
[0080] 2.1 Gene synthesis and vector construction
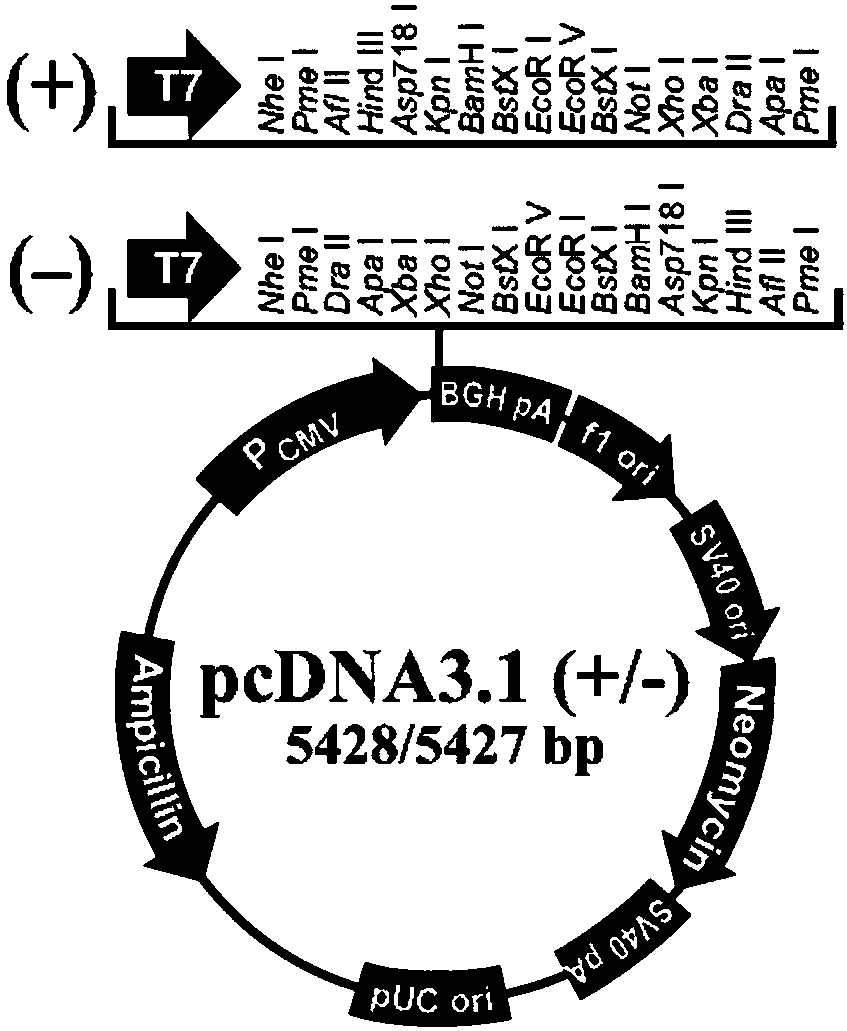
[0081] Synthesize DNA sequences as shown in SEQ ID NO.19-35 respectively. The 5'end of the synthesized DNA sequence contains the Kozak sequence (CCCACCATGG) and the signal peptide (METDTLLLWVLLLWVPGSTG); and the 5'end contains EcoRI restriction site and 3' HindIII restriction site. Combine the synthesized DNA segment and pcDNA3.1(-) vector plasmid ( figure 1 ) Digest with EcoRI and HindIII restriction enzymes, and perform agarose gel electrophoresis to recover the corresponding digested gene fragments. Then the purified heavy and light chain coding DNA ...
