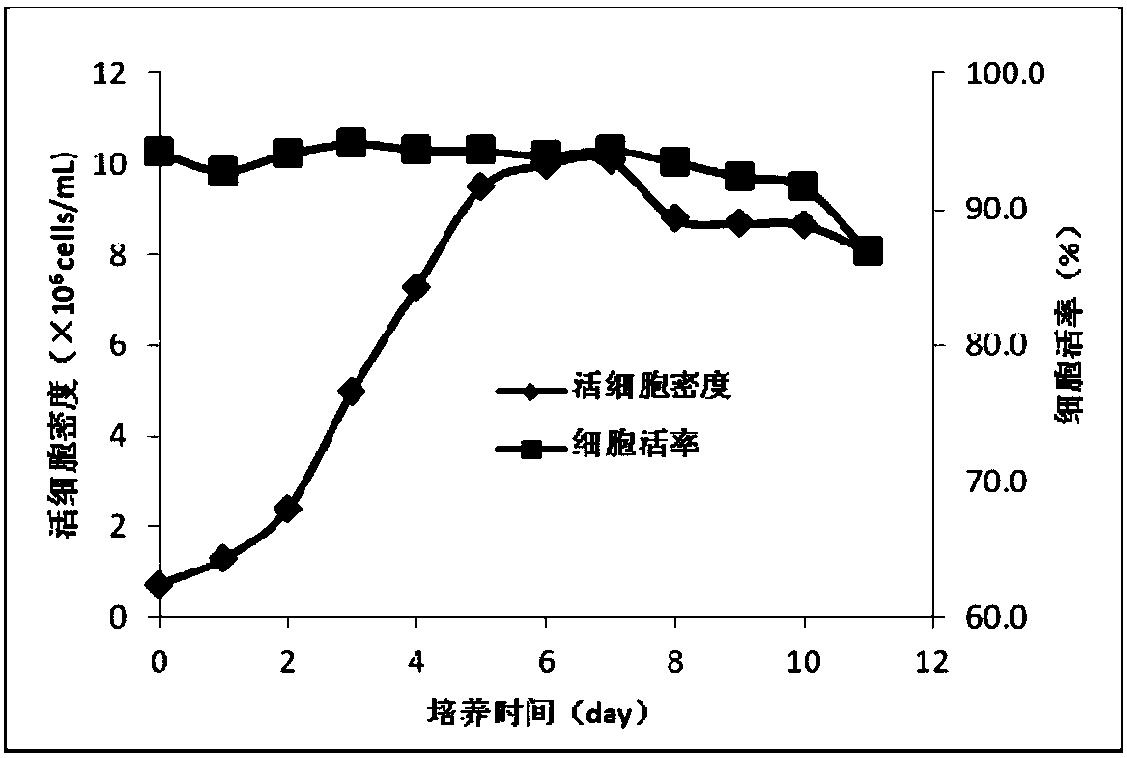
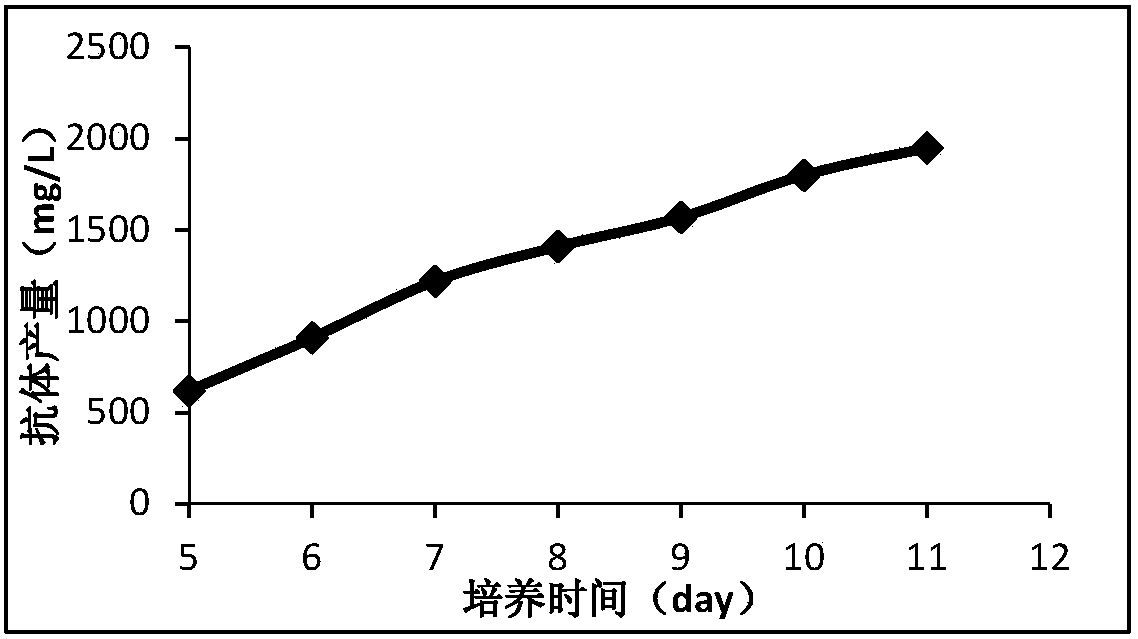
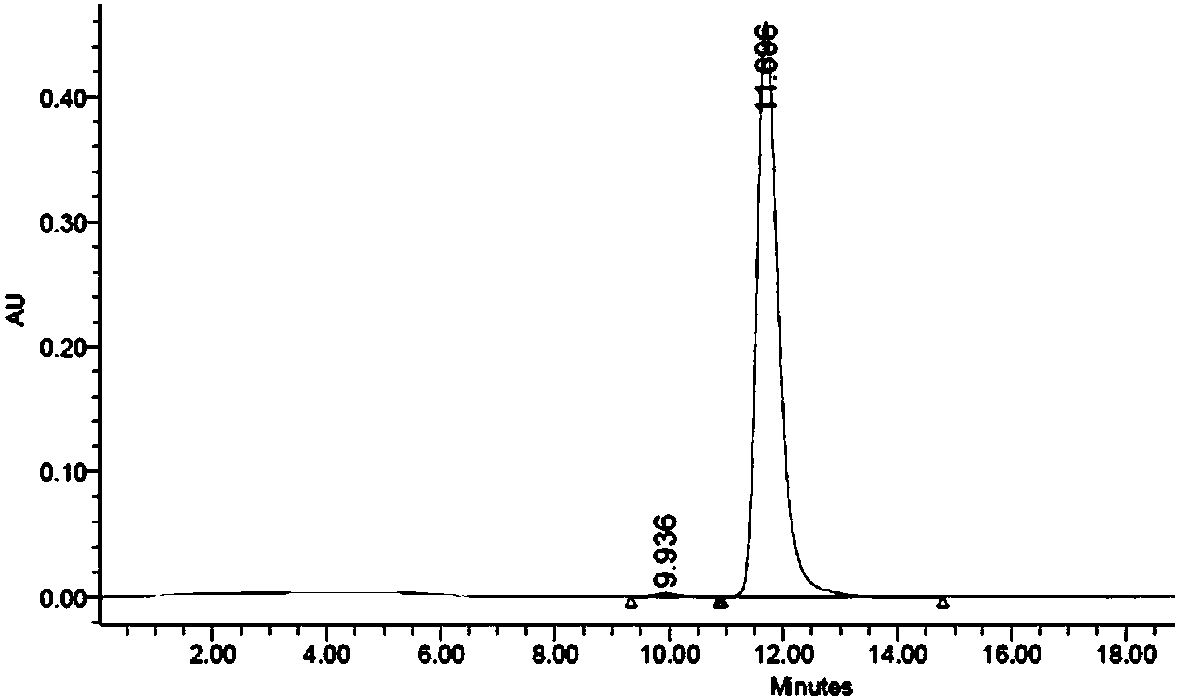
Cell culture medium and method for producing protein
A technology of cell culture and culture medium, applied in the field of biopharmaceuticals, which can solve the problems of potential risks in the stability of product quality between batches, complex components of hydrolyzate, and difficulty in controlling batch-to-batch consistency, and achieve good product consistency and easy quality. Effects of management and productivity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1, cell culture medium
[0060] 1. Preparation of basal medium
[0061] The formulation of the basal medium: CD FortiCHO AGT (Thermo Fisher Company), 12.5g / L; Ex-Cell302 (Sigma-Aldrich Company), 10.8g / L.
[0062] Preparation method: Take 80% of the preparation volume of water for injection in the liquid preparation container, weigh the Ex-cell302 dry powder and CD FortiCHO AGT dry powder required for the culture medium, add them to the liquid preparation container, and stir for 30 minutes. Adjust the pH to 6.9-7.0 with 5M sodium hydroxide, and continue stirring for about 10 minutes. Dilute to the prepared volume, filter and aliquot. Verify sterility after filtration and check filter integrity. Store in a refrigerator at 4°C.
[0063] 2. Preparation of feed medium
[0064] The formula and preparation method of the feed medium are as follows:
[0065] Table 1: Recipe of feed medium
[0066]
[0067]
[0068] Preparation method: Take 80% of the prep...
Embodiment 2
[0070] A cell culture medium comprising basal medium and feed medium,
[0071] The formulation of the basal medium: CD FortiCHO AGT, 5g / L; Ex-Cell 302, 5g / L.
[0072] Formula of feed medium: CD EfficientFeed C AGT, 40g / L; Cell Boost 5, 20g / L; Glutamic acid 0.1g / L; Tyrosine 0.5g / L; Cysteine 0.2g / L; Amino acid 0.1g / L; PF68 1g / L.
Embodiment 3
[0074] A cell culture medium comprising basal medium and feed medium,
[0075] The formulation of the basal medium: CD FortiCHO AGT, 20g / L; Ex-Cell 302, 15g / L.
[0076] Formula of feed medium: CD EfficientFeed C AGT, 80g / L; Cell Boost 5, 70g / L; Glutamic acid 0.8g / L; Tyrosine 1.2g / L; Cysteine 1g / L; Tryptophan Acid 0.8g / L; threonine 0.3g / L; phenylalanine 0.4g / L; PF68 2g / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com