Preparing method and application of mycoplasma bovis inactivated vaccine
A technology of Mycoplasma bovis and inactivated vaccines, which is applied in the field of preparation of Mycoplasma bovis inactivated vaccines, can solve the problems of indistinguishable interaction and insignificant immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
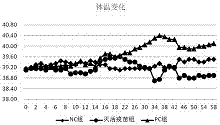
[0023] 1. Study on formaldehyde inactivation conditions for the preparation of Mycoplasma bovis inactivated vaccine
[0024] After recovering the frozen M.bovis strain, inoculate it into the M.bovis liquid medium, culture it under certain conditions, pass passage after 3 days, measure the CCU content in the fourth-generation M.bovis culture, and concentrate it according to a certain ratio get 1.0×10 10 The ccu / ml M.bovis culture is ready for use, add formaldehyde (analytical pure) to the culture to make the final concentration respectively 0.3%, 0.4%, 0.5%, mix well and culture under the same conditions, measure every 12h The content of M.bovisCCU was measured three times for each concentration, and the average value was taken. When the CCU content was 0, the culture was centrifuged at 16000r / min for 30min, and the obtained precipitate was washed 3 times with 0.1mol / L PBS, and then blindly passed in liquid medium at a ratio of 1:10 for 3 generations while inoculating solid me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com