Embodiment 2
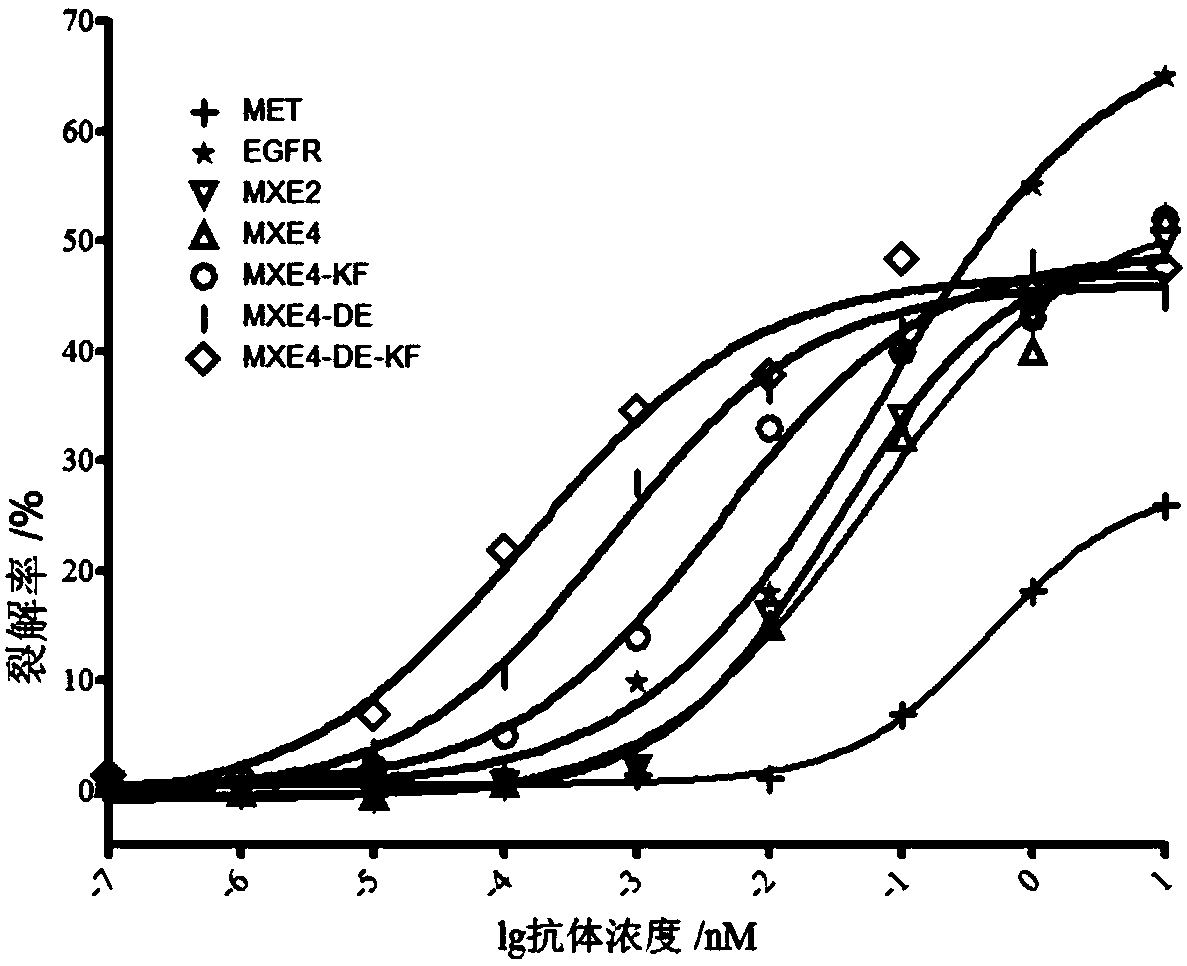
[0096] Example 2 Mass spectrometric identification of bispecific antibodies
[0097] experimental method:
[0098] Take 400μg of each antibody separately, add 10μL G7PNGaseF enzyme digestion buffer, 10μL 10% NP40, 2.5μL PNGaseF, supplemented with ultrapure water, the final volume to 100μL, after mixing, wrap it with a sealing film and place it in a 37℃ water bath Warm bath overnight. The antibody sample from which the N-sugar was cut was desalted by a C4 reverse chromatographic column and analyzed by TripleTOF 5600 (AB Sciex) mass spectrometry from AB Sciex, and the data was analyzed by Analyst TF software.
[0099] result:
[0100] After purification and assembly, the antibody was modified by glycosidase to remove the N glycosyl group. The molecular weight determined by mass spectrometry is shown in Table 3. Table 3 shows that the difference between the purified and assembled antibody and the theoretical value is within the error range of the instrument, indicating that the antibody...
