Mycoplasma hyopneumoniae genetic engineering subunit vaccine as well as preparation method and application thereof
A technology of gene and coding gene, which is applied in the field of animal immune drugs, can solve the problems of high difficulty in operation, difficult cultivation, strong virulence, etc., and achieve the effects of reducing production costs, good application prospects, and high expression levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] For example, in a specific embodiment of the embodiments of the present invention, a method for preparing a genetically engineered subunit vaccine of Mycoplasma hyopneumoniae may specifically include:
[0062] (1) Prepare nucleic acid molecules for encoding P36-DnaK-ffh and P97-P46-DnaJ fusion proteins respectively;
[0063] (2) Clone each nucleic acid molecule prepared in step (1) into a shuttle vector to obtain a corresponding recombinant shuttle vector containing the target gene;
[0064] (3) Transform the recombinant shuttle vectors obtained in step (2) into DH10Bac bacteria, select the recombinant bacteria, extract the genome and transfect Sf9 cells (or other insect cells mentioned above) to obtain recombinant baculoviruses;
[0065] (4) Cultivate the Sf9 cells (or other aforementioned insect cells) and then recombinantly express and produce P36-DnaK-ffh, P97-P46-DnaJ fusion proteins;
[0066] (5) Mixing the P36-DnaK-ffh and P97-P46-DnaJ fusion proteins into an ad...
Embodiment 1
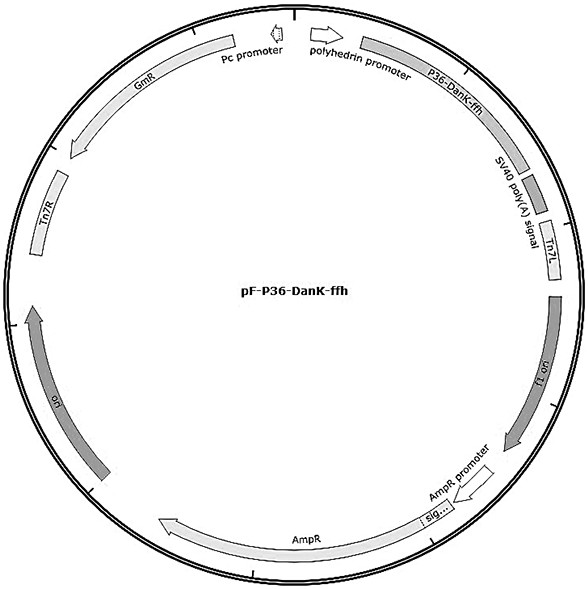
[0071] Example 1 Transfer vector pF-P36-DnaK-ffh construction and identification
[0072] 1. Amplification and purification of P36-DnaK-ffh gene
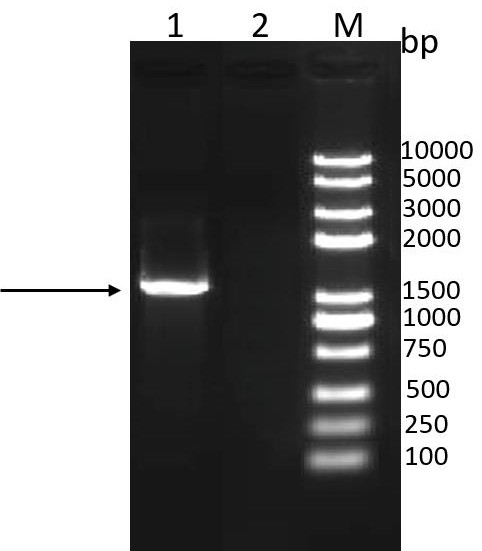
[0073] The codon-optimized P36-DnaK-ffh gene (SEQ ID NO: 1) was synthesized in Nanjing GenScript Biotechnology Co., Ltd. and cloned into the pUC17 vector to obtain the pUC-P36-DnaK-ffh plasmid vector. Using pUC-P36-DnaK-ffh plasmid as template, P36-DnaK-ffh-F, P36-DnaK-ffh-R as upstream and downstream primers for PCR amplification (P36-DnaK-ffh-F, P36-DnaK-ffh- The gene sequence of R is shown in SEQ ID NO: 3, 4), and the amplification system is shown in Table 1.
[0074] Table 1 P36-DnaK-ffh gene amplification system
[0075]
[0076] The reaction conditions were: 95°C pre-denaturation for 5 minutes; 94°C denaturation for 45 seconds, 54°C annealing for 45 seconds, 72°C extension for 1 minute, 35 cycles; 72°C extension for 10 minutes.
[0077] Perform gel electrophoresis on the PCR product to verify the size of the target gene...
Embodiment 2
[0093] Example 2 Construction and Identification of Transfer Vector pF-P97-P46-DnaJ
[0094] 1. Amplification and purification of P97-P46-DnaJ gene
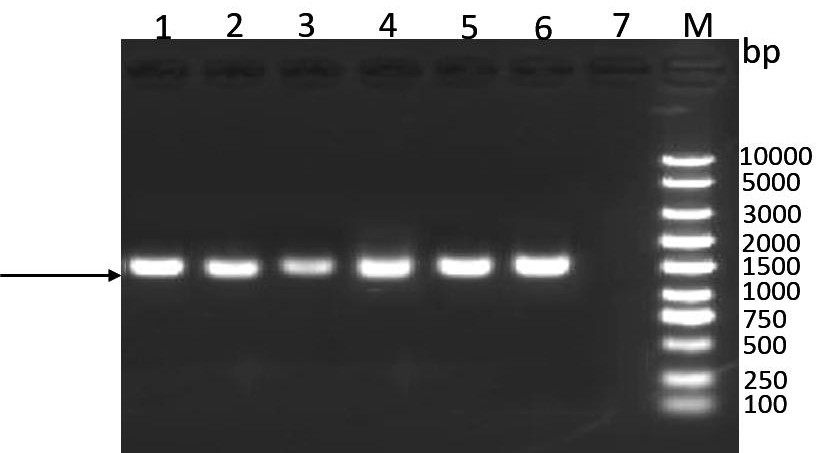
[0095] The codon-optimized P97-P46-DnaJ gene (SEQ IDNO:5) was synthesized in Nanjing GenScript Biotechnology Co., Ltd. and cloned into the pUC17 vector to obtain the pUC-P97-P46-DnaJ plasmid vector. Using pUC-P97-P46-DnaJ plasmid as template, P97-P46-DnaJ-F, P97-P46-DnaJ-R as upstream and downstream primers for PCR amplification (P97-P46-DnaJ-F, P97-P46-DnaJ- The gene sequence of R is shown in SEQ ID NO: 7, 8), and the amplification system is shown in Table 5.
[0096] Table 5 P97-P46-DnaJ gene amplification system
[0097]
[0098] The reaction conditions were: 95°C pre-denaturation for 5 minutes; 94°C denaturation for 45 seconds, 54°C annealing for 45 seconds, 72°C extension for 1 minute, 35 cycles; 72°C extension for 10 minutes.
[0099] Perform gel electrophoresis on the PCR product to verify the size of the target gen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com