Embodiment 3
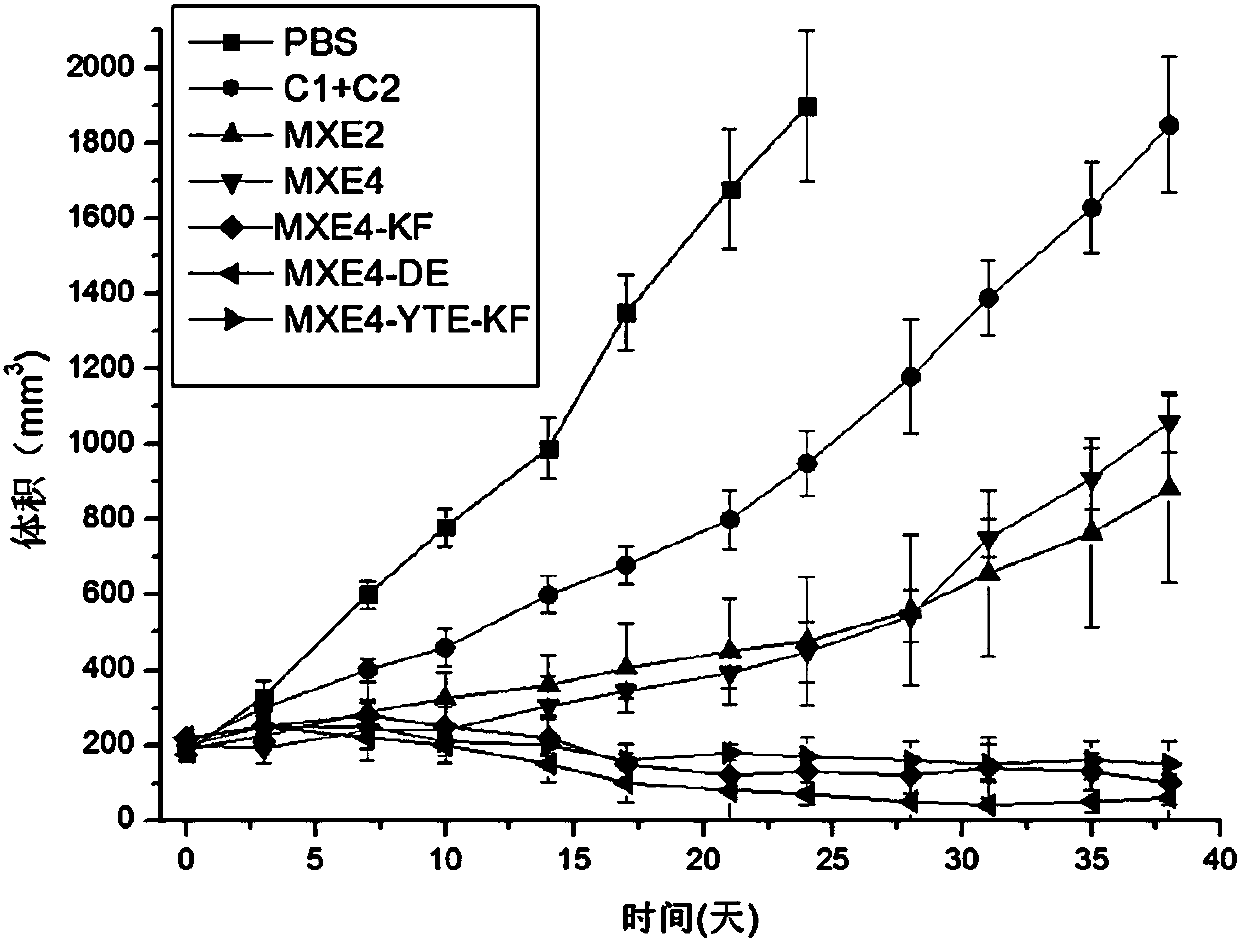
[0104] Example 3 Purity determination of bispecific antibodies
[0105] (1) Molecular exclusion high performance liquid chromatography
[0106] At 200mM KH 2 PO 4 , 250mM KCl, pH 6.0 mobile phase, column temperature 25℃, injection volume: 40μg, flow rate 0.6ml / min, isocratic operation for 25min, analyze bispecific antibody and control antibody samples by molecular exclusion high performance liquid chromatography The purity of the monomer and the ratio of the polymer.
[0107] (2) Sodium dodecyl sulfate capillary electrophoresis (CE-SDS)
[0108] Using sodium dodecyl sulfate capillary electrophoresis (CE-SDS) ultraviolet detection method, under non-reducing conditions, according to the molecular weight, quantitative determination of the purity of recombinant monoclonal antibody products. Take 100μg of the sample, add 5μl of 0.8M iodoacetamide aqueous solution, vortex to mix, incubate at 70°C for 5 minutes, cool to room temperature and centrifuge at 6000 rpm for 1 minute. Take 75μl fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com